Abstract
Background
Coronary plaque vulnerability is associated with the fat attenuation index (FAI) of pericoronary adipose tissue (PCAT), but the associations between vulnerability features and multiparametric indices of PCAT remain unclear. In this study, we aimed to explore the effect of four vulnerability features of atherosclerotic coronary plaque on multiparametric indices of PCAT and evaluate the relative responsiveness of these indices in determining the degree of vascular inflammation.
Methods
A retrospective study was conducted on 443 patients clinically diagnosed with coronary artery disease (CAD) at Bozhou People’s Hospital from January 2022 to August 2023. The most severely plaque-burdened diseased blood vessels were selected, and plaque vulnerability features, including positive remodeling (PR), low attenuation plaque (LAP), punctate calcification (PC), and napkin-ring sign (NRS), were further evaluated. In addition, quantitative measurements of the multiparametric indices of PCAT centered around the plaque, with a vertical diameter of 40 mm, including FAI, total pericoronary fat volume (FV), perivascular water attenuation index (PVWI), FAI of non-PCAT (non-FAI), and volumetric pericoronary characterization index (VPCI), were performed. They were divided into left anterior descending (LAD)/left circumflex artery (LCx) and right coronary artery (RCA) groups; the indices of PCAT between the two groups were compared using t-test or Mann-Whitney U test. Univariate and multivariate linear regression analyses were conducted to evaluate the associations between vulnerability features, diseased vessels, and PCAT multiparametric indices.
Results
A total of 291 eligible patients were included in this study. Multivariate linear regression analysis revealed that PR, PC, LAP, and NRS were all positively correlated with FAI and non-FAI; PR and LAP were positively correlated with PVWI and VPCI, and negatively correlated with FV (all P<0.05). RCA was negatively correlated with non-FAI and PVWI, and positively correlated with VPCI and FV (all P<0.05). The values of non-FAI and PVWI in the LAD/LCx group (n=210) were higher than those in the RCA group (n=81), whereas FV and VPCI values were lower in the LAD/LCx group (all P<0.05). Multivariate linear regression analysis showed that in the LAD/LCx group, the four vulnerability features were positively correlated with FAI, PC and LAP were positively correlated with non-FAI and VPCI, and negatively correlated with FV value, and PR was positively correlated with PVWI (all P<0.05). In the RCA group, PR, PC, and LAP were positively correlated with FAI, PR, PC, and NRS were positively correlated with non-FAI, LAP was positively correlated with VPCI and PVWI, and PR and LAP were negatively correlated with FV (all P<0.05).
Conclusions
Among the five PCAT indices, the FAI and non-FAI demonstrated stronger associations with plaque vulnerability features, whereas VPCI, PVWI, and FV showed weaker associations. Vulnerable plaque features (PR, LAP) induced more severe inflammatory responses in PCAT compared to PC and NRS. Additionally, PCAT indices were influenced by the lesion-bearing vessel. These findings may guide clinical prioritization of inflammatory biomarkers and refine assessments of plaque-related inflammation severity.
Keywords: Atherosclerotic coronary plaques, computed tomography angiography (CT angiography), plaque vulnerability features, pericoronary adipose tissue (PCAT)
Introduction
The incidence of coronary artery disease (CAD), especially acute coronary syndrome, is gradually increasing and remains one of the major causes of death in high-income economies (1,2). The presence of vulnerable plaques in the coronary artery may increase the risk of acute coronary syndrome (3,4). According to further research, among the four plaque vulnerability features, namely, positive remodeling (PR), low attenuation plaque (LAP), punctate calcification (PC), and napkin-ring sign (NRS) (5), PR and LAP are associated with the risk of future cardiovascular events (6-8), with LAP load being the strongest predictor. Relevant evidence indicates that patients may develop a fivefold increased risk of myocardial infarction when the LAP load exceeds 4% (9). In recent years, some new technologies have been applied to the diagnosis of CAD, such as radiomics, deep learning, and intravascular optical coherence tomography (10,11). Similarly, a new concept has been proposed, pericoronary adipose tissue (PCAT), which is defined as a circular area with a radial distance from the outer vessel wall equal to the coronary artery diameter (12). Studies have shown that plaque vulnerability is related to PCAT computed tomography (CT) attenuation, also known as the fat attenuation index (FAI). The FAI value related to vulnerable plaques is higher than that associated with nonvulnerable plaques (5,13,14). Furthermore, as a “sensor” of coronary inflammation, PCAT can be used to accurately determine the inflammatory state of blood vessels, with the FAI increase in PCAT suggesting a high degree of inflammation around vulnerable plaques. However, FAI can only reveal the “lipid phase CT attenuation degree” of PCAT. With in-depth research, new indicators of PCAT have been proposed, for example, perivascular water attenuation index (PVWI) to reveal the “aqueous phase CT attenuation degree” of PCAT; total pericoronary fat volume (FV) to reveal the FV of PCAT. In addition, the peripheral area surrounding PCAT constitutes the non-PCAT region, where the FAI measured within the non-PCAT region is defined as non-FAI, and the volumetric pericoronary characterization index (VPCI) is a derivative indicator reflecting the inflammatory attenuation gradient between PCAT and non-PCAT (15,16). Although the concept of these indices has been proposed, many studies have focused on the relationship between vulnerable plaques and FAI (5,13,17), whereas other indices are rarely discussed. Moreover, there has been no systematic study on the relationship between the vulnerability features and other indices of PCAT. Therefore, the associations between the four plaque vulnerability features and PCAT and the relative responsiveness of PCAT indices to inflammation need to be further demonstrated. This study aimed to explore the associations between coronary plaque vulnerability features and PCAT multiparametric indices and evaluate the association strength of PCAT indices and the degree of vascular inflammation. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1002/rc).
Methods
General data
This work was designed as a retrospective cross-sectional study. The included cases were all inpatients with clinically diagnosed CAD in the Department of Cardiovascular Medicine of Bozhou People’s Hospital from January 2022 to August 2023. A total of 443 patients underwent coronary CT angiography (CCTA) within 7 days before and after admission, and the presence of coronary plaques was confirmed. Patients who met the following criteria were excluded: (I) previous medical or surgical treatment for CAD; (II) other heart diseases (e.g., congenital heart disease, dilated cardiomyopathy, etc.); (III) poor image quality of CCTA that would have restricted accurate evaluation of plaque features. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Medical Ethics Committee of Bozhou People’s Hospital (ethical approval No. 2024-105) and the requirement for individual consent for this analysis was waived due to the retrospective nature.
Instruments and methods
A GE Revolution CT scanner (GE Healthcare, Chicago, IL, USA) was used in CCTA, within the scanning range from the region below the carina of the trachea to 2 cm below the diaphragm. A total of 60–80 mL of the contrast agent iohexol (350 mg I/mL) was injected through the right elbow vein at a flow rate of 5 mL/s, followed by 40 mL physiological saline. Threshold tracking trigger scanning was performed, with the ascending aorta as the monitoring point, a trigger threshold of 120 Hounsfield units (HUs), using retrospective electrocardiogram-gating, tube voltage of 100 kV, tube current of Smart mA (300–545 mA), layer thickness of 0.625 mm, and layer spacing of 0.625 mm, Reconstruction type was set as standard algorithm. After scanning, the images were uploaded to the GE AW4.71 workstation to obtain the best frozen images, and subsequently transferred to the Deepwise vascular CT imaging diagnosis system [Artificial Intelligence (AI)-Assisted Diagnosis System, version 3.0.0.1, Deepwise, Hangzhou, China] for AI postprocessing. The diagnostic system has been certified by the US Food and Drug Administration.
Evaluation of plaque vulnerability features
The postprocessed images were used as a basis for the evaluation of atherosclerotic coronary plaque vulnerability features. All image evaluations were performed by radiologist with over 10 years of working experience. Plaque vulnerability features included the following: (I) PR (remodeling index >1.05); (II) PC (maximum diameter of calcified plaques ≤3 mm); (III) LAP (CT value <30 HU in low-density areas within plaques); and (IV) NRS: low-density plaques surrounded by high-density rings (9).
Measurement of PCAT and non-PCAT parameter indices
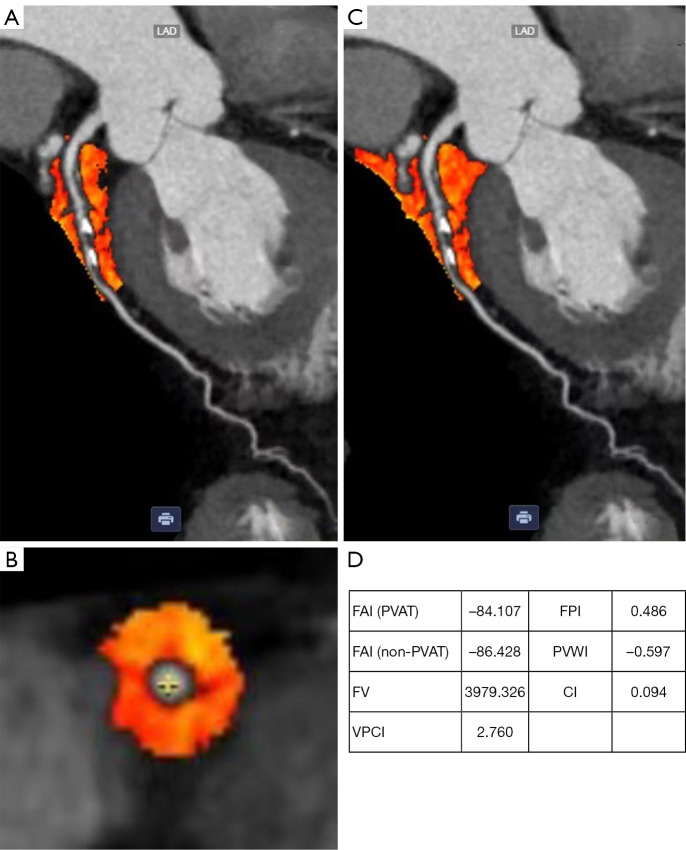
Quantitative measurement of the PCAT and non-PCAT regions centered around the most severely plaque-burdened, with a vertical diameter of 40 mm, were performed using the Deepwise AI-Assisted Diagnosis System, and all measurement indices are automatically generated by Deepwise AI. The PCAT region was defined as a circular area with a radial distance from the outer vessel wall equal to the coronary artery diameter (Figure 1A,1B) (12); the non-PCAT region was a circular area extending 20 mm outward from the centerline of a blood vessel (excluding the PCAT region) (Figure 1C). The FAI, FV, and PVWI of the PCAT region and non-FAI of the non-PCAT region were automatically generated by the Deepwise AI-Assisted Diagnosis System. Specifically, the CT attenuation range for the measurement of FAI, FV, and non-FAI was set to −190 to −30 HU and that for PVWI to −15 to –15 HU. In addition, VPCI was calculated using the formula VPCI = [100 × (FAI − non-FAI)/|FAI|] (15) (Figure 1D).
Figure 1.
PCAT, non-PCAT, and example diagrams of multiparametric indices. (A,B) PCAT region (labeled in orange); (C) PCAT and non-PCAT regions (labeled in orange); (D) example diagrams of multiparametric indices of PCAT and non-PCAT. CI, calcified index; FAI, fat attenuation index; FPI, fiber plaque index; FV, fat volume; PCAT, pericoronary adipose tissue; PVAT, perivascular adipose tissue; PVWI, perivascular water attenuation index; VPCI, volumetric pericoronary characterization index.
Statistical analysis
The software SPSS 23.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis in this study. The measurement data were presented by mean ± standard deviation () or median and interquartile range. The normality of quantitative data was tested using the Kolmogorov-Smirnov test. According to the left and right coronary arteries, the cases were divided into two groups: the left anterior descending (LAD)/left circumflex artery (LCx) group and the right coronary artery (RCA) group. For comparison between LAD/LCx and RCA groups, t-test was used for normally distributed data, and Mann-Whitney U test was used for non-normally distributed data. Plaque vulnerability features and RCA were used as independent variables, and PCAT parameters were used as dependent variables. Univariate linear regression analysis was used to observe the relationship between the two. The independent variables with P<0.05 in univariate analysis were included in the multivariate linear regression model to further observe the relationship between the variables. A two-tailed P value of <0.05 was considered statistically significant.
Results
Of the 443 patients that were initially identified, 291 patients were included in this study. The most severely plaque-burdened vessels from the enrolled 291 patients, including 193 LAD, 17 LCx, and 81 RCA, were selected for this study. Figure 2 presents the workflow chart. Table 1 shows the demographic data and plaque vulnerability features of 291 patients in this study.
Figure 2.
Study workflow with inclusion and exclusion criteria. CAD, coronary artery disease; CCTA, coronary computed tomography angiography; LAP, low attenuation plaque; NRS, napkin-ring sign; PC, punctate calcification; PR, positive remodeling.
Table 1. Summary of patient demographic data and plaque vulnerability features.
| Patient demographics | Diseased blood vessels (n=291) |
|---|---|
| Male | 152 (52.2) |
| Age (years) | 65 [54, 72] |
| Hypertension | 187 (64.3) |
| Current smoker | 69 (23.7) |
| Diabetes | 70 (24.1) |
| BMI (kg/m2) | 25.05±3.76 |
| Family history of cardiovascular disease | 22 (7.6) |
| Multivessel disease | 50 (17.2) |
| PR | 145 (49.8) |
| PC | 145 (49.8) |
| LAP | 142 (48.8) |
| NRS | 88 (30.2) |
Data were presented as n (%), median [interquartile range], or mean ± standard deviation. BMI, body mass index; LAP, low attenuation plaque; NRS, napkin-ring sign; PR, positive remodeling; PC, punctate calcification.
Associations of plaque vulnerability features and diseased blood vessels with PCAT multiparametric indices
The diseased blood vessels were divided into two categories: the LAD/LCx and the RCA. Then, univariate linear regression analysis was performed, with the four plaque vulnerability features and the classification of diseased blood vessels as independent variables and PCAT multiparametric indices as dependent variables. The results showed that RCA had a positive effect on FV and VPCI, and negative effects on non-FAI and PVWI (all P<0.05), but no effect on FAI (P=0.999). The four vulnerability features had positive effects on FAI, non-FAI, and VPCI; PR, LAP, and NRS had negative effects on FV and a positive effect on PVWI (all P<0.05), but PC showed no significant association with either FV (P=0.458) or PVWI (P=0.890) (Figure 3).
Figure 3.

Comparison of associations between RCA, PR, PC, LAP, NRS, and PCAT parameters (FAI, FV, non-FAI, PVWI, VPCI). RCA has no effect on FAI; PC has no effect on FV and PVWI; other features have effects on PCAT parameters. FAI, fat attenuation index; FV, fat volume; LAP, low attenuation plaque; NRS, napkin-ring sign; PC, punctate calcification; PCAT, pericoronary adipose tissue; PR, positive remodeling; PVWI, perivascular water attenuation index; RCA, right coronary artery; VPCI, volumetric pericoronary characterization index.
Independent variables with P<0.05 in univariate analysis were included in multivariate linear regression analysis. It was found that RCA in diseased blood vessels had a negative effect on non-FAI and PVWI but a positive effect on VPCI and FV, with statistically significant differences (all P<0.05). Meanwhile, PR, PC, LAP, and NRS all exhibited a positive effect on FAI and non-FAI (all P<0.05), whereas PR and LAP positively affected VPCI and PVWI and negatively affected FV, with statistically significant differences (all P<0.05). PC and NRS had no significant influence on VPCI, PVWI and FV, and the differences were not statistically significant (all P>0.05) (Table 2).
Table 2. Multivariate linear regression analysis results on associations of plaque vulnerability features and diseased blood vessels with PCAT multiparametric indices.
| Dependent variable | Independent variable | Constant (95% CI) | β value | R2 value | t value | P value |
|---|---|---|---|---|---|---|
| FAI | PR | 2.852 (1.605 to 4.098) | 0.214 | 0.453 | 4.503 | <0.001 |
| PC | 2.054 (0.891 to 3.217) | 0.154 | 3.477 | 0.001 | ||
| LAP | 5.228 (3.666 to 6.791) | 0.393 | 6.585 | <0.001 | ||
| NRS | 2.572 (0.926 to 4.218) | 0.178 | 3.076 | 0.002 | ||
| FV | PR | −431.202 (−606.368 to −256.035) | −0.256 | 0.330 | −4.845 | <0.001 |
| LAP | −500.745 (−715.476 to −286.014) | −0.297 | −4.590 | <0.001 | ||
| NRS | −135.185 (−364.042 to 93.673) | −0.074 | −1.163 | 0.246 | ||
| RCA | 559.728 (380.699 to 738.757) | 0.298 | 6.154 | <0.001 | ||
| Non-FAI | PR | 1.740 (0.396 to 3.083) | 0.138 | 0.302 | 2.549 | 0.011 |
| PC | 2.418 (1.144 to 3.692) | 0.191 | 3.737 | <0.001 | ||
| LAP | 2.496 (0.817 to 4.175) | 0.197 | 2.926 | 0.004 | ||
| NRS | 2.459 (0.690 to 4.227) | 0.179 | 2.737 | 0.007 | ||
| RCA | −5.123 (−6.524 to −3.722) | −0.363 | −7.197 | <0.001 | ||
| PVWI | PR | 0.151 (0.045 to 0.258) | 0.158 | 0.227 | 2.789 | 0.006 |
| LAP | 0.159 (0.028 to 0.290) | 0.167 | 2.397 | 0.017 | ||
| NRS | 0.046 (−0.093 to 0.186) | 0.044 | 0.653 | 0.515 | ||
| RCA | −0.419 (−0.528 to −0.310) | −0.394 | −7.566 | <0.001 | ||
| VPCI | PR | 1.557 (0.404 to 2.711) | 0.140 | 0.332 | 2.657 | 0.008 |
| PC | 0.019 (−1.075 to 1.113) | 0.002 | 0.034 | 0.973 | ||
| LAP | 3.411 (1.969 to 4.852) | 0.307 | 4.657 | <0.001 | ||
| NRS | 0.495 (−1.023 to 2.013) | 0.041 | 0.642 | 0.522 | ||
| RCA | 4.905 (3.702 to 6.108) | 0.396 | 8.026 | <0.001 |
CI, confidence interval; FAI, fat attenuation index; FV, fat volume; LAP, low attenuation plaque; NRS, napkin-ring sign; PC, punctate calcification; PCAT, pericoronary adipose tissue; PR, positive remodeling; PVWI, perivascular water attenuation index; RCA, right coronary artery; VPCI, volumetric pericoronary characterization index.
Comparison of PCAT multiparametric indices between the LAD/LCx and RCA groups
In view of the aforementioned associations of diseased blood vessels with non-FAI, FV, PVWI, and VPCI, further comparison was conducted on the differences in PCAT and non-PCAT indices between LAD/LCx and RCA. No statistically significant difference was observed in the FAI between the two groups (t=−0.001, P=0.999). The LAD/LCx group had higher non-FAI and PVWI but lower FV and VPCI than the RCA group, with statistically significant differences (all P<0.05) (Table 3 and Figure 4).
Table 3. Comparison of PCAT multiparametric indices between the LAD/LCx and RCA groups.
| Group | FAI (HU) | FV (mm3) | Non-FAI (HU) | VPCI | PVWI (HU) |
|---|---|---|---|---|---|
| LAD/LCx | −84.34±6.29 (−85.19 to −83.48) |
3,375.95±777.55 (3,270.17 to 3,481.73) |
−88.07 (−91.65 to −84.19) |
3.96 (1.62 to 6.46) |
−0.58 (−0.82 to −0.25) |
| RCA | −84.34±7.59 (−86.02 to −82.66) |
3,872.40±980.97 (3,708.74 to 4,102.20) |
−91.96±6.44 (−93.38 to −90.53) |
9.38±6.74 (7.89 to 10.87) |
−0.98 (−1.29 to −0.65) |
| t/U | −0.001 | −4.525 | 5,201.00 | 4,521.00 | 4,374.00 |
| P value | 0.999 | <0.001 | <0.001 | <0.001 | <0.001 |
Data were presented as mean ± standard deviation (95% CI) or median (interquartile range). CI, confidence interval; FAI, fat attenuation index; FV, fat volume; HU, Hounsfield unit; LAD, left anterior descending; LCx, left circumflex; PCAT, pericoronary adipose tissue; PVWI, perivascular water attenuation index; RCA, right coronary artery; VPCI, volumetric pericoronary characterization index.
Figure 4.
Comparison of PCAT multiparametric indices between the LAD/LCx and RCA groups. The LAD/LCx group achieved higher non-FAI and PVWI values and lower FV and VPCI values than the RCA group. FAI, fat attenuation index; FV, fat volume; HU, Hounsfield unit; LAD, left anterior descending; LCx, left circumflex; PCAT, pericoronary adipose tissue; PVWI, perivascular water attenuation index; RCA, right coronary artery; VPCI, volumetric pericoronary characterization index.
Differences in the effect of plaque vulnerability features of LCA (LAD/LCx) and RCA on PCAT multiparametric indices
In both the LAD/LCx and RCA groups, univariate linear regression analysis showed that PC had no effect on FV, PVWI, and VPCI (all P>0.05). The other vulnerability features had positive effects on FAI, non-FAI, PVWI, and VPCI, and a negative effect on FV (all P<0.05) (Figures 5,6).
Figure 5.

The association between the vulnerability features and the parameters of PCAT of the LAD/LCx group. PC has no effect on FV, PVWI, and VPCI; the other features had effects on PCAT parameters. FAI, fat attenuation index; FV, fat volume; LAD, left anterior descending; LAP, low attenuation plaque; LCx, left circumflex; NRS, napkin-ring sign; PC, punctate calcification; PCAT, pericoronary adipose tissue; PR, positive remodeling; PVWI, perivascular water attenuation index; VPCI, volumetric pericoronary characterization index.
Figure 6.

The association between the vulnerability features and the parameters of PCAT of the RCA group. PC has no effect on FV, PVWI, and VPCI; the other features had effects on PCAT parameters. FAI, fat attenuation index; FV, fat volume; LAP, low attenuation plaque; NRS, napkin-ring sign; PC, punctate calcification; PCAT, pericoronary adipose tissue; PR, positive remodeling; PVWI, perivascular water attenuation index; RCA, right coronary artery; VPCI, volumetric pericoronary characterization index.
Independent variables with P<0.05 in univariate analysis were included in multivariate linear regression analysis. It was found that in the LAD/LCx group, all four plaque vulnerability features had positive effects on FAI (all P<0.05). PC and LAP had positive effects on non-FAI (all P<0.05). PR and LAP had positive effects on VPCI and negative effects on FV; PR had a positive effect on PVWI, with statistically significant differences (all P<0.05) (Table S1).
Meanwhile, in the RCA group, PR, PC, and LAP had positive effects on FAI; PR, PC, and NRS exhibited the same effects on non-FAI and LAP on VPCI; PVWI, PR, and LAP had negative effects on FV, with statistically significant differences (all P<0.05) (Table S2).
The effects of plaque vulnerability features on PCAT multiparametric indices can be summarized from another perspective as follows. In the LAD/LCx + RCA group, FAI and non-FAI were affected by all four vulnerability features, and VPCI, PVWI, and FV were affected only by PR and LAP (all P<0.05). In the LAD/LCx group, FAI was affected by all four vulnerability features; non-FAI was affected by PC and LAP; VPCI and FV were influenced by PR and LAP; PVWI was affected only by PR (all P<0.05). In addition, in the RCA group, FAI was affected by PR, PC, and LAP, non-FAI by PC, LAP, and NRS, and FV by PR and LAP, whereas VPCI and PVWI were affected only by LAP (all P<0.05) (Table 4).
Table 4. Summary of the effects of plaque vulnerability features on PCAT multiparametric indices in the three groups classified by blood vessels.
| Vulnerability features | Indices of PCAT | Group | The change mechanism of PCAT multiparametric indices | ||
|---|---|---|---|---|---|
| LAD/LCx + RCA | LAD/LCx | RCA | |||
| PR | FAI | ↑ | ↑ | ↑ | FAI ↑: PCAT inflammation → fat cell edema, adipose tissue fibrosis → “fat phase” CT value ↑; PVWI ↑: PCAT inflammation → fat cell edema, adipose tissue fibrosis → “aqueous phase” CT value ↑; FV ↓: PCAT inflammation → inhibition of adipogenesis → fat volume ↓; non-FAI ↑: Inflammation involving non-PCAT→ “fat phase” CT value in non-PCAT ↑; VPCI ↑: inflammatory gradient between PCAT and non-PCAT ↑ |
| PVWI | ↑ | ↑ | – | ||
| FV | ↓ | ↓ | ↓ | ||
| Non-FAI | ↑ | – | ↑ | ||
| VPCI | ↑ | ↑ | – | ||
| PC | FAI | ↑ | ↑ | ↑ | |
| Non-FAI | ↑ | ↑ | ↑ | ||
| LAP | FAI | ↑ | ↑ | ↑ | |
| PVWI | ↑ | – | ↑ | ||
| FV | ↓ | ↓ | ↓ | ||
| Non-FAI | ↑ | ↑ | – | ||
| VPCI | ↑ | ↑ | ↑ | ||
| NRS | FAI | ↑ | ↑ | – | |
| Non-FAI | ↑ | – | ↑ | ||
“↑” indicates an increase in value; “↓” indicates a decrease in value; and “–” indicates no significant change in value; “→” means “lead to”. FAI, fat attenuation index; FV, fat volume; LAD, left anterior descending; LAP, low attenuation plaque; LCx, left circumflex; NRS, napkin-ring sign; PC, punctate calcification; PCAT, pericoronary adipose tissue; PR, positive remodeling; PVWI, perivascular water attenuation index; RCA, right coronary artery; VPCI, volumetric pericoronary characterization index.
Discussion
In this study, among the five PCAT indices, the FAI and non-FAI demonstrated stronger associations with plaque vulnerability features, whereas VPCI, PVWI, and FV showed weaker associations. Vulnerability characteristics PR and LAP affected the five PCAT indices, whereas PC and NRS only affected FAI and non-FAI indices. In addition, the diseased vessels (LAD/LCx and RCA) were also the influencing factor of PCAT indices.
Clinical significance of PCAT and related multiparametric indices
PCAT lies adjacent to the walls of coronary arteries, two of which show a close anatomical relationship. PCAT adipocytes are embedded in the vascular wall to varying degrees and have been reported to be a part of vascular wall (18). PCAT comprises lipid-containing cells (adipocytes), stromal cells (preadipocytes, inflammatory cells, fibroblasts, etc.), and interstitial tissue (16). During inflammation of the vascular wall, inflammatory factors may spread to the PCAT, which leads to certain changes, namely, (I) inhibited adipogenesis and reduced adipocyte volume and lipid content; (II) increased extracellular fluid and tissue edema; and (III) fibrosis of adipose tissue owing to the progression of inflammation (16,19); all of these changes may increase FAI, namely, a transition from the “lipid phase” to the “aqueous phase”.
FAI indicates the CT value of the PCAT region within the range of −190 to −30 HU, also known as the range of fat density, which was thus defined as the “lipid phase CT value” in the present study. Meanwhile, PVWI serves as an indicator of the CT value in the PCAT region within the range of −15 to –15 HU, similar to the CT value range of water, which was thus defined as the “aqueous phase CT value” in this study. Furthermore, FV serves as an index of the total FV within the PCAT range. In the theoretical context above, aggravated inflammation of the vascular wall can cause an increase in FAI, which has been extensively investigated and confirmed by various studies (20,21). Similarly, PVWI may theoretically increase with the augmentation of inflammation reaction, and conversely, FV may decrease due to the inhibition of adipogenesis. However, to the best of our knowledge, no prior study has investigated this relationship. Moreover, FAI only indicates the CT values within but not those outside the PCAT range. Critically, the inflammatory factors released from the vascular wall may affect other regions beyond the PCAT region. Antonopoulos et al. (15) proposed the concept of non-PCAT to gain insights into the peripheral inflammation of PCAT. Non-PCAT refers to the outermost layer of PCAT, and its CT value is non-FAI. When non-PCAT regions are affected by inflammation, their corresponding non-FAI value will exhibit elevation. Since inflammatory activity diminishes gradually outward from the vascular wall, the PCAT region theoretically demonstrates higher inflammatory severity compared to adjacent non-PCAT areas. VPCI, an inflammation gradient index, is based on the differences in the CT values (FAI values) between PCAT and non-PCAT regions and is generally calculated using the formula VPCI = [100 × (FAI − non-FAI)/|FAI|]. Accordingly, a larger VPCI value will be observed with the increase in the differences between FAI and non-FAI, reflecting a greater inflammatory gradient between these regions.
Influence of atherosclerotic coronary plaque vulnerability features on PCAT multiparametric indices and assessment of association strength between parameters and inflammatory severity
A significant association exists between the presence of vulnerable plaques and elevated FAI (5,13,21-23); in addition, vulnerable plaques may induce a high risk of non-obstructive acute myocardial infarction and Tako-Tsubo syndrome (24). Moreover, the risk of cardiovascular events will significantly increase when vulnerable plaques and elevated FAI occur simultaneously (25,26). LAP and NRS are considered alternative indicators of necrotic cores and thin-cap atherosclerotic plaque in CCTA imaging (27-29). LAP and NRS are frequently detected in CAD patients with elevated high-sensitivity C-reactive protein levels (17); LAP is also an independent predictor of FAI (27). Collectively, plaque vulnerability features are related to FAI values.
In this study, in the LAD/LCx + RCA group, in addition to the aforementioned vulnerable features LAP and NRS being associated with FAI, PR and PC demonstrated associations with FAI. Furthermore, the vulnerable features PR and LAP are linked to elevated non-FAI, VPCI, PVWI, and reduced FV, whereas PC and NRS are associated with increased non-FAI but showed no evident influence on VPCI, PVWI, and FV. These findings imply that PR and LAP have greater effects on PCAT, whereas PC and NRS have a relatively smaller influence on PCAT. Alternatively, among PCAT multiparametric indices, FAI and non-FAI were affected by all four plaque vulnerability features. However, VPCI, PVWI, and FV were only affected by PR and LAP. Thus, among the five indicators of PCAT, FAI, non-FAI, and PCAT demonstrated stronger associations with inflammatory severity, whereas VPCI, PVWI, and FV exhibited relatively weaker associations.
Relative responsiveness differences in PCAT multiparametric indices between LCA and RCA
Xu et al. (30) have shown that in patients without CAD, clinical risk factors [smoking, hyperlipidemia, body mass index (BMI), etc.] have different effects on the PCAT of LCA and RCA, indicating that there are also differences in PCAT between LCA and RCA. In our study, comparison of PCAT multiparametric indices between LCA and RCA, except for the nonsignificant difference in FAI between groups, showed that the LAD/LCx group had higher non-FAI and PVWI but lower FV and VPCI than the RCA group. This finding may be explained by the thicker myocardial tissue of the left anterior wall, which caused LAD/LCx to be closer to the myocardium and myocardial bridge (31,32). Consequently, less adipose tissue was observed around LAD/LCx, but the opposite result was observed in RCA, which resulted in a significantly higher FV value of the RCA group than the LAD/LCx group. With respect to the above findings, compared with the RCA group, the LAD/LCx group had a smaller non-PCAT range, which was close to the PCAT region. Given the gradual attenuation of inflammation from the inner region outward, the non-FAI measured in the LAD/LCx group was closer to the FAI, with the value of non-FAI being higher. By contrast, fat distribution around the RCA was more abundant, which supports the more authentic measurement of non-FAI in the RCA group, along with a lower non-FAI value. Meanwhile, VPCI is mainly related to the difference between FAI and non-FAI. In our study, the difference between FAI and non-FAI in the RCA group was greater than that in the LAD/LCx group, which also reveals a higher VPCI value in the former group than in the latter group. Notably, in this study, the PVWI of the LAD/LCx group was higher than that of the RCA group, and no significant difference was observed in the FAI values between the two groups. These results, to some extent, may imply the higher sensitivity of PVWI than FAI in determining the inflammation of PCAT. However, such a finding contradicts the previous understanding and requires further verification.
In the LAD/LCx group, PR was associated with the increase in FAI, VPCI, and PVWI and the decrease in FV. LAP was associated with the increase in FAI, non-FAI, and VPCI and the decrease in FV. PC was associated with the increase FAI and non-FAI; NRS was only associated with the increase in FAI. Meanwhile, FAI was affected by all four plaque vulnerability features; non-FAI, VPCI, and FV were affected by two features; PVWI was only affected by PR. These findings suggest that PR and LAP have the greatest influence on PCAT, followed by PC, and NRS shows the smallest influence. Moreover, among indicators of the degree of inflammation in PCAT, FAI exhibited the highest relative responsiveness, followed by non-FAI, VPCI, and FV, whereas PVWI had the lowest relative responsiveness.
Simultaneously, in the RCA group, LAP was associated with the increase in FAI, VPCI, and PVWI and decrease in FV; PR and PC were associated with the increase in FAI and non-FAI, and NRS was only associated to the increase non-FAI. From another perspective, FAI and non-FAI were affected by three plaque vulnerability features, FV were affected by PR and LAP, whereas VPCI and PVWI were only affected by LAP. Thus, LAP had the greatest effect on PCAT, followed by PR and PC, and NRS had the smallest influence. In addition, FAI and non-FAI displayed more relative responsiveness in detecting the degree of inflammation in PCAT, followed by FV, whereas VPCI and PVWI exhibited lower relative responsiveness.
The intergroup comparison between the LAD/LCx and RCAs groups revealed that PR and LAP had a greater impact on PCAT, whereas PC and NRS had a comparatively smaller impact. For this reason, PR and LAP may be early or progressive manifestations of plaques, during which inflammation increases in severity within and around the vascular wall (33). Moreover, the RCA group exhibited a more significant effect of LAP on PCAT than the PR, which was consistent with the findings of Williams et al. and Tzolos et al. (9,34), who mentioned that LAP load is the strongest predictor of a future cardiovascular event. It should be noted that the associations between PR, LAP, NRS, and PCAT indices (primarily VPCI, PVWI, and non-FAI) differed between the LAD/LCx and RCA groups. For instance, in the LAD/LCx group, PR demonstrated associations with PVWI but not with non-FAI, whereas LAP showed associations with non-FAI but not with PVWI. Similar contradictory patterns were observed in the RCA group, revealing multiple paradoxical relationships. These inconsistencies are difficult to reconcile through pathophysiological mechanisms alone and may reflect the inherent instability of the aforementioned parameters, which necessitates validation in larger cohorts.
Limitations
The limitations of this study should be considered. First, the relatively small sample size and lack of validation in independent external cohorts. Second, differences in the measurement results of PCAT parameters (FAI, FV, etc.) in various AI-assisted platforms, which may have led to possible selection bias. Third, adoption of fixed tube voltage (100 kV), which requires possible modification of PCAT multiparametric indices when using other scanning parameters given the influence of tube voltage on adipose tissue attenuation. Fourth, absence of histopathological or biochemical validation for inflammation. Finally, the cross-sectional design precluded assessment of prospective patient outcomes (e.g., major adverse cardiovascular events).
Conclusions
Among the five PCAT indices, the FAI and non-FAI demonstrated stronger associations with plaque vulnerability features, whereas VPCI, PVWI, and FV showed weaker associations. Vulnerable plaque features (PR, LAP) induced more severe inflammatory responses in PCAT compared to PC and NRS. Additionally, PCAT indices were influenced by the lesion-bearing vessel. These findings may guide clinical prioritization of inflammatory biomarkers and refine assessments of plaque-related inflammation severity.
Supplementary
The article’s supplementary files as
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Medical Ethics Committee of Bozhou People’s Hospital (ethical approval No. 2024-105) and the requirement for individual consent for this analysis was waived due to the retrospective nature.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1002/rc
Funding: This work was supported by the Health Research Project of Bozhou City (No. bzwjw2023c016).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1002/coif). The authors have no conflicts of interest to declare.
Data Sharing Statement
Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1002/dss
References
- 1.World Health Organization. Disease burden and mortality estimates. WHO: Geneva, Switzerland; 2018. [Google Scholar]
- 2.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J Am Coll Cardiol 2020;76:2982-3021. 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuvaraj J, Lin A, Nerlekar N, Munnur RK, Cameron JD, Dey D, Nicholls SJ, Wong DTL. Pericoronary Adipose Tissue Attenuation Is Associated with High-Risk Plaque and Subsequent Acute Coronary Syndrome in Patients with Stable Coronary Artery Disease. Cells 2021;10:1143. 10.3390/cells10051143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferencik M, Mayrhofer T, Bittner DO, Emami H, Puchner SB, Lu MT, Meyersohn NM, Ivanov AV, Adami EC, Patel MR, Mark DB, Udelson JE, Lee KL, Douglas PS, Hoffmann U. Use of High-Risk Coronary Atherosclerotic Plaque Detection for Risk Stratification of Patients With Stable Chest Pain: A Secondary Analysis of the PROMISE Randomized Clinical Trial. JAMA Cardiol 2018;3:144-52. 10.1001/jamacardio.2017.4973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun JT, Sheng XC, Feng Q, Yin Y, Li Z, Ding S, Pu J. Pericoronary Fat Attenuation Index Is Associated With Vulnerable Plaque Components and Local Immune-Inflammatory Activation in Patients With Non-ST Elevation Acute Coronary Syndrome. J Am Heart Assoc 2022;11:e022879. 10.1161/JAHA.121.022879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motoyama S, Ito H, Sarai M, Kondo T, Kawai H, Nagahara Y, Harigaya H, Kan S, Anno H, Takahashi H, Naruse H, Ishii J, Hecht H, Shaw LJ, Ozaki Y, Narula J. Plaque Characterization by Coronary Computed Tomography Angiography and the Likelihood of Acute Coronary Events in Mid-Term Follow-Up. J Am Coll Cardiol 2015;66:337-46. 10.1016/j.jacc.2015.05.069 [DOI] [PubMed] [Google Scholar]
- 7.Andreini D, Magnoni M, Conte E, Masson S, Mushtaq S, Berti S, Canestrari M, Casolo G, Gabrielli D, Latini R, Marraccini P, Moccetti T, Modena MG, Pontone G, Gorini M, Maggioni AP, Maseri A; . Coronary Plaque Features on CTA Can Identify Patients at Increased Risk of Cardiovascular Events. JACC Cardiovasc Imaging 2020;13:1704-17. 10.1016/j.jcmg.2019.06.019 [DOI] [PubMed] [Google Scholar]
- 8.Lu ZF, Yin WH, Schoepf UJ, Abrol S, Ma JW, Yu XB, Zhao L, Su XM, Wang CS, An YQ, Xiao ZC, Lu B. Residual Risk in Non-ST-Segment Elevation Acute Coronary Syndrome: Quantitative Plaque Analysis at Coronary CT Angiography. Radiology 2023;308:e230124. 10.1148/radiol.230124 [DOI] [PubMed] [Google Scholar]
- 9.Williams MC, Kwiecinski J, Doris M, McElhinney P, D'Souza MS, Cadet S, et al. Low-Attenuation Noncalcified Plaque on Coronary Computed Tomography Angiography Predicts Myocardial Infarction: Results From the Multicenter SCOT-HEART Trial (Scottish Computed Tomography of the HEART). Circulation 2020;141:1452-62. 10.1161/CIRCULATIONAHA.119.044720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang J, Zhou K, Chu MP, Wang Y, Yang G, Li H, Chen W, Yin K, Xue Q, Zheng C, Gu R, Li Q, Chen X, Sheng Z, Chu B, Mu D, Yu H, Zhang B. Automated detection and classification of coronary atherosclerotic plaques on coronary CT angiography using deep learning algorithm. Quant Imaging Med Surg 2024;14:3837-50. 10.21037/qims-23-1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, Nan N, Tong X, Chen H, Zhang X, Li S, Zhang M, Gao B, Wang X, Song X, Chen D. Validation of biomechanical assessment of coronary plaque vulnerability based on intravascular optical coherence tomography and digital subtraction angiography. Quant Imaging Med Surg 2024;14:1477-92. 10.21037/qims-23-1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oikonomou EK, Marwan M, Desai MY, Mancio J, Alashi A, Hutt Centeno E, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet 2018;392:929-39. 10.1016/S0140-6736(18)31114-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuki H, Sugiyama T, Suzuki K, Kinoshita D, Niida T, Nakajima A, Araki M, Dey D, Lee H, McNulty I, Nakamura S, Kakuta T, Jang IK. Coronary Inflammation and Plaque Vulnerability: A Coronary Computed Tomography and Optical Coherence Tomography Study. Circ Cardiovasc Imaging 2023;16:e014959. 10.1161/CIRCIMAGING.122.014959 [DOI] [PubMed] [Google Scholar]
- 14.Zhang R, Ju Z, Li Y, Gao Y, Gu H, Wang X. Pericoronary fat attenuation index is associated with plaque parameters and stenosis severity in patients with acute coronary syndrome: a cross-sectional study. J Thorac Dis 2022;14:4865-76. 10.21037/jtd-22-1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antonopoulos AS, Sanna F, Sabharwal N, Thomas S, Oikonomou EK, Herdman L, et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med 2017;9:eaal2658. 10.1126/scitranslmed.aal2658 [DOI] [PubMed] [Google Scholar]
- 16.Antoniades C, Kotanidis CP, Berman DS. State-of-the-art review article. Atherosclerosis affecting fat: What can we learn by imaging perivascular adipose tissue? J Cardiovasc Comput Tomogr 2019;13:288-96. 10.1016/j.jcct.2019.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai X, Deng J, Yu M, Lu Z, Shen C, Zhang J. Perivascular fat attenuation index and high-risk plaque features evaluated by coronary CT angiography: relationship with serum inflammatory marker level. Int J Cardiovasc Imaging 2020;36:723-30. 10.1007/s10554-019-01758-8 [DOI] [PubMed] [Google Scholar]
- 18.Akoumianakis I, Antoniades C. The interplay between adipose tissue and the cardiovascular system: is fat always bad? Cardiovasc Res 2017;113:999-1008. 10.1093/cvr/cvx111 [DOI] [PubMed] [Google Scholar]
- 19.Marcelin G, Silveira ALM, Martins LB, Ferreira AV, Clément K. Deciphering the cellular interplays underlying obesity-induced adipose tissue fibrosis. J Clin Invest 2019;129:4032-40. 10.1172/JCI129192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki K, Kinoshita D, Yuki H, Niida T, Sugiyama T, Yonetsu T, Araki M, Nakajima A, Seegers LM, Dey D, Lee H, McNulty I, Takano M, Kakuta T, Mizuno K, Jang IK. Higher Noncalcified Plaque Volume Is Associated With Increased Plaque Vulnerability and Vascular Inflammation. Circ Cardiovasc Imaging 2024;17:e015769. 10.1161/CIRCIMAGING.123.015769 [DOI] [PubMed] [Google Scholar]
- 21.Kinoshita D, Suzuki K, Yuki H, Niida T, Fujimoto D, Minami Y, Dey D, Lee H, McNulty I, Ako J, Ghoshhajra B, Ferencik M, Kakuta T, Jang IK. Coronary artery disease reporting and data system (CAD-RADS), vascular inflammation and plaque vulnerability. J Cardiovasc Comput Tomogr 2023;17:445-52. 10.1016/j.jcct.2023.09.008 [DOI] [PubMed] [Google Scholar]
- 22.Ichikawa K, Miyoshi T, Osawa K, Miki T, Morimitsu Y, Akagi N, Nakashima M, Ito H. Association between higher pericoronary adipose tissue attenuation measured by coronary computed tomography angiography and nonalcoholic fatty liver disease: A matched case-control study. Medicine (Baltimore) 2021;100:e27043. 10.1097/MD.0000000000027043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boussoussou M, Vattay B, Szilveszter B, Simon J, Lin A, Vecsey-Nagy M, Konkoly G, Merkely B, Maurovich-Horvat P, Dey D, Kolossváry M. The effect of patient and imaging characteristics on coronary CT angiography assessed pericoronary adipose tissue attenuation and gradient. J Cardiovasc Comput Tomogr 2023;17:34-42. 10.1016/j.jcct.2022.09.006 [DOI] [PubMed] [Google Scholar]
- 24.Gaibazzi N, Martini C, Botti A, Pinazzi A, Bottazzi B, Palumbo AA. Coronary Inflammation by Computed Tomography Pericoronary Fat Attenuation in MINOCA and Tako-Tsubo Syndrome. J Am Heart Assoc 2019;8:e013235. 10.1161/JAHA.119.013235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oikonomou EK, Desai MY, Marwan M, Kotanidis CP, Antonopoulos AS, Schottlander D, Channon KM, Neubauer S, Achenbach S, Antoniades C. Perivascular Fat Attenuation Index Stratifies Cardiac Risk Associated With High-Risk Plaques in the CRISP-CT Study. J Am Coll Cardiol 2020;76:755-7. 10.1016/j.jacc.2020.05.078 [DOI] [PubMed] [Google Scholar]
- 26.Ichikawa K, Miyoshi T, Nakashima M, Nishihara T, Osawa K, Miki T, Toda H, Yoshida M, Ito H. Prognostic value of pericoronary adipose tissue attenuation in patients with non-alcoholic fatty liver disease with suspected coronary artery disease. Heart Vessels 2022;37:1977-84. 10.1007/s00380-022-02107-x [DOI] [PubMed] [Google Scholar]
- 27.Yan H, Zhao N, Geng W, Hou Z, Gao Y, Lu B. The Perivascular Fat Attenuation Index Improves the Diagnostic Performance for Functional Coronary Stenosis. J Cardiovasc Dev Dis 2022;9:128. 10.3390/jcdd9050128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seifarth H, Schlett CL, Nakano M, Otsuka F, Károlyi M, Liew G, Maurovich-Horvat P, Alkadhi H, Virmani R, Hoffmann U. Histopathological correlates of the napkin-ring sign plaque in coronary CT angiography. Atherosclerosis 2012;224:90-6. 10.1016/j.atherosclerosis.2012.06.021 [DOI] [PubMed] [Google Scholar]
- 29.Motoyama S, Kondo T, Anno H, Sugiura A, Ito Y, Mori K, Ishii J, Sato T, Inoue K, Sarai M, Hishida H, Narula J. Atherosclerotic plaque characterization by 0.5-mm-slice multislice computed tomographic imaging. Circ J 2007;71:363-6. 10.1253/circj.71.363 [DOI] [PubMed] [Google Scholar]
- 30.Xu R, Jing M, Zhu H, Xi H, Ren W, Zhou J. Relationship between different clinical characteristics and pericoronary adipose tissue attenuation values quantified from coronary computed tomographic angiography (CCTA) in patients without coronary heart disease (CHD). Quant Imaging Med Surg 2024;14:4054-66. 10.21037/qims-23-1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma ES, Ma GL, Yu HW, Wu W, Li K. Assessment of myocardial bridge and mural coronary artery using ECG-gated 256-slice CT angiography: a retrospective study. ScientificWorldJournal 2013;2013:947876. 10.1155/2013/947876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu Y, Liu H, Zhu Z, Wang S, Liu Q, Qiu J, Xing W. Assessment of myocardial bridging and the pericoronary fat attenuation index on coronary computed tomography angiography: predicting coronary artery disease risk. BMC Cardiovasc Disord 2023;23:145. 10.1186/s12872-023-03146-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakajima A, Sugiyama T, Araki M, Seegers LM, Dey D, McNulty I, Lee H, Yonetsu T, Yasui Y, Teng Y, Nagamine T, Nakamura S, Achenbach S, Kakuta T, Jang IK. Plaque Rupture, Compared With Plaque Erosion, Is Associated With a Higher Level of Pancoronary Inflammation. JACC Cardiovasc Imaging 2022;15:828-39. 10.1016/j.jcmg.2021.10.014 [DOI] [PubMed] [Google Scholar]
- 34.Tzolos E, Williams MC, McElhinney P, Lin A, Grodecki K, Flores Tomasino G, et al. Pericoronary Adipose Tissue Attenuation, Low-Attenuation Plaque Burden, and 5-Year Risk of Myocardial Infarction. JACC Cardiovasc Imaging 2022;15:1078-88. 10.1016/j.jcmg.2022.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as
Data Availability Statement
Available at https://qims.amegroups.com/article/view/10.21037/qims-24-1002/dss