Abstract
Water scarcity has become challenging and has greatly impacted crop production globally. This study focused on adverse effects of drought on a delicate vegetable crop Coriandrum sativum L. which is also used in spices worldwide. Furthermore, the role of magnesium oxide nanoparticles (MgONPs) in improving drought resilience in C. sativum was also analyzed. For this, a two factorial pot experiment was designed in a completely randomized fashion with five replicates. For drought, the field capacity of stressed pots was maintained at below 40%. MgONPs were foliar sprayed using a manual hand sprayer at 300 mg L–1. Meanwhile, for comparative analysis, precursor molecules used for synthesis of MgONPs (i.e., MgSO4) were also applied via a foliar spray at the same level. Results showed that drought severely reduced growth (shoot length; 17%, root length; 34%), whole plant fresh (34%) and dry (23%) weights, photosynthetic pigments (total chlorophyll; 37%), and chlorophyll fluorescence and performance index (74%). It also reduced photosynthesis rate (56%), transpiration rate (48%), and stomatal conductance (56%), whereas drought stress elevated the level of stress markers (relative membrane permeability; 43%, malondialdehyde; 32% and H2O2; 34%) and antioxidants (catalase; 26%, superoxide dismutase; 81%, peroxidase; >2 folds). Total phenolics and flavonoids were also increased by 23 and 51% in drought stressed plants. On the other side, MgSO4 and MgONPs successfully mitigated harsh effects of drought, but the MgONPs were more efficient in improving drought resilience of C. sativum . MgONPs improved photosynthetic pigments, chlorophyll fluorescence, gas exchange attributes, antioxidant defense, and ultimately growth of C. sativum in both control and drought conditions. These findings suggest that MgONPs can potentially be used for improving drought resilience in crop plants.


Introduction
Drought stress is a major abiotic factor that significantly hampers agricultural productivity and plant growth. Water availability is directly linked to food crop production, with over 40% of global agricultural land relying on irrigation to sustain yields. Water constitutes 80–95% of plant biomass and plays a fundamental role in various physiological processes. However, the increasing degradation of water resources and rising food demands have exacerbated the adverse effects of drought on crop production. Human-induced climate change has further intensified the frequency and severity of droughts worldwide, making it a pressing concern for sustainable agriculture.
The impact of drought stress is multifaceted and varies based on factors such as soil moisture availability, temperature fluctuations, light intensity, and rainfall patterns. Under water-deficit conditions, plants experience disruptions in essential physiological functions, leading to reduced yield and quality. Drought stress causes nutrient deficiencies, osmotic imbalances, and oxidative stress due to the excessive accumulation of reactive oxygen species (ROS), which damage lipids, proteins, and nucleic acids. Consequently, stomatal regulation, chloroplast function, and photosynthetic efficiency are severely compromised. Plants have evolved complex adaptive mechanisms to withstand drought, but their response varies depending on species, developmental stage, genetic makeup, and drought duration. Enhancing plant resilience to drought through cost-effective and sustainable approaches is crucial to ensuring global food security. Drought stress limits cell extensibility, inhibits growth due to reduced turgor pressure, and triggers physiological drought conditions that impair root function and water uptake. These stressors lead to a decline in protein content, antioxidant enzyme activity, and overall plant vigor while increasing proline, malondialdehyde (MDA), and hydrogen peroxide (H2O2) levels, ultimately causing cellular damage.
Magnesium (Mg) is an essential macronutrient found in various rock minerals and seawater, primarily available in the soil as Mg2+ ions. It plays a critical role in plant growth, particularly in photosynthesis and carbohydrate metabolism. Approximately 35% of foliar Mg is localized within chloroplasts, where it is vital for chlorophyll synthesis, grana development, and thylakoid membrane stability. Mg also regulates ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) activity, which is a key enzyme in the Calvin cycle. Mg deficiency results in chlorosis, impaired carbohydrate transport, and reduced productivity, necessitating the use of magnesium fertilizers to restore plant health.
Nanotechnology has emerged as a promising tool in sustainable agriculture, offering various benefits, such as soil quality enhancement, climate change mitigation, and improved nutrient uptake efficiency. MgONPs have shown potential in improving plant resilience to drought stress by enhancing photosynthetic efficiency, regulating osmotic balance, and stimulating antioxidant defense systems. These nanoparticles facilitate root growth, upregulate aquaporin expression, modulate intracellular water metabolism, and maintain ion homeostasis under water-limiting conditions. Their relatively low toxicity, ecofriendly nature, and high surface reactivity make them suitable candidates for agricultural applications. Additionally, MgONPs contribute to the removal of heavy metals and pollutants from the environment, further reinforcing their role in sustainable crop production.
Coriandrum sativum L. (Coriander), an important herb from the Apiaceae family, is widely cultivated for its culinary, medicinal, and aromatic properties. , Native to the Mediterranean region, coriander is extensively grown worldwide as a vegetable and medicinal plant. It is rich in bioactive compounds such as terpenes and flavonoids, which contribute to its antioxidant, anti-inflammatory, and neuroprotective properties. Coriander is traditionally used in the treatment of digestive, respiratory, and urinary ailments. However, its growth and productivity are highly susceptible to water stress, necessitating an in-depth understanding of its physiological responses to drought and potential mitigation strategies.
Despite extensive research on the beneficial effects of MgONPs on plant growth, their role in improving drought resilience in C. sativum remains unexplored. This study hypothesizes that MgONPs can effectively mitigate the adverse effects of drought on coriander by enhancing its physiological and biochemical responses. Therefore, the objective of this research is to synthesize MgONPs and evaluate their potential in improving drought tolerance in C. sativum through antioxidant regulation, redox balancing, and photosynthetic efficiency enhancement.
Results
Characterization of Synthesized Magnesium Oxide Nanoparticles
UV–Visible Spectroscopy Analysis
Ultraviolet–visible (UV–Vis) spectroscopy constitutes an essential analytical methodology for the examination of nanoparticles, yielding valuable information regarding their optical characteristics, dimensions, and electronic configurations. Upon exposure to UV–Vis radiation, nanoparticles absorb distinct wavelengths, which is contingent upon their material composition, dimensions, morphology, and surface attributes, resulting in the emergence of characteristic absorption peaks. The localization of this peak is influenced by variables such as the particle dimensions, morphology, and properties of the surrounding medium. The MgO NPs show a strong absorption peak at 220 nm (Figure A).
1.
Characterization of magnesium oxide nanoparticles (MgONPs), (A) UV–vis spectrophotometer analysis, (B) energy-dispersive X-ray spectroscopy (EDX), (C) scanning electron microscopy (SEM), (D) histogram showing particle size, and (E) Fourier-transform infrared spectroscopy (FTIR).
Energy-Dispersive X-ray
Energy-dispersive X-ray (EDX) microanalysis represents a sophisticated and highly specialized technique employed for the purpose of conducting elemental analysis, which is intrinsically linked to the field of electron microscopy and is fundamentally predicated upon the generation of distinctive characteristic X-rays that serve to elucidate and make apparent the presence of various elements that may be found within the samples under examination. Energy-dispersive X-ray spectroscopy constitutes an analytical methodology, wherein X-ray photons emitted from a sample are identified and transmitted into a voltage signal that is directly proportional to their energy, thereby facilitating the generation of a histogram that illustrates the quantity of detected X-rays in correlation with their energy levels. The samples contain Mg (6.75%), O (43.69%), Ca (3.14%), and C (46.41%). The presence of Mg and O confirms that these are MgONPs (Figure B).
Scanning Electron Microscopy
Scanning electron microscopy (SEM) is a method used for morphological analysis and direct visualization of nanoparticles (JEOL 5600LV, Tokyo, Japan). It can also be used to examine complex, inorganic, biological, carbon-based, and organic materials as well as identify the morphological conditions of nanomaterials. This approach provides insight into the size distribution but lacks insight into the population average. The nanoparticle solution should be dried before being coated with conductive metals. A concentrated fine electron beam is used to scan the materials and determine the surface properties. However, electron beams can harm polymers. SEM involves the examination of a concentrated beam of electrons directed across the surface of a sample to generate an image of exceptional detail. Upon interaction with the sample, the electron beam produces various signals, including secondary electrons, backscattered electrons, and X-rays. These emitted signals yield critical insights into the surface topography, composition, and structural characteristics of a sample. SEM captures these emitted signals, which undergo processing to render a magnified representation of the sample, typically achieving resolution at the nanometer scale. SEM is prevalent across disciplines such as materials science, biology, and various industrial sectors. The MgO nanoparticles have a thin, sheet-like structure (Figure C,D).
Fourier Transform Infrared Spectroscopy
The abbreviation FTIR stands for “Fourier Transform Infrared”. FTIR spectra can be collected by absorbing electromagnetic radiation from 500 nm to 4000 cm–1. The FTIR spectrometer collects data across a wide range. The absorption bands at 3419 cm–1, 2158 cm–1, and 2389 cm–1 indicate that the presence of N–H or −OH functional groups in Mg(OH)2 arises from the propensity of MgO to adsorb H2O and CO2 onto its surface upon exposure to atmospheric conditions. The wide spectral band observed at the threshold of 1585 cm–1 could be attributed to the N–H bond, which is instigated by a bending vibrational mode in an aromatic amine. The band detected at a limit of 725 confirms that MgO nanoparticles are formed (Figure E).
Effects of Foliar Spray with MgSO4 and MgONPs on the Growth and Biomass Attributes of C. sativum Grown under Drought Conditions
Figure shows that, compared with the control, drought stress reduced the growth of C. sativum plants; specifically, it significantly reduced shoot length (34%), root length (17%), fresh weight (34%), and dry weight (23%). Foliar spraying of MgSO4 and MgONPs improved the growth of C. sativum plants under both control and drought conditions. MgSO4 increased the aforementioned attributes by 7, 6, 12, and 23%, whereas MgONPs increased these attributes by 25, 6, 15, and 24%, respectively, when applied under normal conditions. Similarly, these supplements also improved growth attributes under drought conditions. However, the effect of MgONPs was statistically more significant, as compared with the drought treatment; MgONPs improved the shoot length, root length, fresh weight, and dry weight of C. sativum plants by 39%, 22%, 52%, and 47%, respectively (Figure A–E).
2.
Effects of MgSO4 and MgONPs on growth attributes of C. sativum under drought stress, where (A) C. sativum plants under different treatments, (B) Shoot length, (C) Root length, (D) Plant fresh weight, and (E) Plant dry weight. Data presented in the graph bars is average of 3 replicates ± standard error in the form of error bars. Bars sharing similar letters obtained after Duncan’s multiple range test (DMRT) do not differ at the significance level (p ≤ 0.05).
Effects of Foliar Spray with MgSO4 and MgONPs on the Photosynthetic Pigments of C. sativum Grown under Drought Conditions
Drought stress significantly decreased the contents of photosynthetic pigments in C. sativum plants, as it reduced the contents of chlorophyll a (Chl a), chlorophyll b (Chl b), and total chlorophyll by 29%, 64%, and 37%, respectively, compared with those in the control. However, the carotenoid content increased by 28% in drought-stressed plants. The application of MgSO4 and MgONPs improved the levels of Chl a, b, and total Chl in both the control (normal) and drought-stressed plants, which were equal to their respective controls. For example, foliar MgONP spray at 300 mg L–1 improved Chl a, Chl b, and total Chl by 45%, 31%, and 41%, respectively, in normal plants and by 90%, 206%, and 106%, respectively, in drought-stressed plants. Foliar spraying of MgSO4 and MgONPs on the other side reduced the carotenoid content by 12% and 20%, respectively, in drought-stressed plants compared with that in drought-susceptible plants without foliar spraying (Figure A–D).
3.
Effects of MgSO4 and MgONPs on pigmentation in C. sativum under drought stress, where (A) Chlorophyll a, (B) Chlorophyll b, (C) Total chlorophyll, and (D) Carotenoids. Data presented in the graph bars is average of 3 replicates ± standard error in the form of error bars. Bars sharing similar letters obtained after DMRT do not differ at the significance level (p ≤ 0.05).
Effect of Foliar Spray with MgSO4 and MgONPs on the Quantum Yield of Photosystem II (ΦPSII) and the Photosynthetic Machinery of C. sativum Grown under Drought Conditions
Drought stress has been shown to significantly compromise the functionality of photosystem II (PSII), a critical component of the photosynthetic machinery, as evidenced by the observable reductions in Fv/Fm (12%), Fv/Fo (41%), performance index (PI; 74%), TRo/ABS (12%), and ETo/TRo (25%) and concurrent increases in ABS/RC (51%) and Dlo/CS (124%). This collectively signifies the occurrence of photoinhibition, a decrease in the efficiency of electron transport, and an increase in the energy dissipation processes. In contrast, the application of MgSO4 and MgONPs effectively mitigated these detrimental effects, leading to a partial restoration of the aforementioned parameters. For example, compared with no treatment, MgONP supplementation increased the Fv/Fm, Fv/Fo, PI, TRO/ABS, and ETo/TRo of drought-stressed plants by 13%, 64%, 210%, 13%, and 17%, respectively, while it decreased the ABS/RC and DIo/CS by 33% and 54%, respectively. These findings indicate an improvement in the overall physiological state of the plants under drought conditions. These findings underscore the crucial protective roles played by MgSO4 and MgO-NPs in alleviating the adverse effects of drought-induced photodamage, enhancing the efficiency of energy transfer processes, and sustaining the performance of PSII (Figure A,B).
4.
(A) Effect of MgSO4, MgONPs, and Drought (D) treatments normalized with control (normal) plants and (B) Effect of MgSO4 and MgONPs on drought-stressed plants normalized with Drought (D) plants. Data presented in the radar graphs is average of 3 replicates.
Effects of Foliar Spray with MgSO4 and MgONPs on the Gas-Exchange Attributes, Water Use Efficiency, and Relative Water Content of C. sativum Grown under Drought Conditions
Compared with the control, drought stress disrupted the gas-exchange attributes, as evidenced by a significant reduction in the net photosynthesis rate (P n; 56%), transpiration rate (T r; 48%), and stomatal conductance (g s; 56%) of C. sativum. Compared with the control, drought stress also increased the intercellular CO2 (Ci; 25%) level. Foliar sprays of MgSO4 and MgONPs reversed these negative effects of drought in C. sativum compared with those in nontreated stressed plants. However, the greatest improvements in the gas-exchange attributes of stressed plants were observed with the foliar spraying of MgONPs. Compared with stress alone, it improved the P n, T r, and g s by 144%, 109%, and 194%, respectively, while at the same time, it reduced the Ci by 28% (Figure A–D). These findings were also supported by observations of the water use efficiency (WUE) and relative water content (RWC) of C. sativum. Figure E,F shows that, compared with the control, drought stress not only caused a reduction in RWC (22%) but also reduced WUE (14%) in stressed plants. On the other hand, the application of MgSO4 and MgONPs improved both the RWC (23 and 33%, respectively) and WUE (7 and 16%, respectively) in drought-stressed C. sativum plants compared with those in stress-only plants.
5.
Effects of MgSO4 and MgONPs on gas-exchange attributes of C. sativum under drought stress, where (A) Net photosynthesis rate (P n), (B) Transpiration rate (T r), (C) Stomatal conductance (g s), (D) Intercellular CO2 (Ci), (E) WUE, and (F) RWC. Data presented in the graph bars is average of 3 replicates ± standard error in the form of error bars. Bars sharing similar letters obtained after DMRT do not differ at the significance level (p ≤ 0.05).
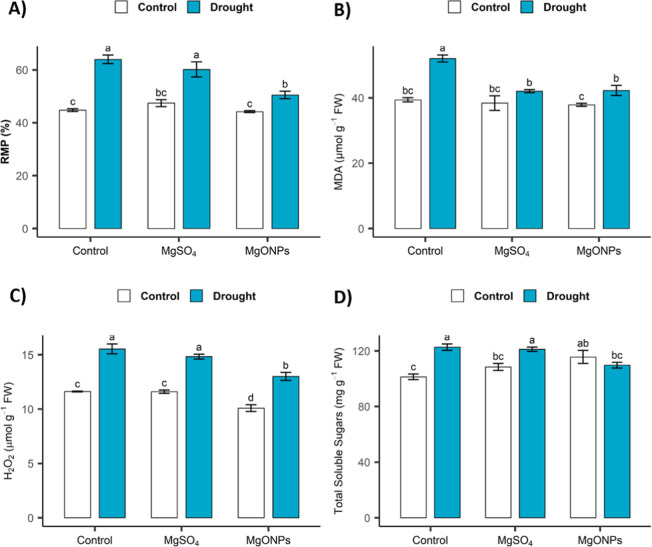
Effects of Foliar Spray with MgSO4 and MgONPs on the Relative Membrane Permeability and Malondialdehyde, Hydrogen Peroxide, and Total Soluble Sugar Contents of C. sativum Grown under Drought Conditions
Drought stress in C. sativum plants was evident from significant increases in the levels of stress markers, such as relative membrane permeability (RMP; 43%), malondialdehyde (MDA; 32%), and hydrogen peroxide (H2O2; 34%), compared with those in control plants. Foliar sprays of both Mg supplements effectively remediated these adverse effects of drought, but MgONPs were more effective. Compared with the control, MgSO4 application reduced RMP, MDA, and H2O2 by 6%, 19%, and 5%, respectively, in the plants under drought conditions. However, foliar spraying of MgONPs reduced these attributes by 21%, 19%, and 16%, respectively, under the same conditions. Compared with the control plants, the drought-stressed plants presented increased total soluble sugar (TSS) contents (21%). Additionally, the foliar spray of MgSO4 and MgONPs increased the TSS content in the control (7 and 14%, respectively) plants compared with that in the nontreated plants. Under drought conditions, MgSO4 had a negligible effect, but compared with the control treatment, the MgONP treatment caused a reduction in the TSS by 11% (Figure A–D).
6.
Effects of MgSO4 and MgONPs on stress markers and soluble sugars in C. sativum under drought stress, where (A) RMP, (B) Malondialdehyde (MDA) content, (C) Hydrogen peroxide, and (D) Total soluble sugars. Data presented in the graph bars is average of 3 replicates ± standard error in the form of error bars. Bars sharing similar letters obtained after DMRT do not differ at the significance level (p ≤ 0.05).
Effect of Foliar Spray with MgSO4 and MgONPs on Total Soluble Protein Content and Antioxidant Enzyme Activities in C. sativum Grown under Drought Conditions
Compared with those of the control plants, the total soluble protein content of C. sativum plants under drought conditions increased by 33%. This is also evident from the increased activities of antioxidant enzymes in stressed plants, as most enzymes are proteins. Compared with those of control plants, the activities of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) were increased by 81%, 525%, and 26%, respectively, in drought-stressed plants. Foliar sprays of MgSO4 and MgONPs further increased the TSP content in drought-stressed plants by 10% and 30%, respectively, compared to that in stress-only plants. However, these treatments reduced the activities of SOD (17 and 36%, respectively) and POD (16 and 52%, respectively) but increased the activity of CAT by 16 and 54%, respectively, under the same conditions (Figure A–D).
7.
Effects of MgSO4 and MgONPs on soluble proteins and antioxidant enzymes activities in C. sativum under drought stress, where (A) Total soluble proteins (TSPs), (B) Catalase (CAT), (C) Peroxidase (POD), and (D) Super oxide dismutase (SOD). Data presented in the graph bars is average of 3 replicates ± standard error in the form of error bars. Bars sharing similar letters obtained after DMRT do not differ at the significance level (p ≤ 0.05).
Effects of Foliar Spray with MgSO4 and MgONPs on the Total Phenolic, Flavonoid, and Proline Contents of C. sativum Grown under Drought Conditions
Some of the secondary metabolites (total phenolics and flavonoids) were increased in the drought-stressed plants compared to the control plants. These metabolites were increased by 23% and 51%, respectively. Compared with control plants, drought-stressed plants also presented increased proline content (77%), an indicator of osmotic stress. The results of this study revealed that the foliar spray of MgSO4 and MgONPs increased the total phenolic, flavonoid, and proline contents under control conditions but significantly decreased these attributes under drought conditions. For example, compared with no drought, MgSO4 application reduced these attributes by 7%, 13%, and 13%, respectively. Similarly, the MgONPs lowered the total phenolic, flavonoid, and proline contents by 12%, 38%, and 33%, respectively (Figure A–C).
8.
Effects of MgSO4 and MgONPs on secondary metabolites and nutritional ions in C. sativum under drought stress, where (A) Leaf proline, (B) Total phenolics, (C) Total flavonoids, (D) Shoot Ca2+, (E) Shoot K+, and (F) Shoot Mg2+. Data presented in the graph bars is average of 3 replicates ± standard error in the form of error bars. Bars sharing similar letters obtained after DMRT do not differ at the significance level (p ≤ 0.05).
Effect of Foliar Spray with MgSO4 and MgONPs on the Nutritional Ion (K+, Ca2+, and Mg2+) Content of C. sativum Grown under Drought Conditions
C. sativum plants subjected to drought stress presented reduced amounts of nutritional ions in their shoots. In the shoots of these plants, the K+ and Ca2+ contents were 27 and 15% lower than those in the control plants. Foliar spray of Mg supplements increased the nutritional ions under both the control and stress conditions. Similarly, in the control plants, MgSO4 application increased Ca2+ and Mg2+ by 25% and 77%, respectively, compared with those in the nontreated control plants. Similarly, MgONPs increased K+, Ca2+, and Mg2+ in the shoots of these plants by 18%, 35%, and 89%, respectively, under the same conditions. Under drought conditions, MgSO4 increased these nutrients by 20%, 14%, and 40%, respectively, whereas MgONPs increased these nutrients by 34%, 36%, and 49%, respectively, compared with those in stress-only plants (Figure D–F).
Pearson’s Correlation and Principal Component Analysis
The Pearson correlation data in Figure show a linear relationship among all the studied attributes of C. sativum under the effects of MgSO4, MgONPs, and drought stress. Blue and yellow represent positive and negative correlations, respectively, among the different attributes. The shaded color bars depict the extent of the relationships among attributes ranging from 0 to 1, with the dark color indicating highly positive or negative relationships. Statistically significant linear relationships among the different studied attributes are expressed with signs ns, *, **, and *** at p > 0.05, p ≤ 0.05, p ≤ 0.01, and p ≤ 0.001, respectively. The results revealed that most of the stress indicators, such as RMP, MDA, and H2O2, were highly negatively correlated with the growth- and photosynthesis-related attributes as well as the nutrient contents of the C. sativum plants examined in this study. Furthermore, the contents of TSS, TSP, SOD, POD, CAT, total phenolics, flavonoids, and even proline, which are all indicators of stress, were also negatively correlated with growth attributes. These findings revealed the effects of drought conditions on the growth of C. sativum plants. Principal component analysis (PCA) further validated these findings. Figure A shows the percentage of explained variables (attributes of C. sativum) in total of five principal components (PCs), with the first two components (PC1; 64.1% and PC2; 15.2%) contributing the most, with a total of 79.3%. Figure B shows the PCA results of the individual treatments applied in this study, including the control (1), MgSO4 (2), MgONP (3), Drought (D; 4), D + MgSO4 (5), and D + MgONP (6) treatments. As Drought (4) was well separated from all the other treatments, drought clearly had different and significant effects on the studied attributes of C. sativum. Furthermore, D + MgONPs (6), which are nearer to the control (1), successfully remediated the adverse effects of drought. In Figure , PCA of all the studied parameters of C. sativum revealed that these parameters are grouped into two categories on the basis of their alignment with PC1 and PC2. The parameters aligned with PC1 were positively correlated with each other but negatively correlated with the parameters aligned with PC2. A similar situation occurs when the parameters are aligned with PC2.
9.

Pearson’s correlation among all studied parameters ofC. sativum under the effect of MgSO4, MgONPs, and drought (various abbreviations used are SL, shoot length; RL, root length; PFW, plant fresh weight; PDW, plant dry weight; Chl, chlorophyll; P n, photosynthesis rate; T r, transpiration rate; g s, stomatal conductance; C i, intercellular CO2; WUE, water use efficiency; RWC, relative water content; RMP, relative membrane permeability; MDA, malondialdehyde; H2O2, hydrogen peroxide; TSS, Total soluble sugar; TSP, Total soluble protein; SOD, superoxide dismutase; POD, Peroxidase; CAT, Catalase; K, Potassium; Ca, Calcium; and Mg, Magnesium). The signs ns (nonsignificant), *, **, and *** showed statistically significant linear relation at p > 0.05, p ≤ 0.05, p ≤ 0.01, and p ≤ 0.001, respectively.
10.
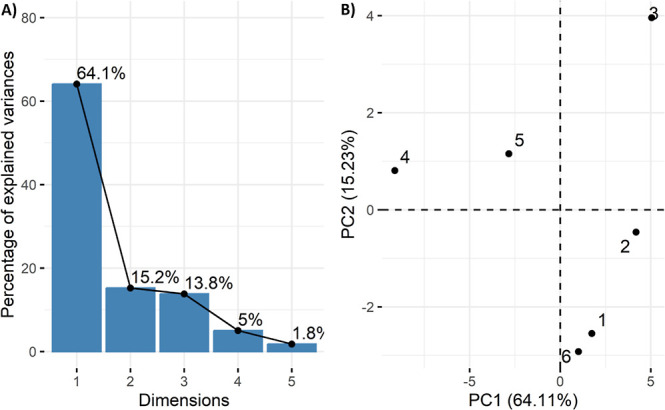
Principal component analysis (PCA). (A) Distribution of explained variables in all PCs and (B) PCA of individual treatment applied in this study, i.e., Control (1), MgSO4 (2), MgONPs (3), Drought (D) (4), D + MgSO4 (5), and D + MgONPs (6).
11.
Principal component analysis of all studied parameters of C. sativum under the effect of MgSO4, MgONPs, and drought (various abbreviations used are the same as given in Figure ).
Discussion
Drought stress severely impairs plant growth and productivity by reducing water availability, leading to physiological and biochemical disruptions. In C. sativum, our study found that drought stress significantly decreased root and shoot lengths, indicating its detrimental effect on plant development. However, the application of magnesium oxide nanoparticles (MgONPs) substantially improved plant biomass, suggesting that MgONPs play a crucial role in mitigating drought-induced stress by enhancing physiological resilience and metabolic stability.
Chlorophyll is a fundamental component of photosynthesis, and its synthesis is highly dependent on magnesium availability. Without sufficient magnesium, plants experience impaired light absorption and reduced photosynthetic efficiency. Our results demonstrated a decline in the chlorophyll content under drought stress, which was significantly restored with MgONP application. This aligns with previous findings in Zea mays and other crops, where MgONPs enhanced chlorophyll biosynthesis and carotenoid content, contributing to improved photosynthetic performance and plant vigor. The decline in photosynthetic parameters such as chlorophyll fluorescence (Fv/Fm), photosystem II efficiency (ϕPSII), net photosynthetic rate, intercellular CO2 concentration, and water use efficiency under drought stress underscores the severity of physiological impairment in untreated plants. However, our results demonstrated that MgONP application mitigated these adverse effects, leading to sustained photosynthetic activity and improved plant performance. One of the primary ways MgONPs enhance drought tolerance is through improved stomatal regulation. Drought stress typically causes stomatal closure to minimize water loss, but this also restricts carbon dioxide uptake, leading to reduced photosynthetic efficiency. Our study revealed that MgONPs increased stomatal conductance, which facilitated better gas exchange and sustained photosynthetic activity under drought conditions. This improvement in stomatal regulation likely stems from the role of magnesium in activating enzymes involved in guard cell function and osmotic balance.
ROS accumulation is another major consequence of drought stress, leading to oxidative damage in plant cells. Our findings showed that plants under drought stress exhibited elevated ROS levels, which triggered an increase in antioxidant enzyme activity, including catalase (CAT), peroxidase (POD), and ascorbate peroxidase (APX). The foliar application of MgONPs further enhanced the activity of these enzymes, effectively scavenging ROS and protecting the cellular structures from oxidative stress. Similar effects have been observed in S. melongena, soyabean, and tomato plants treated with CuO, MgO, and ZnO nanoparticles under abiotic stresses, highlighting the universal role of nanoparticles in oxidative stress mitigation. ,, Moreover, the significant reduction in the malondialdehyde (MDA) content in MgONP-treated plants indicates reduced lipid peroxidation, further confirming the protective effects of MgONPs on plant cell membranes under drought stress.
The accumulation of osmo-protectants such as proline, flavonoids, and TSSs is another adaptive mechanism in plants exposed to drought stress. These compounds aid in osmotic adjustment, stabilizing cellular membranes and maintaining water uptake. Our study found that MgONP-treated plants exhibited higher levels of proline and phenolics, further supporting their role in drought resilience. Additionally, the increase in total soluble sugars observed in MgONP-treated plants suggests enhanced carbon assimilation and energy availability, which contribute to improved root growth and water absorption. Magnesium is an essential macronutrient that plays a critical role in plant metabolism, and its deficiency exacerbates drought stress effects. Our findings revealed that MgONPs alleviated magnesium deficiency by enhancing ion homeostasis, particularly for key inorganic ions such as K, Mg, and Ca, which are vital for maintaining cellular turgor and metabolic functions. This study provides compelling evidence that MgONPs enhance drought tolerance in C. sativum by improving chlorophyll synthesis, stomatal regulation, ROS scavenging, osmoprotectant accumulation, and ion homeostasis. These findings highlight the potential of MgONPs as an effective strategy for improving crop resilience under water-limited conditions, paving the way for their application in sustainable agriculture, particularly in arid and semiarid regions. Use of nanoparticles or nano fertilizers is a cost-effective technique. This is due to specialized properties related to nanoparticles such as slow-release mechanism, enhanced nutrient use efficiency, biodegradability, bioavailability, and efficient uptake due to their small size and increased surface area and contact ratio. Nanoparticles are nontoxic and ecofriendly and have great impact on mitigation of abiotic stresses in crops. ,
Conclusions
This study demonstrates the significant potential of magnesium oxide nanoparticles (MgONPs) in enhancing drought resilience in Coriandrum sativum L. under water scarcity conditions. The results indicate that drought stress adversely affects the growth and physiological attributes of coriander, leading to reduced biomass, decreased photosynthetic efficiency, and increased oxidative stress markers. However, the application of MgONPs effectively mitigated these detrimental effects, improving key parameters such as photosynthetic pigments, gas exchange, and antioxidant defense mechanisms. Notably, MgONPs outperformed the precursor compound MgSO4 in enhancing plant resilience, indicating their superior efficacy. These findings highlight the promising role of MgONPs as biostimulants in agricultural practices, particularly for crops facing drought stress. The use of MgONPs could be a viable strategy for improving crop productivity and sustainability in arid and semiarid regions, paving the way for further research into their application across various crop species.
Materials and Methodology
Synthesis of MgO Nanoparticles
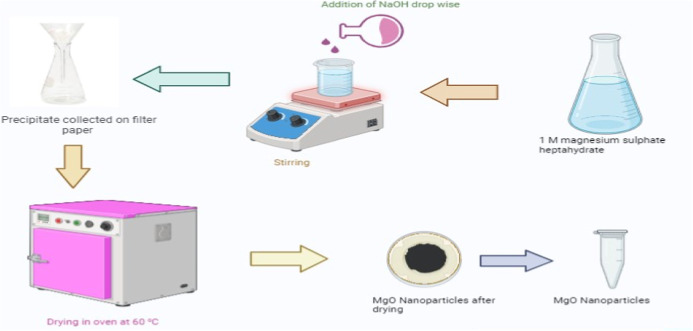
The chemicals used in this study were magnesium sulfate heptahydrate and sodium hydroxide. The chemicals used in this experiment were highly purified. The coimmunoprecipitation method was used in this study. One M magnesium sulfate heptahydrate solution was added to a conical flask. The conical flask was placed on a magnetic stirrer for 6 h at room temperature. The 2 M NaOH solution was added dropwise to magnesium sulfate heptahydrate with continuous stirring. A white precipitate formed at the bottom of the conical flask. Whatman filter paper was used to filter these precipitates. The precipitates on the filter paper were collected and placed at 100 °C in a drying oven. The weight of the sample was used to compute the actual yield of the product, following that the dried samples were kept in a muffle furnace for 7 h at 500 °C to get final stable product (Figure ). For confirmation of synthesized nanoparticles, UV–vis spectrophotometer analysis, EDX, SEM, and FTIR characterization techniques were employed.
12.
Magnesium oxide nanoparticle (MgONP) synthesis process.
Experimental Layout and Study Design
This experiment was conducted in the botanical garden of the University of Education, Lahore. The experiment was conducted under natural conditions from October 2024. This was a two-factorially completely randomized design experiment with three replicates. Seeds were procured from the Ayub Agricultural Research Institute, Faisalabad. The garden soil was collected, air-dried, and sieved to remove debris. The soil used in this experiment had a loamy texture, a pH of 6.7, and an EC of 1.30 dS m–1. Each pot (15 × 20 cm) contained 5 kg of soil. Nearly 10 healthy seeds were sown in each pot over equal distances. After germination, the plants were thinned to ensure that 4–5 seedlings of the same size were equidistant from each other. Two weeks after germination, the following treatments were executed: the control (normal plants), foliar spray of MgSO4 and MgONPs (300 mg L–1 for both), drought (D), and drought with the foliar spray of MgSO4 and MgONPs. The concentrations of MgSO4 and MgONPs were selected on the basis of studies conducted by Sharavdorj et al. and Ojagh and Moaveni, respectively. C. sativum plants were subjected to drought stress by maintaining the moisture content of the soil below 40% of the field capacity of the soil. MgSO4 and MgONP solutions were prepared in 1% Tween 20 and sprayed via a manual hand sprayer until the plants were fully wet. The control plants were sprayed with the same quantity of distilled water. During this process, the pots were carefully covered with a piece of cloth to prevent moisture invasion via spray. These treatments were applied three times during the entire experiment at intervals of 1 week. Two weeks after the treatments were completed, the growth indices and various physiological and biochemical attributes of the studied plants were measured.
Determination of Chlorophyll Content
A fresh leaf sample weighing 0.5 g was homogenized in an 80% acetone solution. Subsequent to the filtration process, the resulting extract was analyzed via UV–vis spectroscopy at various wavelengths (645 and 663 nm) to determine the optical density (OD). The total chlorophyll content was derived from the cumulative values of chlorophyll a and chlorophyll b. Carotenoid content was calculated following the method of Lichtenthaler.
Assessment of the Chlorophyll Fluorescence and Quantum Yield of Photosystem II
Chlorophyll fluorescence was measured in the field via an automated OS30P+ ADC Bioscientific fluorometer. The fully developed young leaves were adapted in the dark for 20 min by using specific clips. The leaves were subsequently exposed to a saturation pulse of 3500 μmol, but the intensity of the modulation light was reduced to 40%. The Fv/Fm and PIABS, which are combinations of biophysical parameters, were recorded. The performance index was determined by measuring the absorbance.
Assessment of Gas-Exchange Attributes
An Infrared Gas Analyzer (IRGA) LCpro SD (ADC Bio Scientific Ltd., Hoddesdon, UK) was employed for the assessment of gas-exchange measurements. Fully mature and juvenile leaves were introduced into a sealed leaf chamber, and following a three min acclimatization period to the chamber’s microclimate, the metrics for photosynthetic rate, transpiration rate, intercellular CO2, and stomatal conductance were recorded. The leaf chamber’s microclimate was set as follows: ambient CO2 concentration (C ref) ranging from 420 to 460 μmol mol–1, photosynthetically active radiation (PAR) or Q leaf measured at 915 μmol m–2 s–1, leaf chamber temperature (T ch) maintained between 24 and 27 °C, leaf temperature recorded at 25 to 27 °C, molar gas flow rate within the leaf chamber (U) at 201 μmol s–1, ambient pressure (P) documented at 999 kPa, and leaf area quantified at 6.25 cm2.
Determination of RWC
The relative water content of the samples was determined by randomly selecting a third mature leaf from each sample. The fresh mass of all of the leaf samples was obtained first to provide a baseline weight. The leaves were then placed in water for 12 to 24 h to allow them to reach their full turgidity state. Leaves from this immersion period were left to desiccate cautiously on tissue paper, with delicately cleaning any excess droplets on the surfaces of the leaves for accurate weighing. From then onward, the turgid weight of each sample was recorded. The leaf samples were subsequently placed in an oven at 80 °C to dry, and their dry weights were measured once a constant weight was reached. Finally, the relative water content of every leaf was determined via the formula established by Huang et al. This value yields the difference in fresh weight minus dry weight, further subtracting turgid weight from it, which ultimately provides a relative measure of the water status of the leaves.
Determination of Relative Membrane Permeability
The fully developed leaves of the coriander plants were used to determine the relative membrane permeability of the plants. The fresh leaves were separated into 2 mm-diameter leaf discs after being cleansed with distilled water. The leaf discs were then placed in the test tube along with ten mL of distilled water. Following two h of incubation at 25 °C, the tube EC (EC0) was determined. After the test tubes were incubated overnight at 4 °C, EC1 was measured. EC2 was then extracted from the samples by autoclaving them at 100 °C for an hour
Determination of Malondialdehyde Content
In this experimental study, the method described by Heath and Packer was used to determine the content of malondialdehyde (MDA). Five mL of a 5% trichloroacetic acid (TCA) solution and 0.5 g of fresh plant material composed the materials used in this protocol. The sample was ground at 40 °C for a period of 10 min at a rotational speed of 10,000 rpm, after which the homogenate was centrifuged. For further investigation, the obtained supernatant was carefully removed and stored. A liquid comprising a component of plant extract and thiobarbituric acid (TBA) was made by the addition of 0.5 g of TBA into 100 mL of distilled water. The same volume of the TBA solution was then mixed with the solution of the plant extract, and the resulting mixture was left at 27 °C for 30 min before being subjected to thermal treatment for 30 min in a water bath. Following the cooling cycle, the absorbance of the solution was observed by using a spectrophotometer (PG Instruments Limited T60UV) at corresponding wavelengths of 600 nm and 523 nm. This technique allowed for the identification of the MDA concentration and the monitoring of potential alterations in the samples. The appropriate mathematical equation was applied to the mathematical computations needed to arrive at the final values.
Assessment of Hydrogen Peroxide
The biosynthesis of H2O2 was measured after the reaction with potassium iodide (KI) via a spectrophotometer. One mL of 100 mM K-phosphate buffer and 4 mL of reagent were used to prepare the reaction mixture. Fresh double-distilled water, H2O containing 1 mM KI, w/v and 1 was added. For 60 min, mL of the 0.1% trichloroacetic acid (TCA) leaf extract supernatant was kept in the dark. The blank containing 0.1% TCA was absorbed while the reaction was at 390 nm, and a mixture was observed. The amount of H2O2 was quantified via comparison with the standard H2O2 curve.
Determination of Total Soluble Sugars
Fresh leaves (0.5 g) were powdered into liquid nitrogen, 5 mL of 95% ethanol was added to remove the sugar, and the mixture was centrifuged for 15 min at 4000 rpm. This solution was refrigerated for 1 week at temperatures lower than 4 °C. To make the new enthrone reagent, 150 mg of enthrone was mixed with 100 mL of 72% H2SO4. Next, three milliliters of the enthrone reagent were combined with 0.1 mL of the stored ethanolic extract, and the mixture was placed in a water bath set at 95 °C. A UV spectrophotometer was used to determine the absorbance at 625 nm.
Assessment of Total Soluble Proteins
Using the Bradford technique, 0.1 g of plant material was used to determine the soluble protein content. The plant sample was homogenized first. After that, the sample was extracted with 5 mL of phosphate buffer. The mixture was then centrifuged after grinding to obtain the extract. The Bradford reagent mixture was mixed with 850 mL of distilled water, 100 mL of phosphoric acid, 50 mL of 95% ethanol, and 0.1 g of Coomassie brilliant blue, after which the mixture was filtered, and the protein assay was continued on the plant extract; 2 mL of Bradford reagent was added to the test tube, and color development was allowed at 27 °C for 5 min. PG Instruments Limited was used to read the absorbance of the solution at 595 nm, a method widely applied for the quantification of protein concentration since the intensity of the color change is proportional to the quantity of proteins in the sample.
Determination of Antioxidant Activity
The catalytic activities of catalase and peroxidase were determined via a modified protocol of Chance and Maehly. The catalase (CAT) solution was prepared via the following steps: a cuvette was completely filled with 1.9 mL of 5.9 mM hydrogen peroxide, 0.1 mL of enzyme extract, and 1000 μL of 50 mM potassium phosphate buffer and adjusted to pH 7.0. All of the constituents should be carefully measured so that all of the reactions are accurate. The cuvette was then placed in a UV/visible spectrophotometer. With this instrument, the absorbance is read over time, as hydrogen peroxide is broken down by the action of the enzyme catalase. More precisely, at 30 s intervals for a total of 150 s, absorbance readings were taken at a wavelength of 240 nm. The latter wavelength corresponds to the peak absorbance of hydrogen peroxide. This process affects catalase activity via the rate of decomposition of H2O.
The following reagents were mixed to prepare a peroxidase (POD) reaction mixture of 2 mL: enzyme extract, 0.1 mL; guaiacol solution at 20 mM, 600 μL; potassium phosphate buffer, 50 mM, pH 5.0, 700 μL; guaiacol solution at 20 mM, 600 μL; and hydrogen peroxide solution, 40 mM, 600 μL. All these reagents must be measured and added to the volumes described so that the appropriate reaction conditions can be met. Once the reagents are combined, the resulting solution must be mixed fairly well, so that uniformity is achieved. Following preparation, the absorbance changes of the reaction mixture were monitored at 470 nm via a UV/vis spectrophotometer. Absorbance readings for each sample were taken over a 30 s window so that the enzymatic activity can be detected by the reduction of H2O2 by peroxidase catalysis and the oxidation of guaiacol, with the resulting color change being measurable. This method can then be used to assess the amount of activity of the enzyme peroxidase within a sample.
SOD activity was analyzed based on amount of photoreduction prevented by nitro blue tetrazolium (NBT). First, 50 mM sodium carbonate, 50 mM sodium phosphate buffer (pH 7.6), 100 μL of crude extract, 12 mM l-methionine, 0.1 mM EDTA, 50 mM NBT, and 10 μM riboflavin were added to reach a final volume of 3.0 mL. This mixture was incubated and exposed to white light for 15 min at room temperature. After incubation, the absorbance was measured at 560 nm. The photochemical degradation of NBT was decreased by 50% when one SOD unit (U) was used. ,
Assessment of Total Phenolics
For each of the plant extracts, a sample mixture of 2.25 mL of 10% Folin–Ciocalteu reagent with 300 μL of the sample was prepared. The reaction was allowed to initiate by incubating the mixture at ambient temperature for 5 min. In this mixture, 6% Na2CO3 (2.25 mL) was added and incubated again for 90 min at room temperature. After this, absorbance was read at 725 nm using a UV/vis spectrophotometer. Total phenolics were expressed as units of gallic acid per gram dry weight of the sample (μg GAE/g DW).
Determination of Total Flavonoids
The flavonoid contents were estimated following the methods described by Beketov et al. The plant extract (0.2 mL) was mixed with 90% ethanol (4.5 mL), 2% aluminum chloride (0.2 mL), and 33% acetic acid (0.1 mL). The mixture was incubated in the dark for 30 min, and the absorbance at 414 nm was subsequently measured spectrophotometrically.
Determination of Proline Content
To begin the test, 1 g of fresh foliage was ground in 4 mL of 3% sulfo-salicylic acid and incubated at room temperature for 24 h. Thereafter, the mixture was centrifuged to allow it to settle, so that the solid residues separated the supernatant. Ninhydrin and glacial acetic acid were added to the supernatant to start the reaction. The reaction mixture was placed in a water bath and kept at 100 °C for 1 h to allow the full reaction of the system and color development. After being heated, the reaction mixture was cooled in an ice bath to stop the reaction. The precipitate obtained was then recovered through extraction using toluene to separate the organic phase. Finally, the absorbance of the solution was read at 520 nm via a spectrophotometer.
Determination of Inorganic Ions (K+, Ca2+, and Mg2+)
Dried sample (0.1 g) was mixed in 2 mL of H2SO4 in test tubes and kept for 24 h. This mixture was then heated on a hot plate in a digestion flask. H2O2 was added dropwise in this mixture and heated until we get a colorless solution. This solution was diluted to make a total volume 50 mL using distilled water and then filtered using Whatman’s filter paper. Sherwood 360, flame photometer was used for estimation of inorganic ions in prepared samples.
Statistical Analysis
Two-way analysis of variance (ANOVA) followed by DMRT at a significance level of p ≤ 0.05 was performed on R software (RStudio 2024.12.0 + 467). Data presented in the graphs are the mean of three replicates ± standard errors. Pearson’s correlation and principal component analysis were also performed on R software using p ≤ 0.05.
Acknowledgments
The authors would like to extend their sincere appreciation to the Ongoing Research Funding Program, (ORF-2025-236), King Saud University, Riyadh, Saudi Arabia.
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
M.K., Experimentation and Methodology; A.A.S., Supervision and Validation; S.U., Statistical analysis and writingoriginal draft preparation; W.X., Formal analysis; and A.A.A., writingrevised draft preparation. All authors have read and approved the final manuscript.
Ongoing Research Funding Program, (ORF-2025-236), King Saud University, Riyadh, Saudi Arabia.
The authors declare no competing financial interest.
We declare that the manuscript reporting studies does not involve any human participants, human data, or human tissues. So, it is not applicable. Our experiment follows with the relevant institutional, national, and international guidelines and legislation.
References
- Chandra P., Wunnava A., Verma P., Chandra A., Sharma R. K.. Strategies to Mitigate the Adverse Effect of Drought Stress on Crop Plants-Influences of Soil Bacteria: A Review. Pedosphere. 2021;31(3):496–509. doi: 10.1016/S1002-0160(20)60092-3. [DOI] [Google Scholar]
- Zia R., Nawaz M. S., Siddique M. J., Hakim S., Imran A.. Plant Survival under Drought Stress: Implications, Adaptive Responses, and Integrated Rhizosphere Management Strategy for Stress Mitigation. Microbiol. Res. 2021;242:126626. doi: 10.1016/j.micres.2020.126626. [DOI] [PubMed] [Google Scholar]
- O’Connell E.. Towards Adaptation of Water Resource Systems to Climatic and Socio-Economic Change. Water Resour. Manag. 2017;31(10):2965–2984. doi: 10.1007/s11269-017-1734-2. [DOI] [Google Scholar]
- Seleiman M. F., Al-Suhaibani N., Ali N., Akmal M., Alotaibi M., Refay Y., Dindaroglu T., Abdul-Wajid H. H., Battaglia M. L.. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants. 2021;10(2):259–325. doi: 10.3390/plants10020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold U.. Plant Life in Extreme Environments: How Do You Improve Drought Tolerance? Front. Plant Sci. 2018;9:543. doi: 10.3389/fpls.2018.00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman A., Weng J., Li P., Shah I. H., Rahman S. u., Khalid M., Manzoor M. A., Chang L., Niu Q.. Green Synthesized Zinc Oxide Nanoparticles Confer Drought Tolerance in Melon (Cucumis Melo L.) Environ. Exp. Bot. 2023;212:105384. doi: 10.1016/j.envexpbot.2023.105384. [DOI] [Google Scholar]
- Sulaiman, Ahmad A., Noor Hassim M. F.. Effects of Silica Nanoparticles on Morpho-Histological and Antioxidant Activities of Rice Seedlings under Drought Stress. South Afr. J. Bot. 2024;168:497–508. doi: 10.1016/j.sajb.2024.03.052. [DOI] [Google Scholar]
- Singh D., Thapa S., Singh J. P., Mahawar H., Saxena A. K., Singh S. K., Mahla H. R., Choudhary M., Parihar M., Choudhary K. B., Chakdar H.. Prospecting the Potential of Plant Growth-Promoting Microorganisms for Mitigating Drought Stress in Crop Plants. Curr. Microbiol. 2024;81(3):84. doi: 10.1007/s00284-023-03606-4. [DOI] [PubMed] [Google Scholar]
- Zeeshan M., Wang X., Salam A., Wu H., Li S., Zhu S., Chang J., Chen X., Zhang Z., Zhang P.. Selenium Nanoparticles Boost the Drought Stress Response of Soybean by Enhancing Pigment Accumulation, Oxidative Stress Management and Ultrastructural Integrity. Agronomy. 2024;14(7):1372. doi: 10.3390/agronomy14071372. [DOI] [Google Scholar]
- Khan M. T., Ahmed S., Shah A. A., Noor Shah A., Tanveer M., El-Sheikh M. A., Siddiqui M. H.. Influence of Zinc Oxide Nanoparticles to Regulate the Antioxidants Enzymes, Some Osmolytes and Agronomic Attributes in Coriandrum Sativum l. Grown under Water Stress. Agronomy. 2021;11(10):2004. doi: 10.3390/agronomy11102004. [DOI] [Google Scholar]
- Omar A. A., Heikal Y. M., Zayed E. M., Shamseldin S. A. M., Salama Y. E., Amer K. E., Basuoni M. M., Abd Ellatif S., Mohamed A. H.. Conferring of Drought and Heat Stress Tolerance in Wheat (Triticum Aestivum L.) Genotypes and Their Response to Selenium Nanoparticles Application. Nanomaterials. 2023;13(6):998. doi: 10.3390/nano13060998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylavarapu, R. ; Sikora, F. J. ; Moore, K. P. . Walkley-Black Method. In Soil Test Methods from the Southeastern United States; Southern Cooperative Series Bulletin, 2014; Vol. 158. [Google Scholar]
- Cakmak I., Yazici A. M.. Magnesium: A Forgotten Element in Crop Production. Better Crops Plant Food. 2010;94(2):23–25. [Google Scholar]
- Faiz S., Yasin N. A., Khan W. U., Shah A. A., Akram W., Ahmad A., Ali A., Naveed N. H., Riaz L.. Role of Magnesium Oxide Nanoparticles in the Mitigation of Lead-Induced Stress in Daucus Carota: Modulation in Polyamines and Antioxidant Enzymes. Int. J. Phytoremediation. 2022;24(4):364–372. doi: 10.1080/15226514.2021.1949263. [DOI] [PubMed] [Google Scholar]
- Kandhol N., Jain M., Tripathi D. K.. Nanoparticles as Potential Hallmarks of Drought Stress Tolerance in Plants. Physiol. Plant. 2022;174(2):e13665. doi: 10.1111/ppl.13665. [DOI] [PubMed] [Google Scholar]
- Ahmed T., Noman M., Manzoor N., Shahid M., Hussaini K. M., Rizwan M., Ali S., Maqsood A., Li B.. Green Magnesium Oxide Nanoparticles-Based Modulation of Cellular Oxidative Repair Mechanisms to Reduce Arsenic Uptake and Translocation in Rice (Oryza Sativa L.) Plants. Environ. Pollut. 2021;288:117785. doi: 10.1016/j.envpol.2021.117785. [DOI] [PubMed] [Google Scholar]
- Ahmed S., Kumar A.. Research Progress in Synthesis Strategies of Magnesium Oxide Nanoparticles for Water Treatment Application; Water Research & Technology. Environ. Sci. 2024;10:577. doi: 10.1039/D3EW00528C. [DOI] [Google Scholar]
- Hanif S., Sajjad A., Javed R., Zia M.. The Role of Proline and Betaine Functionalized Zinc Oxide Nanoparticles as Drought Stress Regulators in Coriandrum Sativum: An in Vivo Study. Discover Plants. 2024;1(1):46–17. doi: 10.1007/s44372-024-00046-7. [DOI] [Google Scholar]
- Fatemi H., Esmaiel Pour B., Rizwan M.. Foliar Application of Silicon Nanoparticles Affected the Growth, Vitamin C, Flavonoid, and Antioxidant Enzyme Activities of Coriander (Coriandrum Sativum L.) Plants Grown in Lead (Pb)-Spiked Soil. Environ. Sci. Pollut. Res. 2021;28(2):1417–1425. doi: 10.1007/s11356-020-10549-x. [DOI] [PubMed] [Google Scholar]
- Agrawal, M. ; Singhal, M. ; Jasoria, Y. ; Chaudhary, H. ; Prajapati, B. G. . Pharmacological Aspects of Essential Oils; CRC Press, 2024; pp 227–242. [Google Scholar]
- Laribi B., Kouki K., M’Hamdi M., Bettaieb T.. Coriander (Coriandrum Sativum L.) and Its Bioactive Constituents. Fitoterapia. 2015;103:9–26. doi: 10.1016/j.fitote.2015.03.012. [DOI] [PubMed] [Google Scholar]
- Kadhim, A. J. Effect of Biofertilizers and Animal Manure on Morphophysiological Characteristics and Amount of Coriander (Coriandrum sativum L.) Essential Oil under Drought Stress Conditions; IOP Publishing, 2021; Vol. 735, p 012047. [Google Scholar]
- Abbas Z., Hassan M. A., Huang W., Yu H., Xu M., Chang X., Fang X., Liu L.. Influence of Magnesium Oxide (MgO) Nanoparticles on Maize (Zea Mays L.) Agronomy. 2024;14(3):617. doi: 10.3390/agronomy14030617. [DOI] [Google Scholar]
- Baca Cabrera J. C., Hirl R. T., Schäufele R., Macdonald A., Schnyder H.. Stomatal Conductance Limited the CO2 Response of Grassland in the Last Century. BMC Biol. 2021;19(1):50. doi: 10.1186/s12915-021-00988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan Z., Jan R., Asif S., Farooq M., Jang Y. H., Kim E. G., Kim N., Kim K. M.. Exogenous Melatonin Induces Salt and Drought Stress Tolerance in Rice by Promoting Plant Growth and Defense System. Sci. Rep. 2024;14(1):1214. doi: 10.1038/s41598-024-51369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S., Ulhassan Z., Shahbaz H., Kaleem Z., Yousaf M. A., Ali S., Sheteiwy M. S., Waseem M., Ali S., Zhou W.. Application of Magnesium Oxide Nanoparticles as a Novel Sustainable Approach to Enhance Crop Tolerance to Abiotic and Biotic Stresses. Environ. Sci. Nano. 2024;11(8):3250–3267. doi: 10.1039/D4EN00417E. [DOI] [Google Scholar]
- Mittler R., Zandalinas S. I., Fichman Y., Van Breusegem F.. Reactive Oxygen Species Signalling in Plant Stress Responses. Nat. Rev. Mol. Cell Biol. 2022;23(10):663–679. doi: 10.1038/s41580-022-00499-2. [DOI] [PubMed] [Google Scholar]
- Faizan M., Bhat J. A., El-Serehy H. A., Moustakas M., Ahmad P.. Magnesium Oxide Nanoparticles (MgO-NPs) Alleviate Arsenic Toxicity in Soybean by Modulating Photosynthetic Function, Nutrient Uptake and Antioxidant Potential. Metals. 2022;12(12):2030. doi: 10.3390/met12122030. [DOI] [Google Scholar]
- Faizan M., Bhat J. A., Chen C., Alyemeni M. N., Wijaya L., Ahmad P., Yu F.. Zinc Oxide Nanoparticles (ZnO-NPs) Induce Salt Tolerance by Improving the Antioxidant System and Photosynthetic Machinery in Tomato. Plant Physiol. Biochem. 2021;161:122–130. doi: 10.1016/j.plaphy.2021.02.002. [DOI] [PubMed] [Google Scholar]
- Maghsoudi K., Emam Y., Ashraf M.. Influence of Foliar Application of Silicon on Chlorophyll Fluorescence, Photosynthetic Pigments, and Growth in Water-Stressed Wheat Cultivars Differing in Drought Tolerance. Turk. J. Bot. 2015;39(4):625–634. doi: 10.3906/bot-1407-11. [DOI] [Google Scholar]
- Gao Y., Zhang J., Wang C., Han K., Hu L., Niu T., Yang Y., Chang Y., Xie J.. Exogenous Proline Enhances Systemic Defense against Salt Stress in Celery by Regulating Photosystem, Phenolic Compounds, and Antioxidant System. Plants. 2023;12(4):928. doi: 10.3390/plants12040928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin M. A. A., Abu-Elsaoud A. M., Nowwar A. I., Abdelwahab A. T., Awad M. A., Hassan S. E. D., Boufahja F., Fouda A., Elkelish A.. Green Synthesis of Magnesium Oxide Nanoparticles Using Endophytic Fungal Strain to Improve the Growth, Metabolic Activities, Yield Traits, and Phenolic Compounds Content of Nigella Sativa L. Green Process. Synth. 2024;13(1):20230215. doi: 10.1515/gps-2023-0215. [DOI] [Google Scholar]
- Fernandes M., RB Singh K., Sarkar T., Singh P., Pratap Singh R.. Recent Applications of Magnesium Oxide (MgO) Nanoparticles in Various Domains. Adv. Mater. Lett. 2020;11(8):1–10. doi: 10.5185/amlett.2020.081543. [DOI] [Google Scholar]
- Al-Khayri J. M., Rashmi R., Surya Ulhas R., Sudheer W. N., Banadka A., Nagella P., Aldaej M. I., Rezk A. A.-S., Shehata W. F., Almaghasla M. I.. The Role of Nanoparticles in Response of Plants to Abiotic Stress at Physiological, Biochemical, and Molecular Levels. Plants. 2023;12(2):292. doi: 10.3390/plants12020292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shende S., Rajput V. D., Gade A., Minkina T., Fedorov Y., Sushkova S., Mandzhieva S., Burachevskaya M., Boldyreva V.. Metal-Based Green Synthesized Nanoparticles: Boon for Sustainable Agriculture and Food Security. IEEE Trans. NanoBioscience. 2022;21(1):44–54. doi: 10.1109/TNB.2021.3089773. [DOI] [PubMed] [Google Scholar]
- Sharavdorj K., Jang Y., Byambadorj S.-O., Cho J.-W.. The Effect of MgSO4 and CaSO4 on Seedlings of Forage Crops under Environmental Stress. Plant Physiol. Rep. 2022;27(4):702–716. doi: 10.1007/s40502-022-00691-8. [DOI] [Google Scholar]
- Ojagh S. E., Moaveni P.. Foliar-Applied Magnesium Nanoparticles Modulate Drought Stress through Changes in Physio-Biochemical Attributes and Essential Oil Profile of Yarrow (Achillea Millefolium L.) Environ. Sci. Pollut. Res. 2022;29(39):59374–59384. doi: 10.1007/s11356-022-19559-3. [DOI] [PubMed] [Google Scholar]
- Arnon D. L.. Copper Enzymes in Isolated Chloroplasts. Plant Physiol. 1949;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler, H. K. 34 Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes; Academic Press, 1987; Vol. 148, pp 350–382. [Google Scholar]
- Leitão S. T., Ferreira E., Bicho M. C., Alves M. L., Pintado D., Santos D., Mendes-Moreira P., Araújo S. S., Costa J. M., Vaz Patto M. C.. Maize Open-Pollinated Populations Physiological Improvement: Validating Xtools for Drought Response Participatory Selection. Sustainability. 2019;11(21):6081. doi: 10.3390/su11216081. [DOI] [Google Scholar]
- Rusinowski S., Krzyżak J., Clifton-Brown J., Jensen E., Mos M., Webster R., Sitko K., Pogrzeba M.. New Miscanthus Hybrids Cultivated at a Polish Metal-Contaminated Site Demonstrate High Stomatal Regulation and Reduced Shoot Pb and Cd Concentrations. Environ. Pollut. 2019;252:1377–1387. doi: 10.1016/j.envpol.2019.06.062. [DOI] [PubMed] [Google Scholar]
- Huang Q., Farooq M. A., Hannan F., Chen W., Ayyaz A., Zhang K., Zhou W., Islam F.. Endogenous Nitric Oxide Contributes to Chloride and Sulphate Salinity Tolerance by Modulation of Ion Transporter Expression and Reestablishment of Redox Balance in Brassica Napus Cultivars. Environ. Exp. Bot. 2022;194:104734. doi: 10.1016/j.envexpbot.2021.104734. [DOI] [Google Scholar]
- Ashraf M., Ali Q.. Relative Membrane Permeability and Activities of Some Antioxidant Enzymes as the Key Determinants of Salt Tolerance in Canola (Brassica Napus L.) Environ. Exp. Bot. 2008;63(1–3):266–273. doi: 10.1016/j.envexpbot.2007.11.008. [DOI] [Google Scholar]
- Heath R. L., Packer L.. Photoperoxidation in Isolated Chloroplasts: I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968;125(1):189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Alomrani S. O., Kaleem M., Aslam M., Habib F., Jamal A., Waseem M., Javed T., Wahid A.. Copper Nanoparticles Alleviate Cadmium Stress in Solanum Melongena through Endogenous Melatonin and Regulation of Some Physiochemical Attributes. Sci. Hortic. 2024;323:112546. doi: 10.1016/j.scienta.2023.112546. [DOI] [Google Scholar]
- Gatasheh M. K., Shah A. A., Kaleem M., Usman S., Shaffique S.. Application of CuNPs and AMF Alleviates Arsenic Stress by Encompassing Reduced Arsenic Uptake through Metabolomics and Ionomics Alterations in Elymus Sibiricus. BMC Plant Biol. 2024;24(1):667. doi: 10.1186/s12870-024-05359-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiq H., Shani M. Y., Ashraf M. Y., De Mastro F., Cocozza C., Abbas S., Ali N., Zaib-un-Nisa, Tahir A., Iqbal M., Khan Z., Gul N., Brunetti G.. Copper Oxide Nanoparticles Induced Growth and Physio-Biochemical Changes in Maize (Zea Mays L.) in Saline Soil. Plants. 2024;13(8):1080. doi: 10.3390/plants13081080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S., Ramzan M., Naz G., Ali L., Danish S., Ansari M. J., Salmen S. H.. Effect of Silicon Nanoparticle-Based Biochar on Wheat Growth, Antioxidants and Nutrients Concentration under Salinity Stress. Sci. Rep. 2024;14(1):6380. doi: 10.1038/s41598-024-55924-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance, B. ; Maehly, A. C. . [136] Assay of Catalases and Peroxidases. {black Small Square}. Methods in Enzymology; Elsevier Science & Technology, 1955; Vol. 2,p 764. [Google Scholar]
- Latif M., Ali S., Ahmad Ansari M., Fawad Zahoor A., Nafees M.. Exploring the Potential of Green Synthesized Cerium Oxide Nanoparticles in Mitigating Chromium Toxicity in Maize. J. King Saud Univ. Sci. 2024;36(8):103323. doi: 10.1016/j.jksus.2024.103323. [DOI] [Google Scholar]
- Beauchamp C., Fridovich I.. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Nanehkaran F. M., Razavi S. M., Ghasemian A., Ghorbani A., Zargar M.. Foliar Applied Potassium Nanoparticles (K-NPs) and Potassium Sulfate on Growth, Physiological, and Phytochemical Parameters in Melissa Officinalis L. under Salt Stress. Environ. Sci. Pollut. Res. 2024;31(21):31108–31122. doi: 10.1007/s11356-024-33306-w. [DOI] [PubMed] [Google Scholar]
- Beketov E. V., Pakhomov V. P., Nesterova O. V.. Improved Method of Flavonoid Extraction from Bird Cherry Fruits. Pharm. Chem. J. 2005;39(6):316–318. doi: 10.1007/s11094-005-0143-7. [DOI] [Google Scholar]
- Tahir K., Haroon U., Akbar M., Elahi M., Quraishi U. M.. Tetragonal Crystalline MnO Nanoparticles Alleviate Pb Stress in Wheat by Modulating Antioxidant Enzymes in Leaves. Physiol. Mol. Biol. Plants. 2024;30(8):1401–1411. doi: 10.1007/s12298-024-01488-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.