Abstract
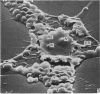
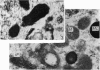
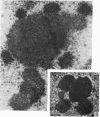
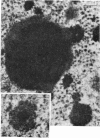
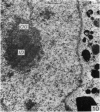
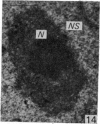
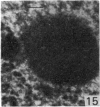
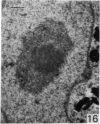
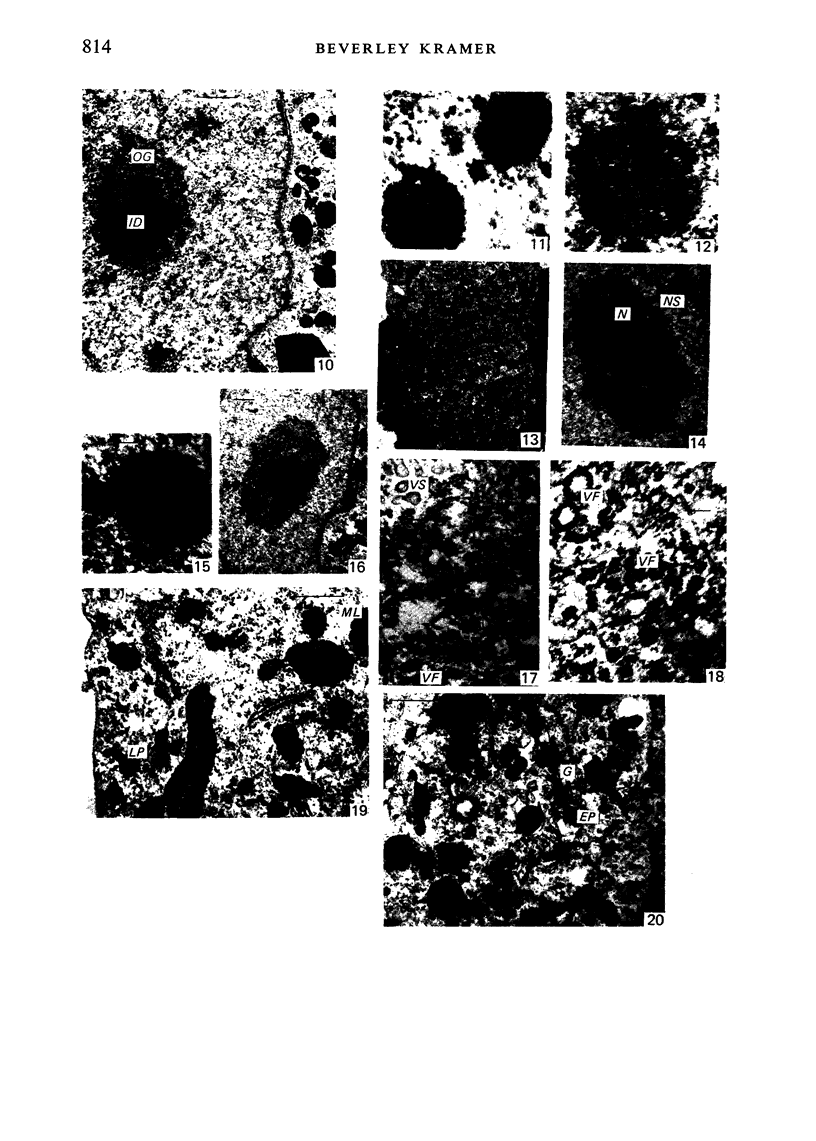
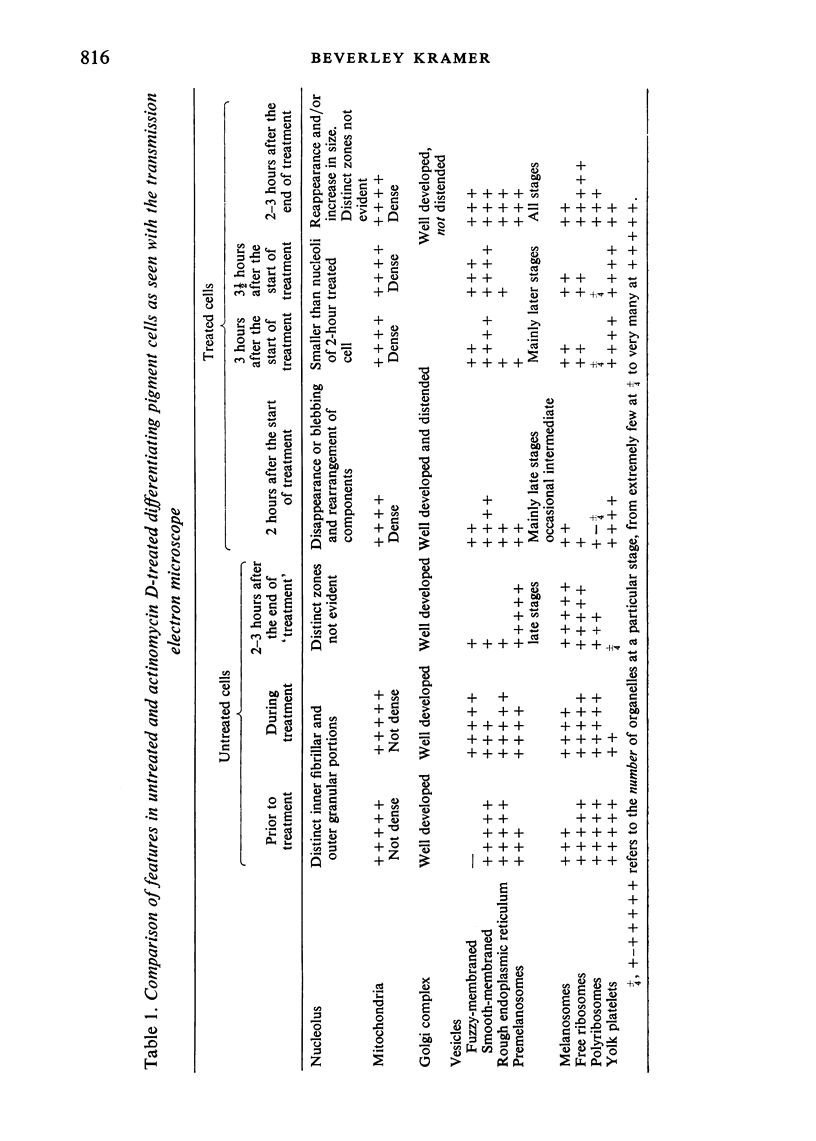
The effect of AMD on the nucleolus and on melanogenesis in differentiating pigment cells of Xenopus laevis was investigated in cultured neural crest cells. Cultures were treated with either 2 or 10 microgram/ml AMD for 41/2 hours. Following treatment the antibiotic was removed. Observations of the cells were made with both scanning and transmission electron microscopes. Actinomycin D almost entirely stopped pigment formation in neural crest cultures during treatment. The morphological sequence in the formation of melanin granules in the untreated pigment cells appears to be as follows: The earliest identifiable premelanosome is membrane-bound and contains very thin laminae and/or small vesciles. The premelanosomes become until they are approximately the size of mature melanin granules and there is thickening of the laminae, which appear to have a periodic substructure. The cells eventually become packed with electron-dense melanin granules. Compared with controls, the cytoplasm of treated cells showed a greater abundance of smooth- than of fuzzy-membraned vesicles, less rough endoplasmic reticulum, dilatation of the Golgi cisternae, and a smaller number of premelanosomes. The necleolus showed segregation and blebbing of its components, decrease in size and even disappearance; sometimes confluence of the components occurred. The most consistent morphological effect of AMD on the nucleolus was the separation of the fibrillar and granular areas. The granular component appeared to undergo marked changes in size and arrangement and is thought to be the source of ribosomal RNA precursors. The alteration in size of the outer component of the nucleolus went hand in hand with disappearance of free ribosomes from the cytoplasm of treated cells and inhibition of pigment synthesis.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew A., Gabie V. Hanging drop culture of Xenopus laevis neural crest. Acta Embryol Exp (Palermo) 1969;2:123–136. [PubMed] [Google Scholar]
- Boyde A., Weiss R. A., Veselý P. Scanning electron microscopy of cells in culture. Exp Cell Res. 1972;71(2):313–324. doi: 10.1016/0014-4827(72)90299-6. [DOI] [PubMed] [Google Scholar]
- Eppig J. J., Jr, Dumont J. N. The distribution of melanosomes in larvae reared from normal and from pigmentless eggs of Xenopus laevis. J Exp Zool. 1971 May;177(1):79–88. doi: 10.1002/jez.1401770109. [DOI] [PubMed] [Google Scholar]
- Eppig J. J., Jr Melanogenesis in amphibians. 3. The buoyant density of oocyte and larval xenopus laevis melanosomes and the isolation of oocyte melanosomes from the eyes of PTU-treated larvae. J Exp Zool. 1970 Dec;175(4):467–475. doi: 10.1002/jez.1401750406. [DOI] [PubMed] [Google Scholar]
- Eppig J. J., Jr Melanogenesis in amphibians. I. A study of the fine structure of the normal and phenylthiourea-treated pigmented epithelium in Rana pipiens tadpole eyes. Z Zellforsch Mikrosk Anat. 1970;103(2):238–246. [PubMed] [Google Scholar]
- Fitzpatrick T. B., Quevedo W. C., Jr, Levene A. L., McGovern V. J., Mishima Y., Oettle A. G. Terminology of vertebrate melanin-containing cells: 1965. Science. 1966 Apr 1;152(3718):88–89. doi: 10.1126/science.152.3718.88. [DOI] [PubMed] [Google Scholar]
- GIRARD M., PENMAN S., DARNELL J. E. THE EFFECT OF ACTINOMYCIN ON RIBOSOME FORMATION IN HELA CELLS. Proc Natl Acad Sci U S A. 1964 Feb;51:205–211. doi: 10.1073/pnas.51.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDSTEIN M. N., SLOTNICK I. J., JOURNEY L. J. In vitro studies with HeLa cell line sensitive and resistant to actinomycin D. Ann N Y Acad Sci. 1960 Oct 5;89:474–483. doi: 10.1111/j.1749-6632.1960.tb20171.x. [DOI] [PubMed] [Google Scholar]
- HACKMANN C. The effect of actinomycins on experimental tumors. Ann N Y Acad Sci. 1960 Oct 4;89:361–367. doi: 10.1111/j.1749-6632.1960.tb20159.x. [DOI] [PubMed] [Google Scholar]
- Hearing V. J., Phillips P., Lutzner M. A. The fine structure of melanogenesis in coat color mutants of the mouse. J Ultrastruct Res. 1973 Apr;43(1):88–106. doi: 10.1016/s0022-5320(73)90072-5. [DOI] [PubMed] [Google Scholar]
- JACOB J., SIRLIN J. L. ELECTRON MICROSCOPE STUDIES ON SALIVARY GLAND CELLS. IV. THE NUCLEUS OF SMITTIA PARTHENOGENETICA (CHIRONOMIDAE) WITH SPECIAL REFERENCE TO THE NUCLEOLUS AND THE EFFECTS OF ACTINOMYCIN THEREON. J Ultrastruct Res. 1964 Oct;11:315–328. doi: 10.1016/s0022-5320(64)90036-x. [DOI] [PubMed] [Google Scholar]
- Kramer B. The effect of actinomycin D on developing pigment cells of Xenopus laevis. Dev Biol. 1972 Oct;29(2):220–226. doi: 10.1016/0012-1606(72)90059-0. [DOI] [PubMed] [Google Scholar]
- Maul G. G., Brumbaugh J. A. On the possible function of coated vesicles in melanogenesis of the regenerating fowl feather. J Cell Biol. 1971 Jan;48(1):41–48. doi: 10.1083/jcb.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu M. C., Twitty V. C. The Differentiation of Gastrula Ectoderm in Medium Conditioned by Axial Mesoderm. Proc Natl Acad Sci U S A. 1953 Sep;39(9):985–989. doi: 10.1073/pnas.39.9.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Penman S., Scherrer K., Becker Y., Darnell J. E. POLYRIBOSOMES IN NORMAL AND POLIOVIRUS-INFECTED HELA CELLS AND THEIR RELATIONSHIP TO MESSENGER-RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):654–662. doi: 10.1073/pnas.49.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S. G., Phillips D. M. Nucleoli of diploid cell strains. Thei normal ultrastructure and the effects of toyocamycin and actinomycin D. J Cell Biol. 1971 Jun;49(3):785–802. doi: 10.1083/jcb.49.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS R. C., MONTGOMERY P. O., KARNEY D. H. Nucleolars "caps'--a morphologic entity produced by the carcinogen 4-nitroquinoline N-oxide. Cancer Res. 1963 May;23:535–538. [PubMed] [Google Scholar]
- Recher L., Briggs L. G., Parry N. T. A reevaluation of nuclear and nucleolar changes induced in vitro by actinomycin D. Cancer Res. 1971 Feb;31(2):140–151. [PubMed] [Google Scholar]
- SCHOEFL G. I. THE EFFECT OF ACTINOMYCIN D ON THE FINE STRUCTURE OF THE NUCLEOLUS. J Ultrastruct Res. 1964 Apr;10:224–243. doi: 10.1016/s0022-5320(64)80007-1. [DOI] [PubMed] [Google Scholar]
- STEVENS B. J. THE EFFECT OF ACTINOMYCIN D ON NUCLEOLAR AND NUCLEAR FINE STRUCTURE IN THE SALIVARY GLAND CELL OF HIRONOMUS THUMMI. J Ultrastruct Res. 1964 Oct;11:329–353. doi: 10.1016/s0022-5320(64)90037-1. [DOI] [PubMed] [Google Scholar]
- Sameshima M., Shiokawa K., Kawakami I. The effect of actinomycin D on nucleolar formationin early Xenopus laevis gastrulae. J Exp Zool. 1970 Jan;173(1):113–119. doi: 10.1002/jez.1401730108. [DOI] [PubMed] [Google Scholar]
- Seiji M., Iwashita S. Intracellular localization of tyrosinase and site of melanin formation in melanocyte. J Invest Dermatol. 1965 Nov;45(5):305–314. doi: 10.1038/jid.1965.135. [DOI] [PubMed] [Google Scholar]
- Smuckler E. A., Benditt E. P. The early effects of actinomycin on rat liver. Changes in ribosomes and polysomes. Lab Invest. 1965 Oct;14(10):1699–1709. [PubMed] [Google Scholar]
- WETTSTEIN F. O., STAEHELIN T., NOLL H. Ribosomal aggregate engaged in protein synthesis: characterization of the ergosome. Nature. 1963 Feb 2;197:430–435. doi: 10.1038/197430a0. [DOI] [PubMed] [Google Scholar]