Abstract
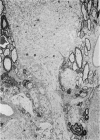
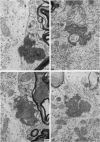
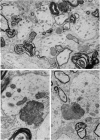
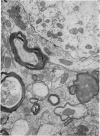
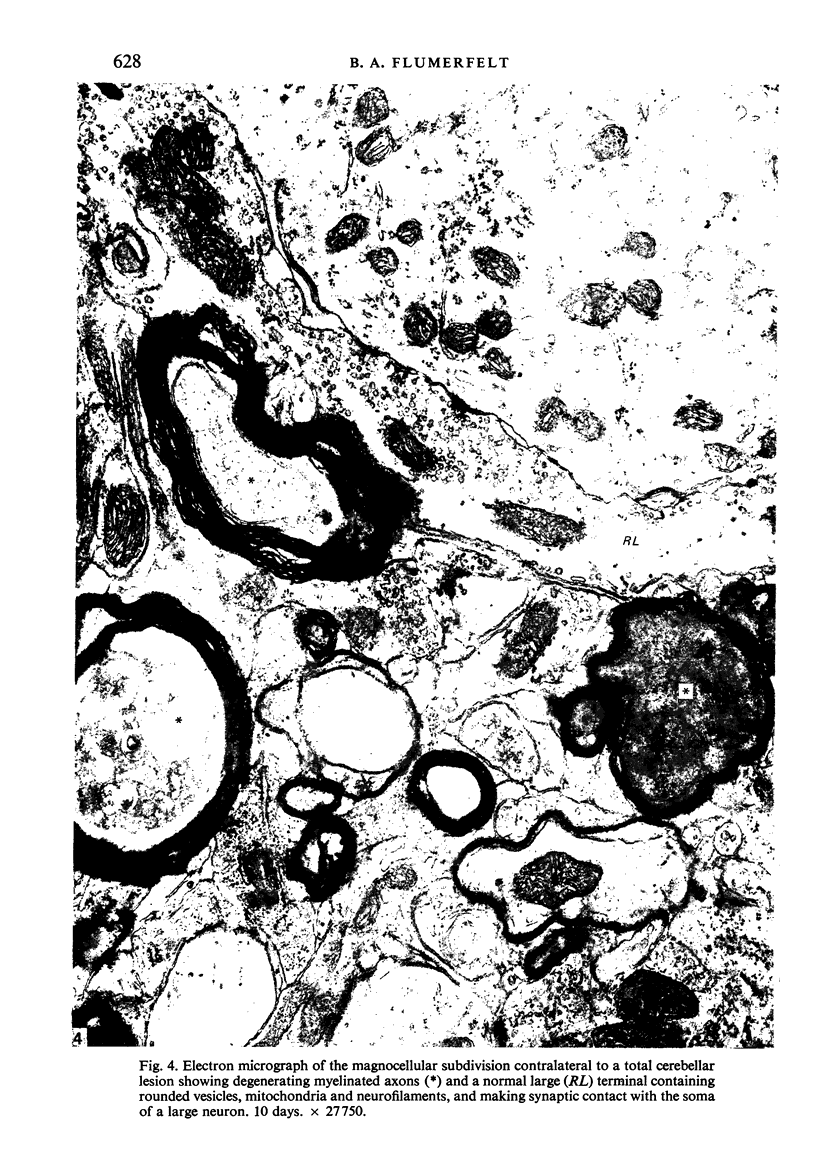
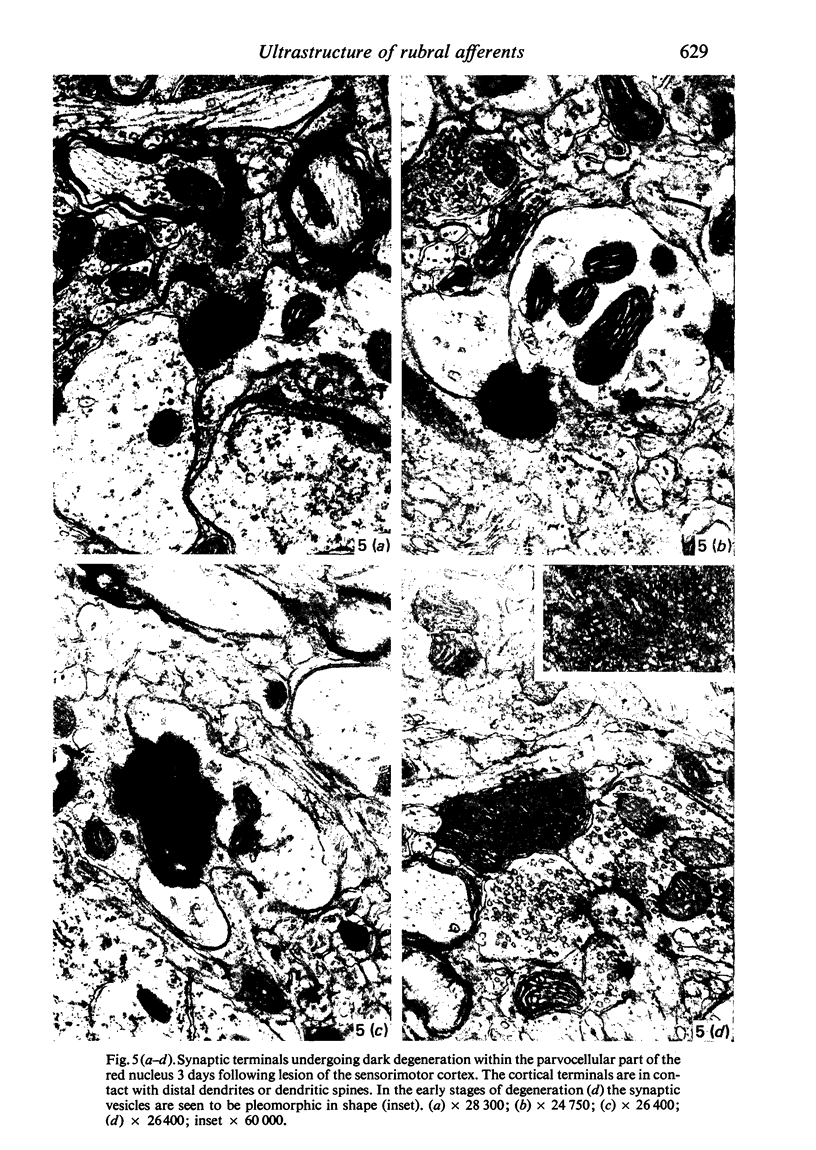
The pattern and mode of termination of afferents to the red nucleus of the rat were investigated with the electron microscope. Lesions were placed by electrocautery in the sensorimotor cortex or were placed electrolytically in the deep cerebellar nuclei and brachium conjunctivum using a stereotaxic approach. With both types of lesion, degenerating fibres of passage, preterminal axons, and synaptic terminals were observed in greatest numbers on the third post-operative day. Following cerebellar lesions, degenerating terminals occurred on the cell bodies and proximal dendrites of large, multipolar neurons in the magnocellular portion of the red nucleus, and on intermediate and small dendrites in the parvocellular portion. It is concluded that the former are interpositus terminals while the latter are dentate (lateralis) terminals ending on rubrospinal and rubrobulbar neurons respectively. Following lesions of the sensorimotor cortex, small degenerating terminals were observed on the distal dendrites and dendritic spines of parvocellular, rubrobulbar neurons. Large terminals containing round vesicles did not undergo degeneration following either type of lesion. These findings suggest the existence of an interpositorubro-spinal pathway in which the interpositus terminals exert a strong influence on the large, caudally placed rubrospinal neurons. The background excitability of the rostrally located rubrobulbar neurons is probably regulated by the distal cortical input while the more proximally located dentate terminals probably exert a stronger discrete influence over their activity.
Full text
PDF
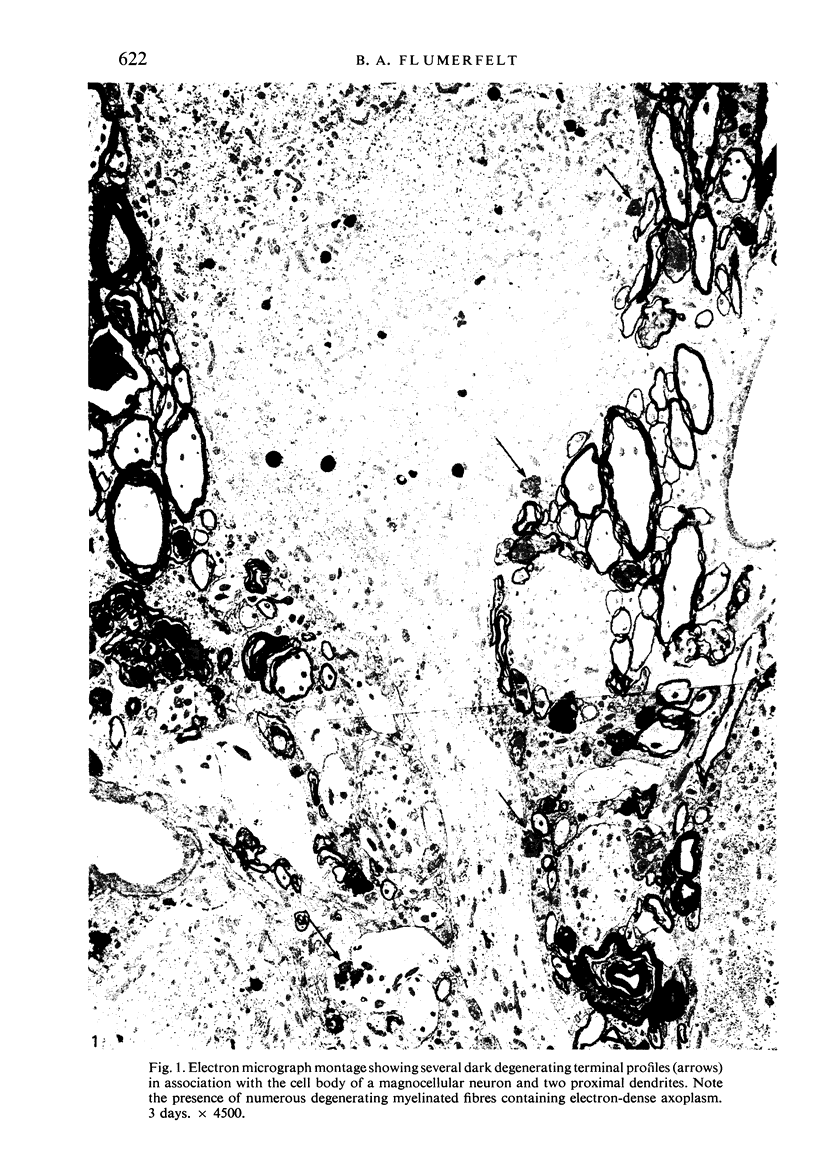

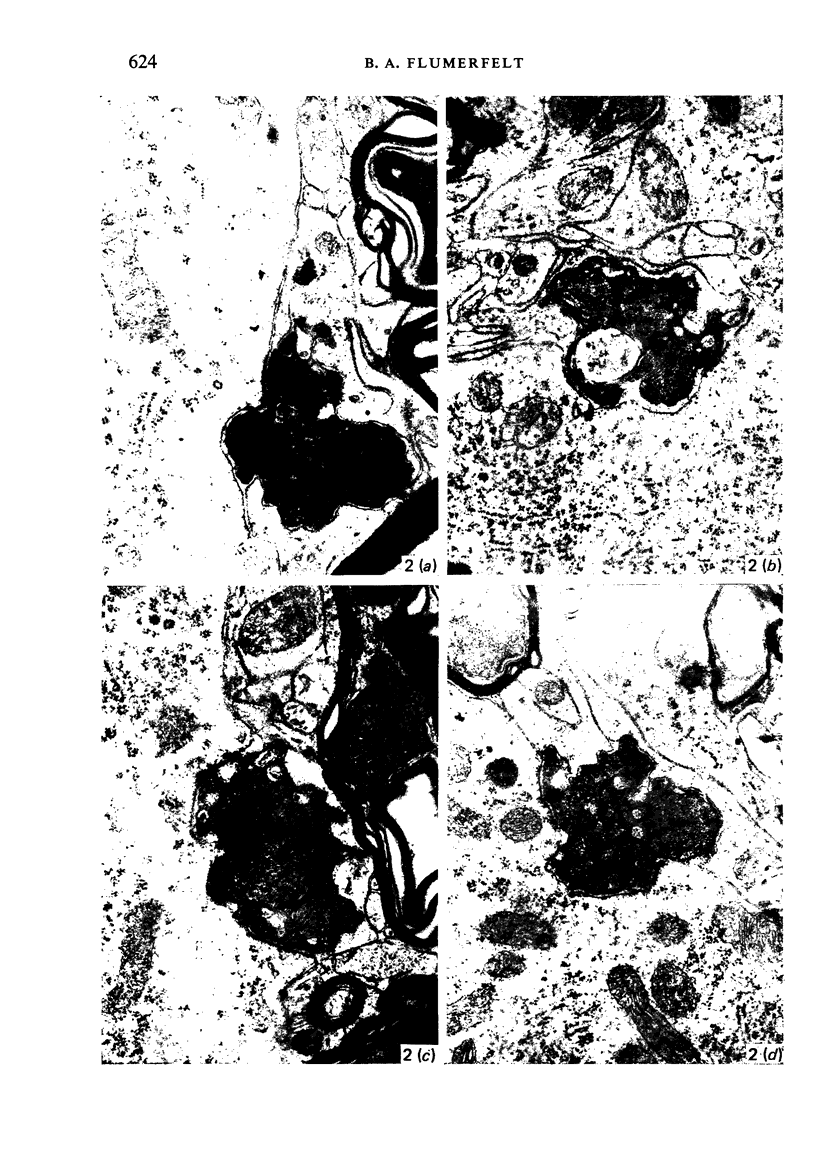

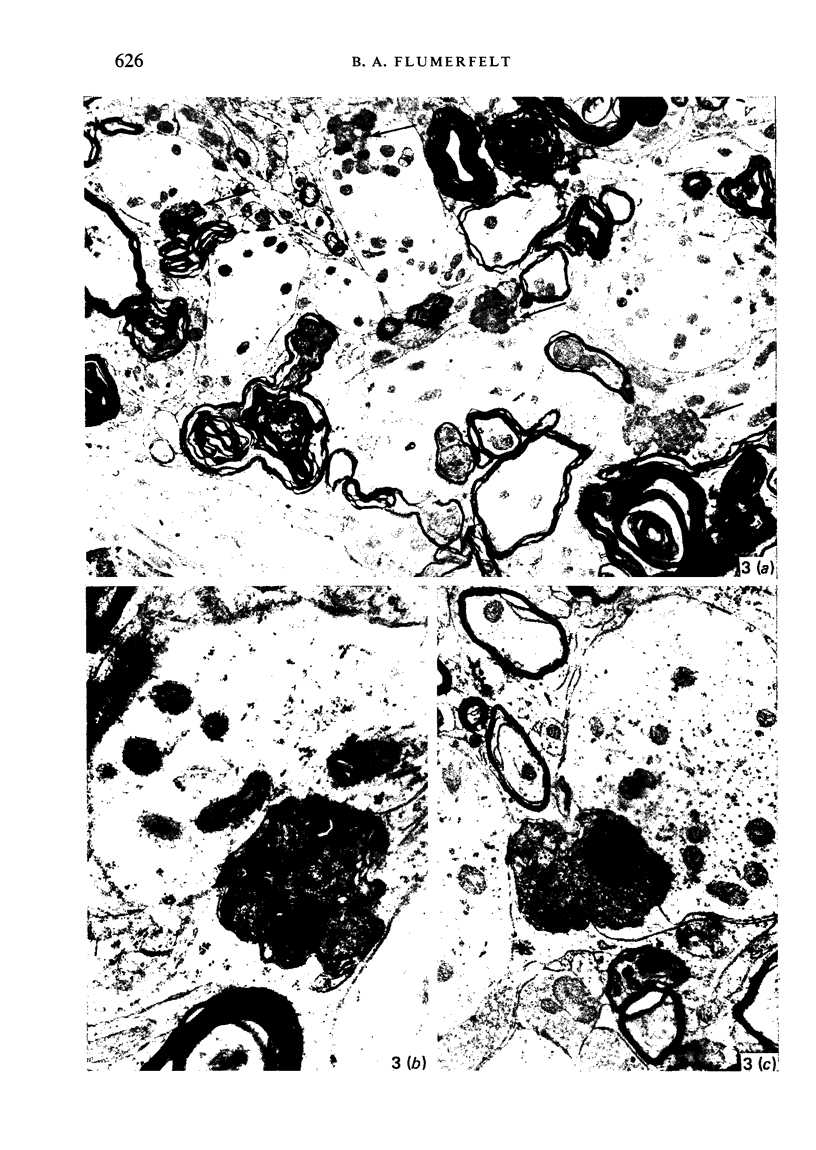





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARNARD J. W., WOOLSEY C. N. A study of localization in the cortico-spinal tracts of monkey and rat. J Comp Neurol. 1956 Aug;105(1):25–50. doi: 10.1002/cne.901050103. [DOI] [PubMed] [Google Scholar]
- Brown L. T. Corticorubral projections in the rat. J Comp Neurol. 1974 Mar 15;154(2):149–167. doi: 10.1002/cne.901540204. [DOI] [PubMed] [Google Scholar]
- Caughell K. A., Flumerfelt B. A. The organisation of the cerebellorubral projection: an experiment study in the rat. J Comp Neurol. 1977 Nov 15;176(2):295–306. doi: 10.1002/cne.901760210. [DOI] [PubMed] [Google Scholar]
- Condé H., Angaut P. An electrophysiological study of the cerebellar projections to the nucleus ventralis lateralis thalami in the cat. 2. Nucleus lateralis. Brain Res. 1970 May 20;20(1):107–119. doi: 10.1016/0006-8993(70)90159-9. [DOI] [PubMed] [Google Scholar]
- Flumerfelt B. A., Caughell K. A. A horseradish peroxidase study of the cerebellorubral pathway in the rat. Exp Neurol. 1978 Jan 1;58(1):95–101. doi: 10.1016/0014-4886(78)90124-3. [DOI] [PubMed] [Google Scholar]
- Gwyn D. G., Flumerfelt B. A. A comparison of the distribution of cortical and cerebellar afferents in the red nucleus of the rat. Brain Res. 1974 Mar 29;69(1):130–135. doi: 10.1016/0006-8993(74)90377-1. [DOI] [PubMed] [Google Scholar]
- Humphrey D. R., Rietz R. R. Cells of origin of corticorubral projections from the arm area of primate motor cortex and their synaptic actions in the red nucleus. Brain Res. 1976 Jun 25;110(1):162–169. doi: 10.1016/0006-8993(76)90217-1. [DOI] [PubMed] [Google Scholar]
- King J. S., Dom R. M., Conner J. B., Martin G. F. An experimental light and electron microscopic study of cerebellorubral projections in the opossum, Didelphis marsupialis virginiana. Brain Res. 1973 Mar 30;52:61–78. doi: 10.1016/0006-8993(73)90650-1. [DOI] [PubMed] [Google Scholar]
- King J. S., Martin G. F., Conner J. B. A light and electron microscopic study of corticorubral projections in the opossum, Didelphis marsupialis virginiana. Brain Res. 1972 Mar 24;38(2):251–265. doi: 10.1016/0006-8993(72)90711-1. [DOI] [PubMed] [Google Scholar]
- Kuypers H. G., Lawrence D. G. Cortical projections to the red nucleus and the brain stem in the Rhesus monkey. Brain Res. 1967 Mar;4(2):151–188. doi: 10.1016/0006-8993(67)90004-2. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P. R., Shute C. C. The distribution of cholinesterase in cholinergic neurons demonstrated with the electron microscope. J Cell Sci. 1966 Sep;1(3):381–390. doi: 10.1242/jcs.1.3.381. [DOI] [PubMed] [Google Scholar]
- Mabuchi M., Kusama T. The cortico-rubral projection in the cat. Brain Res. 1966 Sep;2(3):254–273. doi: 10.1016/0006-8993(66)90048-5. [DOI] [PubMed] [Google Scholar]
- Massion J. The mammalian red nucleus. Physiol Rev. 1967 Jul;47(3):383–436. doi: 10.1152/physrev.1967.47.3.383. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Mizuno N. An electron microscopic study of the interposito-rubral connections in the cat and rabbit. Brain Res. 1971 Dec 10;35(1):283–286. doi: 10.1016/0006-8993(71)90619-6. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Mizuno N., Konishi A. A quantitative electron microscope study of cerebellar axon terminals on the magnocellular red nucleus neurons in the cat. Brain Res. 1978 May 19;147(1):17–27. doi: 10.1016/0006-8993(78)90769-2. [DOI] [PubMed] [Google Scholar]
- Oka H., Jinnai K. Electrophysiological study of parvocellular red nucleus neurons. Brain Res. 1978 Jun 23;149(1):239–246. doi: 10.1016/0006-8993(78)90605-4. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON K. C., JARETT L., FINKE E. H. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol. 1960 Nov;35:313–323. doi: 10.3109/10520296009114754. [DOI] [PubMed] [Google Scholar]
- RINVIK E., WALBERG F. Demonstration of a somatotopically arranged cortico-rubral projection in the cat. An experimental study with silver methods. J Comp Neurol. 1963 Jun;120:393–407. doi: 10.1002/cne.901200303. [DOI] [PubMed] [Google Scholar]
- Reid J. M., Flumerfelt B. A., Gwyn D. G. An ultrastructural study of the red nucleus in the rat. J Comp Neurol. 1975 Aug 1;162(3):363–385. doi: 10.1002/cne.901620306. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Toyama K., Tsukahara N., Kosaka K., Matsunami K. Synaptic excitation of red nucleus neurones by fibres from interpositus nucleus. Exp Brain Res. 1970;11(2):187–198. doi: 10.1007/BF00234322. [DOI] [PubMed] [Google Scholar]
- Tsukahara N., Murakami F., Hultborn H. Electrical constants of neurons of the red nucleus. Exp Brain Res. 1975 Jul 11;23(1):49–64. doi: 10.1007/BF00238728. [DOI] [PubMed] [Google Scholar]
- Welker C. Microelectrode delineation of fine grain somatotopic organization of (SmI) cerebral neocortex in albino rat. Brain Res. 1971 Mar 5;26(2):259–275. [PubMed] [Google Scholar]