Abstract
Anoikis is a programmed cell death that occurs when cells detach from the extracellular matrix (ECM). Its role in psoriasis remains unclear. This study aims to explore the relationship between psoriasis and apoptosis, focusing on anoikis-related mechanisms. Differentially expressed genes (DEGs) in psoriasis were identified using the GEO database and overlapped with anoikis-related genes from GeneCards to find differentially expressed anoikis-related genes (DE-ARGs). A PPI network was constructed, revealing eight hub DE-ARGs. These were then analyzed using GO, KEGG and ssGSEA. Immune infiltration cells and molecules were examined, and single-cell data analysis, immunohistochemistry, qRT-PCR, Western blotting, gene mapping, and drug prediction were performed. Eighteen DE-ARGs were identified, with STAT3 and BIRC5 as key DEGs. Enrichment analysis showed DE-ARGs were related to the regulation of anoikis and positive regulation of epithelial cell proliferation. Notable differences in dendritic and mast cells were observed between psoriasis lesions and no-lesion samples. Elevated expression of BIRC5 and STAT3 in psoriasis lesions was confirmed through qRT-PCR, western blotting and immunohistochemistry. This study establishes a relationship between anoikis and psoriasis, where STAT3 and BIRC5 play important roles. STAT3 and BIRC5 may serve as potential targets for the prevention and treatment of psoriasis.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-13992-3.
Keywords: Psoriasis, Anoikis, DEGs, Biomarkers, Immune microenvironment
Subject terms: Cell biology, Computational biology and bioinformatics, Molecular biology
Introduction
Psoriasis is a chronic, systemic immune-mediated disease characterised by erythematous, induration, scaly, pruritic and often painful skin plaques1. It affects approximately 2–3% of the global population2, imposing a significant burden on individuals and society3. Despite its prevalence, the pathogenesis of psoriasis remains largely unclear. While the disease has a genetic component4, various environmental and intrinsic factors are speculated to contribute to its development5. Psoriasis is marked by persistent inflammation and the inflammation of dendritic cells (DC), macrophages, T cells and neutrophils6. Notably, neutrophil activity and the neutrophil-to-lymphocyte ratio (NLR) are significantly elevated in patients with psoriasis7. The skin of patients with psoriasis shows an increased presence of DCs, specifically plasmacytoid DC (pDC) and myeloid DCs, which play important roles in the early and chronic phases of the disease, respectively8,9. The proportion of activated mast cells is also significantly increased in patients with psoriasis10,11. Additionally, several inflammatory mediators and cytokines, including IL-17, IL-23, TNF and IFN, are involved in the pathogenesis of psoriasis. IL-17, particularly IL-17A, is a key cytokine in psoriasis, targeting keratinocytes and promoting inflammation4,12. TNF-α, produced by T cells, is a crucial component of the TNF-α/IL-23/IL-17 signalling axis in psoriasis13. IFN influences psoriasis through the Janus kinase (JAK)/signal transducer and activator of the transcription (STAT) pathway14. The JAK-STAT signalling pathway is central to psoriasis15,16, with excessive activation of STAT3 playing a significant role in disease pathogenesis17. Psoriasis is also closely linked to the hypoxia inducible factor-1 (HIF-1) signalling pathway and the nucleotide oligomerization domain (NOD)-like receptor pathway18,19.
Anoikis is a form of programmed cell death that occurs when cells detach from the extracellular matrix (ECM) and lose contact with neighbouring cells20–22. Anoikis eliminates mispositioned cells and prevents their adhesion to new matrices, thus limiting dysplasia22. However, anoikis resistance is associated with tumour invasiveness and metastasis21. Molecules and signalling pathways have been identified that mediate anoikis resistance in cancer cells23. In triple-negative breast cancer (TNBC), anoikis resistance is associated with manganese superoxide dismutase (MnSOD), transforming growth factor β1 (TGF-β1), and collagen XIII24. In glioma, anoikis resistance is linked to integrin, IGFR, the Hippo pathway and NF-κB25. Moreover, inflammatory mediators and cytokines play an important part in anoikis resistance. Specifically, IL-17A enhances anoikis resistance and promotes TNBC metastasis26. Similarly, IL-6 accelerates anoikis resistance in oral squamous cell carcinomas27, while TNF-α regulates anoikis and anoikis resistance through binding to its receptor TNFR1/TNFR228.
The relationship between psoriasis and anoikis remains unexplored. However, key inflammatory mediators and cytokines in psoriasis have been reported to be strongly correlated with anoikis resistance. Notably, the JAK-STAT signalling pathway is a key pathway in psoriasis and the excessive activation of STAT3 promotes the development of psoriasis. Furthermore, excessive activation of STAT3 enhances anoikis resistance in tumour cells, while its silencing reduces anoikis resistance in melanoma and pancreatic cancer29,30. Given that IL-17A is a crucial inflammatory factor in psoriasis pathogenesis and enhances anoikis resistance in TNBC, there is a potential link between anoikis resistance and psoriasis. However, the relation between psoriasis and anoikis is yet to be elucidated. Further investigation is needed to elucidate whether anoikis plays a role in psoriasis pathogenesis. This study aims to uncover anoikis-related molecular mechanisms and explore the immune microenvironment in psoriasis through comprehensive bioinformatics analyses.
Material and methods
Data source and pre-processing
A total of 26 anoikis-associated genes were retrieved from the GeneCards database (https://www.genecards.org/), using a relevance score threshold of > 2.0, which indicates a strong correlation between genes and anoikis. Gene expression profile datasets GSE117239 and GSE30999 were obtained from the GEO database (https://www.ncbi.nlm.nih.gov/geo/). The GSE117239 dataset comprised 324 samples, including 240 from patients with psoriasis, while the GSE30999 dataset included 170 samples, with 85 from patients with psoriasis. The inclusion criteria for psoriasis samples were based on histological features such as (1) epidermal hyperplasia, (2) dilated and prominent blood vessels in the dermis, (3) inflammatory infiltration of leukocytes, predominantly in the dermis, (4) loss of the granular cell layer, (5) parakeratosis and (6) normal histology of uninvolved and clinically asymptomatic skin areas. Robust Multiarray Average (RMA) was employed for data pre-processing to adjust for missing values and outliers.
Differentially expressed gene identification
Differentially expressed genes (DEGs) were identified from the GES117239 and GES30999 datasets using the ‘limma’ package in R software (version 4.2.3, URL: https://www.r-project.org), with thresholds set at a P-value < 0.05 and |log2FC|> 1.5. The DEGs from both datasets were intersected, and the resulting DEGs were overlapped with anoikis-associated genes. Visualisation of differentially expressed anoikis-related genes (DE-ARGs) was performed using ‘Heatmap’ and ‘ggplot2’ packages in R, which generated volcano maps, heatmaps, and block diagrams.
Protein–protein interaction
The STRING database (version 12.0, https://cn.string-db.org/) and Cytoscape software (version 3.8.1, URL: https://cytoscape.org) were utilised to construct a protein–protein interaction (PPI) network for the DE-ARGs. To clarify gene interactions, the MCODE analysis plugin was used to identify and visualise hub genes within the PPI network. The ‘corrplot’ package of R software was used for Spearman correlation analysis of the hub genes.
Functional enrichment analysis
Gene Ontology (GO) annotation was performed using the ‘Org.Hs.eg.db’ package in R software. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway gene annotations were retrieved from the KEGG rest API (https://www.kegg.jp/kegg/rest/keggapi.html)31. GO enrichment analysis was conducted across three categories: biological process (BP), cellular component (CC) and molecular function (MF). KEGG analysis was used to identify the enrichment pathways associated with the DEGs. Both GO and KEGG pathways were performed with a threshold of P-value < 0.05 using the ‘clusterProfiler’ package. Results were visualised with the ‘GOplot’ and ‘ggplot2’ packages in R software. Using the Benjamini & Hochberg (BH) method, we controlled the false discovery rate (FDR), ensuring an FDR < 0.1 and P-value < 0.05.
Single-sample gene set enrichment analysis
To further investigate the functional pathways enriched by DE-ARGs, Single-sample Gene Set Enrichment Analysis (ssGSEA) was performed on the eight hub DE-ARGs. Gene expression data were stratified into high and low expression groups based on the mean expression levels of the eight DE-ARGs. The ‘GSEA’ package in R was used for ssGSEA, and the five most clinically significant pathways with the highest enrichment values were visualised.
Immune infiltration analysis
The CIBERSORT (https://cibersortx.stanford.edu/) algorithm was employed to assess the association between 21 immune cell types and the eight DE-ARGs. Expression levels of these 21 immune cells in psoriasis lesions (LS) and no-lesion (NL) samples were compared using R software. The correlation between 29 immune molecules and the 8 DE-ARGs was also explored with the CIBERSORT algorithm and visualised using the ‘ggplot’ package in R.
Single-cell data analysis
The CellAtlasPsoriaticSkin (https://yz-studio.shinyapps.io/psoriaticskincellatlas2/) dataset provided comprehensive cell atlas data, including neural network learning, differential gene expression and GO enrichment components32. We applied CellAtlasPsoriaticSkin to identify 21 major skin cell subtypes involved in cellular inflammation during psoriasis and to visualise gene expression patterns across these cell types.
Drug prediction analysis
The Human Protein Atlas (HPA) (https://www.proteinatlas.org/) was used to obtain immunofluorescence staining images of genes. The Dgibd database (https://dgidb.org/) was queried to identify potential drugs targeting DE-ARGs. Drug candidates were selected based on the following criteria: (1) relevance to Homo sapiens, (2) high correlation with DE-ARGs, (3) reported in the literature and proved to have relevant effects and (4) availability of a chemical formula. Subsequently, potential compounds with specific biological activities were identified, and their structural formulas were obtained from CHEMBL (https://www.ebi.ac.uk/chembl/). Chromosomal locations of three DE-ARGs were identified and visualised using the ‘RCiros’ package in R software. Additionally, miRWalk (http://mirwalk.umm.uni-heidelberg.de/) was used to screen for target mRNAs and miRNAs associated with the three hub genes, and a DNA-mRNA-miRNA axis was constructed using Cytoscape software (version 3.8.1) with the MCODE plugin.
Molecular docking
To explore the binding modes and interactions between potential drugs and target proteins, we performed molecular docking and structural visualization using the computational software AutoDock Vina (version 1.2.2, URL: https://vina.scripps.edu)33. The molecular structures of the compounds were retrieved from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/)34, while the crystal structures of the target proteins STAT3 (PDB ID, 4ZIA; resolution, 2.70 A) and BIRC5 (PDB ID, 1F3H; resolution, 2.58 A) were obtained from the PDB (http://www.rcsb.org/). Binding energy and the number of hydrogen bonds formed during docking were used as evaluation criteria35.
Clinical samples
Skin samples embedded in paraffin were collected from 3 psoriasis patients and 3 healthy controls at the tissue bank of the First Affiliated Hospital of Xi’an Jiaotong University in Xi’an, China. Ethical permit was obtained from Ethics Committee of Xi’an Jiaotong University (Reference number: LLSBPJ-2024-701). The screening conditions for clinical samples of patients with psoriasis were as follows: adult, moderate to severe plaque psoriasis, disease stage in progress, limb samples, untreated, yellow race. Three clinical samples of patients with psoriasis were screened for the next study, from two men and one woman. The clinical samples of the control group were from adults in the department of aesthetic surgery.
Cell Culture and Treatment
The HaCaT cells were cultured using Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. The cell culture was maintained at 37 °C in a humidified atmosphere with 5% carbon dioxide. To create a psoriasis-like environment, the cells were treated with a mixture of cytokines, specifically 10 ng/ml of M5 (a combination of IL-1α, IL-17A, IL-22, oncostatin M, and TNF-α), obtained from PeproTech, USA. This stimulation process lasted for 24 h at the same temperature of 37 °C36.
qRT-PCT
RNA extraction was performed using TRIzol reagent according to the protocol. cDNA was generated and used for qRT-PCR as previously described37. The primer oligonucleotide sequences were as follows: hSTAT3: forward-5′-ACCAGCAGTATAGCCGCTTC-3’, and reverse-5’-GCCACAATCCGGGCAATCT-3′; hBIRC5: forward-5′-AGGACCACCGCATCTCTACAT-3′, and reverse-5′-AAGTCTGGCTCGTTCTCAGTG-3′; hGAPDH: forward 5′-CTCCTCCACCTTTgACgCTg-3′, and reverse 5′-TCCTCTTgTgCTCTTgCTgg-3′. GAPDH was used as an internal control, and Data were analyzed by the 2-ΔΔCT method.
Western blotting
Western blotting was performed as previously described37. The primary antibodies used in the study were as follows: STAT3 (A19566, ABclonal, China, Hubei), BIRC5 (A1551, ABclonal, China, Hubei) and α-Tubulin (KC031236S, Abmart, China, Shanghai). Cells were washed twice with cold PBS and lysed with IP buffer containing protease inhibitor PMSF (Sigma Aldrich, #P7626). The protein lysates were separated by SDS gel electrophoresis, transferred to PVDF membrane and incubated with specific primary antibodies overnight at 4 °C and HRP-conjugated second antibodies for 1 h at room temperature. α-Tubulin was used as an internal control. Band intensities were visualized by ChemiScope 3300 Mini (CLINX Science, Shanghai, China) and quantified by the ImageJ (version 1.54b, URL: https://imagej.net/ij/) software. The antibodies used and their dilutions were listed in supplementary Table S1.
Immunohistochemistry
Normal and psoriasis samples were performed using standard immunohistochemistry procedures. The antibodies (STAT3 and BIRC5) used for Immunohistochemistry were the same as those used for Western blotting and diluted at a ratio of 1:100. The specific antibody information is shown in Table S1. The K-viewer software (version 1.0.5, URL: https://www.kfbio.cn/k-viewer/) was used to scan the stained sections.
Statistical analysis
Analyses of the data from the above database were conducted using the R software. A P-value < 0.05 was deemed statistically significant and adjusted by FDR < 0.1 using the Benjamini & Hochberg method. Gene expression differences between psoriasis and normal groups were assessed via the Wilcoxon test, while DE-ARGs correlations were evaluated using the Pearson correlation test. The results of qRT-PCR were analyzed using Student’s t-test.
Results
Identification of DEGs
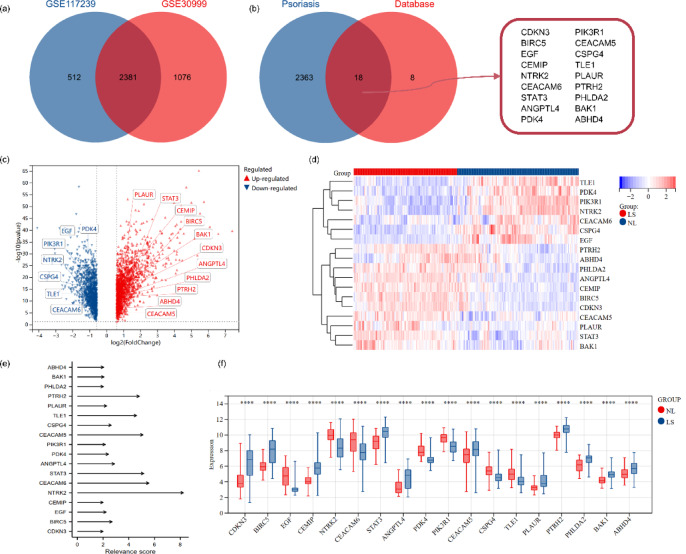
DEGs were identified using the ‘limma’ package in R software with thresholds set at P-value < 0.05 and |log2FC|> 1.5. A total of 2381 DEGs were identified in both the GSE117239 and GSE30999 datasets (Fig. 1a). By overlapping these DEGs with a set of 26 anoikis-related gene sets, 18 DE-ARGs were identified with a relevance score > 2.0 (Fig. 1b,e and Table 1). Among these, 11 DE-ARGs were upregulated and 7 were downregulated in LS compared to NL samples, as shown in the volcano and heat maps (Fig. 1c,d). Among these, STAT3, BIRC5 and CDKN3 were significantly upregulated. The expression patterns of these DE-ARGs were visualised in block diagrams (Fig. 1f), highlighting significant differences between LS and NL groups.
Fig. 1.
Venn diagram and differential expression analysis of 18 differentially expressed anoikis-related genes. (a) Intersection of DEGs from the GSE117239 and GSE30999 datasets; (b) DE-ARGs identified by overlapping DEGs with the anoikis-related gene set (relevance score > 2.0); (c,d) Volcano plot and heat map showing differential expression analysis of the 18 DE-ARGs. LS: psoriasis lesions; NL: no-lesion. (e) Relevance scores of the 18 DE-ARGs; (f) Block diagram illustrating the expression patterns of the 18 DE-ARGs.
Table 1.
18 differentially expressed anoikis-related genes.
| Gene | Change | GSE117239 | GSE30999 | ||||
|---|---|---|---|---|---|---|---|
| logFC | P Value | adj.P.Val | logFC | P Value | adj.P.Val | ||
| EGF | Down | − 1.671644745 | 6.21E–40 | 2.08E–37 | –1.914823529 | 3.89E–23 | 6.41E–22 |
| NTRK2 | Down | − 1.562415828 | 2.03E–17 | 2.26E–16 | –0.810745098 | 1.59E–28 | 4.53E–27 |
| CEACAM6 | Down | − 1.39888069 | 5.94E–12 | 3.16E–11 | –1.213941176 | 8.72E–07 | 2.85E–06 |
| PDK4 | Down | − 1.114416396 | 2.79E–31 | 2.57E–29 | –0.857176471 | 4.17E–22 | 6.18E–21 |
| PIK3R1 | Down | − 1.03985224 | 6.98E–20 | 1.10E–18 | –1.149205882 | 1.78E–28 | 5.00E–27 |
| CSPG4 | Down | − 0.864711877 | 5.83E–16 | 5.31E–15 | –1.080705882 | 1.84E–12 | 1.06E–11 |
| TLE1 | Down | − 0.846855051 | 4.29E–14 | 3.02E–13 | –0.598117647 | 1.04E–21 | 1.49E–20 |
| ABHD4 | Up | 0.610510407 | 9.38E–08 | 2.97E–07 | 0.827529412 | 3.93E–10 | 1.79E–09 |
| BAK1 | Up | 0.691203277 | 1.72E–16 | 1.69E–15 | 0.797411765 | 5.31E–16 | 4.35E–15 |
| PTRH2 | Up | 0.691971486 | 1.68E–12 | 9.62E–12 | 1.062470588 | 1.70E–22 | 2.62E–21 |
| PHLDA2 | Up | 0.745183654 | 5.92E–13 | 3.60E–12 | 1.565882353 | 1.44E–31 | 5.85E–30 |
| PLAUR | Up | 0.833822934 | 6.84E–11 | 3.20E–10 | 1.137960784 | 1.51E–13 | 9.65E–13 |
| CEACAM5 | Up | 0.892901385 | 7.35E–06 | 1.84E–05 | 0.797705882 | 3.69E–11 | 1.85E–10 |
| ANGPTL4 | Up | 1.156615585 | 4.59E–14 | 3.22E–13 | 1.867705882 | 1.72E–29 | 5.46E–28 |
| STAT3 | Up | 1.259483812 | 9.70E–18 | 1.12E–16 | 1.0995 | 5.04E–24 | 9.02E–23 |
| CEMIP | Up | 1.609465896 | 3.46E–26 | 1.47E–24 | 1.093411765 | 2.27E–32 | 1.02E–30 |
| BIRC5 | Up | 1.880395712 | 3.58E–21 | 6.96E–20 | 1.845441176 | 1.99E–38 | 1.72E–36 |
| CDKN3 | Up | 2.261047537 | 6.40E–20 | 1.02E–18 | 3.341588235 | 2.16E–35 | 1.34E–33 |
Construction of PPI network and identification of hub genes
We utilised the STRING database to analyse the interactions among the 18 DE-ARGs with medium confidence and obtained a PPI network with 18 nodes and 20 edges (Fig. 2a). This PPI network was filtered to identify subnetworks with the highest clustering scores, visualised using Cytoscape software and the MCODE plugin (Fig. 2b). The analysis identified STAT3, BIRC5, PIK3R1, EGF, CEACAMS, NTRK2, PLAUR and CDKN3 as hub genes. Subsequently, correlation analysis of these hub genes revealed significant positive correlations between NTRK2 and PIK3R1 (Fig. 2c). BIRC5 showed a significant negative correlation with NTRK2 and PIK3R1 and a positive correlation with STAT3 and PLAUR (P < 0.0001).
Fig. 2.
PPI and correlation analysis of hub differentially expressed anoikis-related genes. (a) PPI network illustrating interactions among the 18 DE-ARGs, constructed using STRING (version 12.0, https://cn.string-db.org/); (b) The subnetwork of eight hub DE-ARGs with the highest clustering scores, identified via Cytoscape software (version 3.8.1, URL: https://cytoscape.org); (c) Correlation analysis demonstrating the relationships between the eight hub DE-ARGs, performed using R software (version 4.2.3, URL: https://www.r-project.org). **P < 0.01, ***P < 0.001, ****P < 0.0001.
Enrichment analysis of DE-ARGs
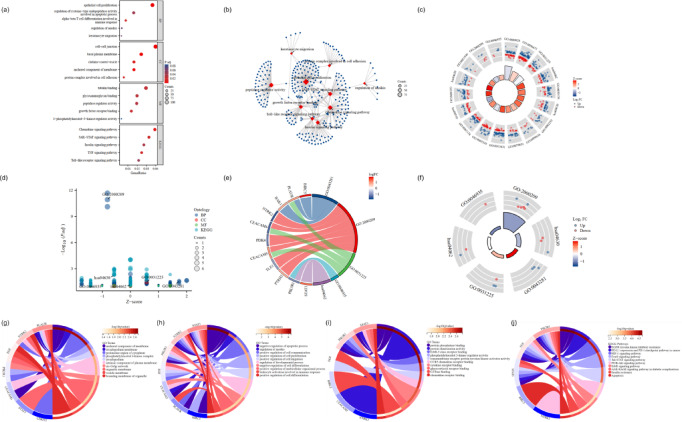
Kyoto Encyclopedia of Genes and Genomes (KEGG) provides the integration and analysis of genome, chemical substances, drugs, diseases and other biological information, such as Genome Atlas, metabolic pathways, signal transduction pathways, disease information, etc. Gene ontology (GO) is composed of molecular function, biological process and cellular component. It can use a structured vocabulary to describe the function, location and product of genes and proteins. KEGG and GO enrichment analyses of the 18 DE-ARGs were conducted to elucidate the underlying mechanism of anoikis in psoriasis via R software. KEGG analysis revealed that the DE-ARGs were predominantly enriched in pathways related to the positive regulation of epithelial cell proliferation, chemokine signalling pathway and JAK-STAT signalling (Fig. 3a,b). The loop graph of GO | KEGG combined with logFC demonstrated that the 18 DE-ARGs were closely associated with the basal plasma membrane, epithelial cell proliferation and chemokine signalling pathway (Fig. 3c). Bubble plots, loop graphs and chordal diagrams illustrated a prominent correlation between DE-ARGs and anoikis regulation (Fig. 3d,e,f). Notably, the chord diagram revealed that STAT3 was linked to several key pathways, including the regulation of anoikis, cysteine-type endopeptidase activity involved in apoptosis, leukocyte activation in immune responses, JAK-STAT signalling and chemokine signalling (Fig. 3g–j). Additionally, STAT3, NTRK2, PIK3R1, CEACAM5, PLAUR and BIRC5 were associated with the negative regulation of apoptotic processes. STAT3, NTRK2 and CEACAM5 were also connected with protein phosphatase binding and protein dimerisation activity. PIK3R1, EGF and STAT3 were relevant to EGFR tyrosine kinase inhibitor resistance, HIF-1 signalling pathway, forkhead box O (FoxO) signalling pathway and Jak-STAT signalling pathway. The HIF-1 signalling pathway and Jak-STAT signalling pathway are closely linked to psoriasis18,19. FoxO pathway is involved in many biological processes, such as cell cycle, cell proliferation, apoptosis, anti-oxidative stress and so on, and has an important connection with the occurrence and development of psoriasis38. Single sample gene set enrichment analysis (ssGSEA) compares the gene expression data of each sample with the immune cell gene set to estimate the relative enrichment of the gene set in the sample, thereby inferring the relative abundance of different immune cell types in the sample. Furthermore, ssGSEA was conducted for the eight DE-ARGs to investigate functional enrichment pathways in detail. The analysis revealed strong correlations with pathways related to type I diabetes mellitus, rig-i-like receptor signalling, NOD-like receptor signalling, Toll-like receptor signalling and the cell cycle (Fig. S1). The NOD-like receptor pathway is closely linked to psoriasis18,19.
Fig. 3.
GO and KEGG analysis of DE-ARGs. (a,b) Bubble plot and network representation of GO and KEGG analysis; (c–f) The loop graph, bubble plot and chordal graph of GO | KEGG combined with logFC analysis. (g–j) Chordal graph illustrating the relationships between DE-ARGs and GO terms. BP, biological process; CC, cellular composition; MF, molecular function; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes.
Immune infiltration analysis of DE-ARGs
The CIBERSORT algorithm was used to investigate the correlation between immune cells and molecules and the eight DE-ARGs. Results revealed that CDKN3, BIRC5, STAT3, CEACAM5 and PLAUR were positively associated with activated dendritic cells. Conversely, CDKN3, BIRC5 and STAT3 were negatively correlated with resting mast cells (Fig. 4a). Among immune molecules, BIRC5 exhibited a strong positive correlation with TNF, IL2RG, IL12RB1, IL12B, IL17A and IL22RA1 (P < 0.0001). STAT3 was positively associated with IFNGR1 and IFNGR2 but negatively correlated with IF17RE (Fig. 4b). Comparison of the expression levels of 21 immune cell types between NL and LS groups revealed notable differences (Fig. 4c). Significant variances were observed in resting mast cells, activated dendritic cells, M1 macrophages, activated NK cells, gamma delta T cells, CD4 memory activated T cells and follicular helper T cells (P < 0.0001). Differential expression levels of these immune cells were also assessed in relation to low and high expression groups of the eight DE-ARGs. In conclusion, we chose BIRC5, STAT3, CDKN3, NTRPK2 and PIK3R1 as target DE-ARGs for further study.
Fig. 4.
Immune infiltration analysis of eight DE-ARGs. (a) Heat map showing the correlation between eight DE-ARGs and 21 types of immune cells; (b) Bubble plot illustrating the relationship between DE-ARGs and 27 immune molecules; (c) Box plot comparing immune cell infiltration levels in NL and LS groups. LS: psoriasis lesions; NL: no-lesion. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Single-cell data analysis
CellAtlasPsoriaticSkin was employed to visualise gene expression patterns of five DE-ARGs (BIRC5, STAT3, CDKN3, NTRPK2 and PIK3R1) within categorised cell types. Stem cycling keratinocytes accumulate in the stratum corneum after proliferation and abnormal differentiation to form scales39. Perivascular inflammatory cells release inflammatory mediators to promote vasodilation and neovascularization40. These two cells are closely related to the formation and development of psoriasis41. CDKN3 and BIRC5 were highly positively associated with stem cycling keratinocytes, while STAT3 and NTRK2 were positively correlated with perivascular inflammatory cells (Fig. 5a). Additionally, tSNE maps displayed the correlation between DE-ARGs and cell clustering, indicating that perivascular inflammatory cells in psoriasis were associated with elevated expression levels of STAT3 and BIRC5 (Fig. 5b–e). Based on these findings, BIRC5 and STAT3 were selected for further experimental validation.
Fig. 5.
tSNE maps of differentially expressed anoikis-related genes. (a) Gene expression patterns of five DE-ARGs grouped by categorical cell information; (b,c) tSNE maps displaying cell clustering locations. (d) tSNE maps of DE-ARGs and cell clustering; (e) tSNE maps demonstrating the correlation between two DE-ARGs.
Cellular experimental validation and immunohistochemistry
Next, we validated the expression of anoikis-related genes, mainly BIRC5 and STAT3, in psoriasis. The primary antibody used in the western blotting experiment were shown in Fig. S2. As shown in Fig. 6a and b, the mRNA and protein level of STAT3 and BIRC5 were both upregulated in M5-induced psoriatic cell models. Besides, the results of immunohistochemistry revealed that both BIRC5 (P < 0.01) and STAT3 (P < 0.001) were highly expressed in the psoriasis lesions samples (Fig. 6c). Thus, these experimental results demonstrated upregulations of STAT3 and BIRC5 in psoriasis.
Fig. 6.
Cellular experimental validation and Immunohistochemistry. (a) Relative mRNA levels of STAT3 and BIRC5 between control and M5-induced HaCaT cells; (b) Western blotting analysis of BIRC5 and STAT3 expression M5-induced HaCaT cells; (c) Immunohistochemical analysis showing cytoplasmic expression differences of BIRC5 and STAT3 in normal and psoriasis tissues. Bar length = 200 μm, **P < 0.01, ***P < 0.001.
Drug prediction analysis
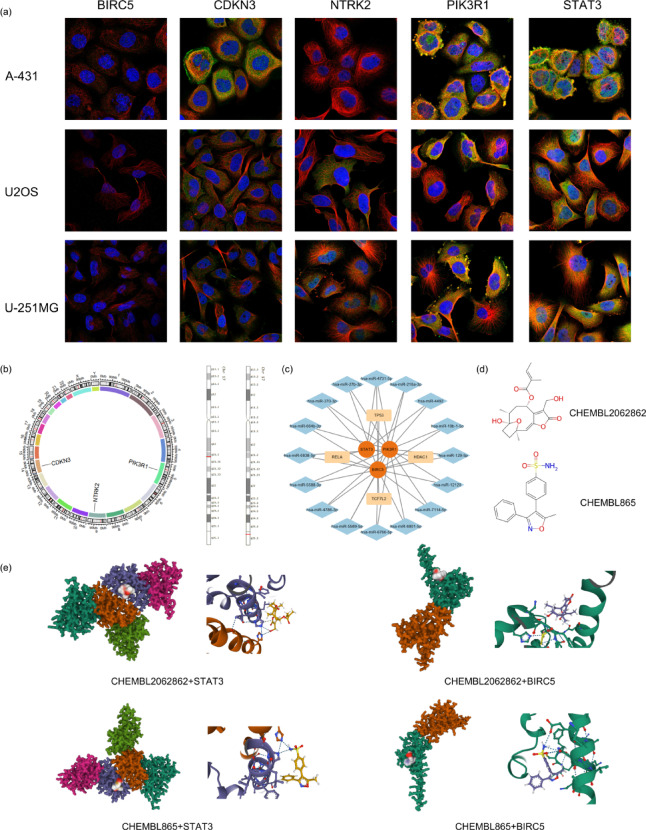
The subcellular localisation of DE-ARG proteins was investigated through immunofluorescence (ICC-IF) and confocal microscopy in various cell lines (Fig. 7a). Chromosomal locations of DE-ARGs were determined (Fig. 7b), revealing four target mRNAs and 16 target miRNAs. TP53, HDAC1, TCF712 and RELA were identified as co-associated with the three hub genes. A DNA-mRNA-miRNA axis centred on these hub genes was constructed (Fig. 7c). Additionally, two compounds with specific biological activities relevant to psoriasis were identified as Valdecoxib and Rel-8Alpha-Tigloyloxyhirsutinolide using CHEMBL (Fig. 7d). The chromosomal locations of BIRC5 and STAT3 on chromosome 17 were highlighted, showing their relevance as drug targets. The binding conformations and interactions of the two compounds with the STAT3 and BIRC5 are obtained with Autodock Vina v.1.2.2 (Fig. 7e). A binding affinity lower than 0 suggests that the compound may bind spontaneously to the target protein, while a value below -5.0 is generally considered indicative of favorable binding activity35. In our analysis, BIRC5 exhibited favorable docking results with both compounds, showing a affinity of -6.021 with Rel-8Alpha-Tigloyloxyhirsutinolide (Table 2).
Fig. 7.
Drug prediction analysis. (a) Subcellular distribution of proteins encoded by five DE-ARGs, generated using the HPA (https://www.proteinatlas.org/); (b) The chromosomal location of DE-ARGs, visualized with R software(version 4.2.3, URL: https://www.r-project.org). BIRC5 and STAT3 are pinpointed on chromosome 17; (c) A DNA-mRNA-miRNA axis centred on three hub genes, constructed using miRWalk (http://mirwalk.umm.uni-heidelberg.de/) and Cytoscape software (version 3.8.1, URL: https://cytoscape.org); (d) Two compounds identified with specific biological activities for psoriasis treatment, screened via the Dgibd database (https://dgidb.org/); (e) Binding models of Valdecoxib and Rel-8α-Tigloyloxyhirsutinolide with STAT3 and BIRC5, respectively, simulated using AutoDock Vina software (version 1.2.2, URL: https://vina.scripps.edu).
Table 2.
Molecular docking.
| Compound | Affinity | |
|---|---|---|
| STAT3 | BIRC5 | |
| Valdecoxib | − 4.212 | − 5.825 |
| Rel-8Alpha-Tigloyloxyhirsutinolide | − 4.33 | − 6.021 |
Discussion
Psoriasis is characterised by chronic inflammation with the infiltration of dendritic cells (DC), macrophages, T cells and neutrophils6. The persistent and pronounced immune cell infiltration alters the extracellular environment, which can influence cell–cell interactions and potentially lead to a type of cell death called anoikis21. Anoikis is intricately linked to immune factors, and evidence suggests that various cytokines and immune mediators, especially IF-17A and IL-6, play significant roles in anoikis resistance26,27. However, the correlation between anoikis and psoriasis progression remains unclear. Our study aimed to explore anoikis-related genes in the context of psoriasis by utilising bioinformatics tools to identify and analyse DE-ARGs. We identified two key genes associated with anoikis in psoriasis, which could provide new avenues for clinical prevention and treatment strategies. Moreover, the dentification and translational applicability of these key biomarkers will be enhanced by the application of artificial intelligence42.
In this study, gene expression profiles from two GEO databases were analysed using bioinformatics approaches to identify DEGs. By overlapping DEGs with anoikis-associated genes from the GeneCards database, we identified 18 DE-ARGs. Functional enrichment analyses, including GO, KEGG and GSEA, revealed that these DE-ARGs were linked to the positive regulation of epithelial cell proliferation, the JAK-STAT signalling pathway and the regulation of anoikis. Consistent with our findings, previous studies have demonstrated that STAT3 is actively involved in various signalling pathways that influence psoriasis pathogenesis by promoting epithelial cell proliferation43,44. Psoriasis is recognised as an inflammatory skin condition in which cytokines such as IL-17, IL-22 and TNF-α play critical roles4. Therefore, we examined the correlations between immune cells and molecules with the eight hub DE-ARGs, visualised DE-ARG expression patterns and conducted drug prediction analyses from genetic, molecular and cellular perspectives. Ultimately, STAT3 and BIRC5 were identified as crucial DE-ARGs strongly associated with both psoriasis and anoikis, suggesting their potential utility as biomarkers. In the context of precision medicine, similar biomarker discoveries have driven the advancement of liquid biopsy strategies in inflammatory diseases45.
The Signal Transducer and Activator of Transcription 3 (STAT3) is a member of the STAT protein family, and its activation has been shown to upregulate vacuolar ATPase, thereby promoting anoikis resistance46. Moreover, STAT3 is involved in IL-6/STAT3 and JAK/STAT3 signalling pathways, which are linked to anoikis resistance in tumours47,48. In our experiments, we observed that STAT3 was upregulated in psoriasis lesions compared to NL samples. STAT3 was strongly associated with the positive regulation of cell proliferation and the JAK-STAT signalling pathway. This is consistent with previous findings that STAT3 promotes psoriasis development through the key JAK-STAT signalling pathway18,19. We also found STAT3 to be positively correlated with IL-17A and strongly negatively correlated with IL17RE. IL-17, especially IL-17A, has been shown to drive inflammation and enhance anoikis resistance26. Additionally, we found that STAT3 was positively associated with activated dendritic cells and negatively related with resting mast cells. Previous research has demonstrated that pDC and myeloid DCs play crucial roles in psoriasis development and that a higher proportion of mast cells transition from dormancy to activation in patients with psoriasis8. Furthermore, STAT3 overexpression has been shown to enhance anoikis resistance. BIRC5, also known as survivin, is a member of the human IAP family49. Studies on circulating tumour cells (CTC) have shown that BIRC5 downregulation promotes anoikis50. Our results indicate that BIRC5 is associated with the negative regulation of apoptotic processes, specifically the regulation of cysteine-type endopeptidase activity involved in apoptosis, and the positive regulation of cell proliferation. BIRC5 is known to inhibit apoptosis and promote mitosis51,52. We also found that BIRC5 was correlated with several immune cells and molecules, including a strong positive correlation with IL-12. IL-12 is a target of microRNAs involved in regulating cell death including anoikis53. Similar to STAT3, BIRC5 was positively associated with activated dendritic cells and negatively related with resting mast cells. Additionally, BIRC5 has been implicated in the KLF12/Sp1/survivin signalling axis, which promotes anoikis resistance54. Although STAT3 and BIRC5 have distinct regulatory mechanisms, both show a strong correlation with psoriasis and anoikis. Interestingly, we also found a positive correlation between BIRC5 and STAT3.
In conclusion, our study identified STAT3, BIRC5, EGF, NTRK2, STAT3, PIK3R1, CEACAM5 and PLAUR as key anoikis-related genes in psoriasis. Among these, BIRC5 and STAT3 emerged as the most critical, with significant links to both psoriasis and anoikis. Our findings on the differential expression of BIRC5 and STAT3 have been supported by analyses at the gene, protein and tissue levels. Based on these results, we also predicted two compounds with potential biological activities for treating psoriasis. However, due to experimental limitations, we have not verified the effectiveness of these compounds, nor further explored how STAT3 and BIRC5 negatively regulate the IL-23/IL-17 cytokine axis through IL-12 to promote anoikis resistance, excessive proliferation and tissue inflammation of keratinocytes in psoriatic skin. Future research is needed to validate these results and further elucidate the connection between psoriasis and anoikis.
Conclusion
We revealed the correlation between anoikis and the development of psoriasis, based on bioinformatics analysis and exploratory experiments, including polymerase chain reaction and western blotting experiments. STAT3 and BIRC5 were identified as the significant anoikis-related biomarkers in psoriasis. Key immune molecules, such as IL-17 and IL-12, were closely associated with STAT3 and BIRC5. Meanwhile, both STAT3 and BIRC5 were related with various metabolic and signalling pathways, including the regulation of anoikis, negative regulation of the apoptotic process and positive regulation of epithelial cell proliferation. Our findings will provide new insights into the pathogenesis of psoriasis, contributing to better prevention and treatment strategies for psoriasis.
Supplementary Information
Acknowledgements
We thank Gene Expression Omnibus (GEO) database and Cancer GenomeAtlas Program (TCGA) database. Thanks to the members of the team for developing and testing the data analysis site Sangerbox, your hard work and talent have enabled this project. We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Abbreviations
- ECM
Extracellular matrix
- DEGs
Differentially expressed genes
- DE-ARGs
Differentially expressed anoikis-related genes
- GO
Gene ontology
- KEGG
Kyoto encyclopedia of genes and genomes
- ssGSEA
Single-sample gene set enrichment analysis
- DC
Dendritic cells
- NLR
Neutrophil-to-lymphocyte ratio
- pDC
Plasmacytoid DC
- JAK
Janus kinase
- STAT
Signal transducer and activator of transcription
- HIF-1
Hypoxia inducible factor-1
- NOD-like receptor
Nucleotide oligomerization domain-like receptor
- FoxO
Forkhead box O
- TNBC
Triple-negative breast cancer
- MnSOD
Manganese superoxide dismutase
- TGF-β1
Transforming growth factor β1
- RMA
Robust multiarray average
- BP
Biological process
- CC
Cellular components
- MF
Molecular functions
- BH
Benjamini and Hochberg
- FDR
False discovery rate
- LS
Psoriasis lesions
- NL
No-lesion
- HPA
Human protein atlas
- EpD_KRTO_StemCyclingKerationcyte
Stem cycling kerationcyte
- Mes PeriVlnflammatory
Merivascular inflammatory cell
- Mes PeriVlnflammatory Psor
Perivascular inflammatory cell in psoriasis
- ICC-IF
Immunofluorescence
- CTC
Circulating tumor cells
Author contributions
Conceptualization, C.C., M.L. and W.B.; investigation, C.C., M.L. and W.B.; resources, W.B., S.W., K.W., X.Z. and L.L.; writing—original draft preparation, W.B. and K.W.; writing—review and editing, C.C.; supervision, C.C. and M.L.; funding acquisition, C.C and M.L. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by joint funds for the innovation of science and technology of Fujian Province (2020Y9022) and Natural Science Foundation of China (82404140).
Data availability
The datasets analyzed during the current study are available in the GeneCards database repository, [https://www.genecards.org/] and GEO database, [https://www.ncbi.nlm.nih.gov/geo/].
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The studies involving humans were approved by Ethics Committee of Xi ‘an Jiaotong University. The studies were conducted in accordance with the local legislation and institutional requirements (No. LLSBPJ-2024–215). The participants provided their written informed consent to participate in this study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wanting Bai, Kunqin Wang and Shaobo Wu have contributed equally to this work.
Contributor Information
Meng Liu, Email: dr_liumeng@126.com.
Caifeng Chen, Email: 1175589670@qq.com.
References
- 1.Korman, N. J. Management of psoriasis as a systemic disease: what is the evidence?. Br. J. Dermatol.182(4), 840–848. 10.1111/bjd.18245 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo, J. et al. Signaling pathways and targeted therapies for psoriasis. Signal Transduct. Target Ther.8(1), 437. 10.1038/s41392-023-01655-6 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffiths, C. E. M., Armstrong, A. W., Gudjonsson, J. E. & Barker, J. Psoriasis. Lancet397(10281), 1301–1315. 10.1016/S0140-6736(20)32549-6 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Ghoreschi, K., Balato, A., Enerbäck, C. & Sabat, R. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet397(10275), 754–766. 10.1016/s0140-6736(21)00184-7 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Kamiya, K., Kishimoto, M., Sugai, J., Komine, M. & Ohtsuki, M. Risk factors for the development of psoriasis. Int. J. Mol. Sci.10.3390/ijms20184347 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rendon, A. & Schakel, K. Psoriasis pathogenesis and treatment. Int. J. Mol. Sci.10.3390/ijms20061475 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang, W. M. & Jin, H. Z. Role of neutrophils in psoriasis. J. Immunol. Res.2020, 3709749. 10.1155/2020/3709749 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamata, M. & Tada, Y. Dendritic cells and macrophages in the pathogenesis of psoriasis. Front. Immunol.13, 941071. 10.3389/fimmu.2022.941071 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou, Y. et al. The epidermal immune microenvironment plays a dominant role in psoriasis development, as revealed by mass cytometry. Cell Mol. Immunol.19(12), 1400–1413. 10.1038/s41423-022-00940-8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang, Y. et al. Immune cell infiltration analysis demonstrates excessive mast cell activation in psoriasis. Front Immunol.12, 773280. 10.3389/fimmu.2021.773280 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou, X. Y., Chen, K. & Zhang, J. A. Mast cells as important regulators in the development of psoriasis. Front Immunol.13, 1022986. 10.3389/fimmu.2022.1022986 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schinocca, C. et al. Role of the IL-23/IL-17 pathway in rheumatic diseases: An overview. Front Immunol.12, 637829. 10.3389/fimmu.2021.637829 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh, R., Koppu, S., Perche, P. O. & Feldman, S. R. The cytokine mediated molecular pathophysiology of psoriasis and its clinical implications. Int. J. Mol. Sci.10.3390/ijms222312793 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He, J. et al. The role and application of three IFN-related reactions in psoriasis. Biomed. Pharmacother.167, 115603. 10.1016/j.biopha.2023.115603 (2023). [DOI] [PubMed] [Google Scholar]
- 15.Tomar, Y., Gorantla, S. & Singhvi, G. Insight into the pivotal role of signaling pathways in psoriasis pathogenesis, potential therapeutic molecules and drug delivery approaches. Drug Discov. Today.28(2), 103465. 10.1016/j.drudis.2022.103465 (2023). [DOI] [PubMed] [Google Scholar]
- 16.Yu, J. et al. Pathogenesis, multi-omics research, and clinical treatment of psoriasis. J. Autoimmun.133, 102916. 10.1016/j.jaut.2022.102916 (2022). [DOI] [PubMed] [Google Scholar]
- 17.Calautti, E., Avalle, L. & Poli, V. Psoriasis: A STAT3-Centric View. Int. J. Mol. Sci.19(1), 171. 10.3390/ijms19010171 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen, L., Cao, S. Q., Lin, Z. M., He, S. J. & Zuo, J. P. NOD-like receptors in autoimmune diseases. Acta Pharmacol Sin.42(11), 1742–1756. 10.1038/s41401-020-00603-2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu, W. J., Li, P., Wang, L. & Xu, Y. C. Hypoxia-inducible factor-1: A potential pharmacological target to manage psoriasis. Int. Immunopharmacol.86, 106689. 10.1016/j.intimp.2020.106689 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Gilmore, A. P. Anoikis. Cell Death Differ.12(Suppl 2), 1473–1477. 10.1038/sj.cdd.4401723 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Paoli, P., Giannoni, E. & Chiarugi, P. Anoikis molecular pathways and its role in cancer progression. Biochim. Biophys. Acta.1833(12), 3481–3498. 10.1016/j.bbamcr.2013.06.026 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Taddei, M. L., Giannoni, E., Fiaschi, T. & Chiarugi, P. Anoikis: An emerging hallmark in health and diseases. J Pathol.226(2), 380–393. 10.1002/path.3000 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Adeshakin, F. O. et al. Mechanisms for modulating anoikis resistance in cancer and the relevance of metabolic reprogramming. Front Oncol.11, 626577. 10.3389/fonc.2021.626577 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tajbakhsh, A., Rivandi, M., Abedini, S., Pasdar, A. & Sahebkar, A. Regulators and mechanisms of anoikis in triple-negative breast cancer (TNBC): A review. Crit. Rev. Oncol. Hematol.140, 17–27. 10.1016/j.critrevonc.2019.05.009 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Zhu, Z. et al. Anoikis resistance in diffuse glioma: The potential therapeutic targets in the future. Front Oncol.12, 976557. 10.3389/fonc.2022.976557 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai, Y. F. et al. Interleukin 17A promotes cell migration, enhances anoikis resistance, and creates a microenvironment suitable for triple negative breast cancer tumor metastasis. Cancer Immunol Immunother.70(8), 2339–2351. 10.1007/s00262-021-02867-x (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karavyraki, M. & Porter, R. K. Evidence of a role for interleukin-6 in anoikis resistance in oral squamous cell carcinoma. Med Oncol.39(5), 60. 10.1007/s12032-022-01664-5 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han, Y. H. et al. Regulation of anoikis by extrinsic death receptor pathways. Cell Commun. Signal21(1), 227. 10.1186/s12964-023-01247-5 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fofaria, N. M. & Srivastava, S. K. Critical role of STAT3 in melanoma metastasis through anoikis resistance. Oncotarget5(16), 7051–7064. 10.18632/oncotarget.2251 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fofaria, N. M. & Srivastava, S. K. STAT3 induces anoikis resistance, promotes cell invasion and metastatic potential in pancreatic cancer cells. Carcinogenesis36(1), 142–150. 10.1093/carcin/bgu233 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanehisa, M., Furumichi, M., Sato, Y., Matsuura, Y. & Ishiguro-Watanabe, M. KEGG: biological systems database as a model of the real world. Nucl. Acids Res.53(D1), D672-d677. 10.1093/nar/gkae909 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao, Y. et al. Integrative single-cell transcriptomic investigation unveils long non-coding RNAs associated with localized cellular inflammation in psoriasis. Front Immunol.14, 1265517. 10.3389/fimmu.2023.1265517 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris, G. M., Huey, R. & Olson, A. J. Using AutoDock for ligand-receptor docking. Curr. Protoc. Bioinf.24(1), 8–14. 10.1002/0471250953.bi0814s24 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Wang, Y. et al. PubChem BioAssay: 2017 update. Nucl. Acids Res.45(D1), D955-d963. 10.1093/nar/gkw1118 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsin, K. Y., Ghosh, S. & Kitano, H. Combining machine learning systems and multiple docking simulation packages to improve docking prediction reliability for network pharmacology. PLoS ONE8(12), e83922. 10.1371/journal.pone.0083922 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu, M. et al. FOXE1 contributes to the development of psoriasis by regulating WNT5A. J. Invest. Dermatol.143(12), 2366–2377. 10.1016/j.jid.2023.04.035 (2023). [DOI] [PubMed] [Google Scholar]
- 37.Liu, M. et al. Cytotoxicity of Saikosaponin A targets HEKa cell through apoptosis induction by ROS accumulation and inflammation suppression via NF-kappaB pathway. Int Immunopharmacol.86, 106751. 10.1016/j.intimp.2020.106751 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Li, B. et al. Dysregulation of Akt-FOXO1 pathway leads to dysfunction of regulatory T cells in patients with psoriasis. J. Invest. Dermatol.139(10), 2098–2107. 10.1016/j.jid.2018.12.035 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Ali, G. et al. Keratinocytes derived from patient-specific induced pluripotent stem cells recapitulate the genetic signature of psoriasis disease. Stem Cells Dev.29(7), 383–400. 10.1089/scd.2019.0150 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee, H. J., Hong, Y. J. & Kim, M. Angiogenesis in chronic inflammatory skin disorders. Int. J. Mol. Sci.10.3390/ijms222112035 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sieminska, I., Pieniawska, M. & Grzywa, T. M. the immunology of psoriasis-current concepts in pathogenesis. Clin. Rev. Allergy Immunol.66(2), 164–191. 10.1007/s12016-024-08991-7 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie, H., Jia, Y. & Liu, S. Integration of artificial intelligence in clinical laboratory medicine: Advancements and challenges. Interdiscip. Med.10.1002/inmd.20230056 (2024). [Google Scholar]
- 43.Huang, W. et al. Protein kinase CK2 promotes proliferation, abnormal differentiation, and proinflammatory cytokine production of keratinocytes via regulation of STAT3 and Akt pathways in psoriasis. Am. J. Pathol.193(5), 567–578. 10.1016/j.ajpath.2023.01.016 (2023). [DOI] [PubMed] [Google Scholar]
- 44.Yang, Z. et al. STAT3/SH3PXD2A-AS1/miR-125b/STAT3 positive feedback loop affects psoriasis pathogenesis via regulating human keratinocyte proliferation. Cytokine144, 155535. 10.1016/j.cyto.2021.155535 (2021). [DOI] [PubMed] [Google Scholar]
- 45.Bao, H., Min, L., Bu, F., Wang, S. & Meng, J. Recent advances of liquid biopsy: Interdisciplinary strategies toward clinical decision-making. Interdiscip. Med.10.1002/inmd.20230021 (2023). [Google Scholar]
- 46.Adeshakin, F. O. et al. Upregulation of V-ATPase by STAT3 activation promotes anoikis resistance and tumor metastasis. J Cancer12(16), 4819–4829. 10.7150/jca.58670 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, L. N. et al. TGF-beta1/SH2B3 axis regulates anoikis resistance and EMT of lung cancer cells by modulating JAK2/STAT3 and SHP2/Grb2 signaling pathways. Cell Death Dis.13(5), 472. 10.1038/s41419-022-04890-x (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang, Z., Yin, M., Wang, R., Liu, X. & Yan, D. Bit1 silencing enhances the proliferation, migration, and invasion of glioma cells through activation of the IL-6/STAT3 pathway. Onco. Targets Ther.13, 2469–2481. 10.2147/OTT.S240081 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pahlavan, Y. et al. Survivin modulatory role in autoimmune and autoinflammatory diseases. J. Cell Physiol.234(11), 19440–19450. 10.1002/jcp.28725 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Dimitrov-Markov, S. et al. Discovery of new targets to control metastasis in pancreatic cancer by single-cell transcriptomics analysis of circulating tumor cells. Mol. Cancer Ther.19(8), 1751–1760. 10.1158/1535-7163.MCT-19-1166 (2020). [DOI] [PubMed] [Google Scholar]
- 51.Zhao, Y. et al. BIRC5 regulates inflammatory tumor microenvironment-induced aggravation of penile cancer development in vitro and in vivo. BMC Cancer22(1), 448. 10.1186/s12885-022-09500-9 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duffy, M. J., O’Donovan, N., Brennan, D. J., Gallagher, W. M. & Ryan, B. M. Survivin: a promising tumor biomarker. Cancer Lett.249(1), 49–60. 10.1016/j.canlet.2006.12.020 (2007). [DOI] [PubMed] [Google Scholar]
- 53.Sadri Nahand, J. et al. Cell death pathways and viruses: Role of microRNAs. Mol. Ther. Nucl. Acids.24, 487–511. 10.1016/j.omtn.2021.03.011 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mak, C. S. et al. MicroRNA-141 enhances anoikis resistance in metastatic progression of ovarian cancer through targeting KLF12/Sp1/survivin axis. Mol. Cancer.16(1), 11. 10.1186/s12943-017-0582-2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available in the GeneCards database repository, [https://www.genecards.org/] and GEO database, [https://www.ncbi.nlm.nih.gov/geo/].