Abstract
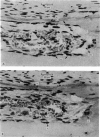
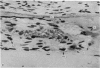
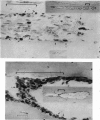
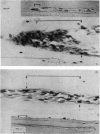
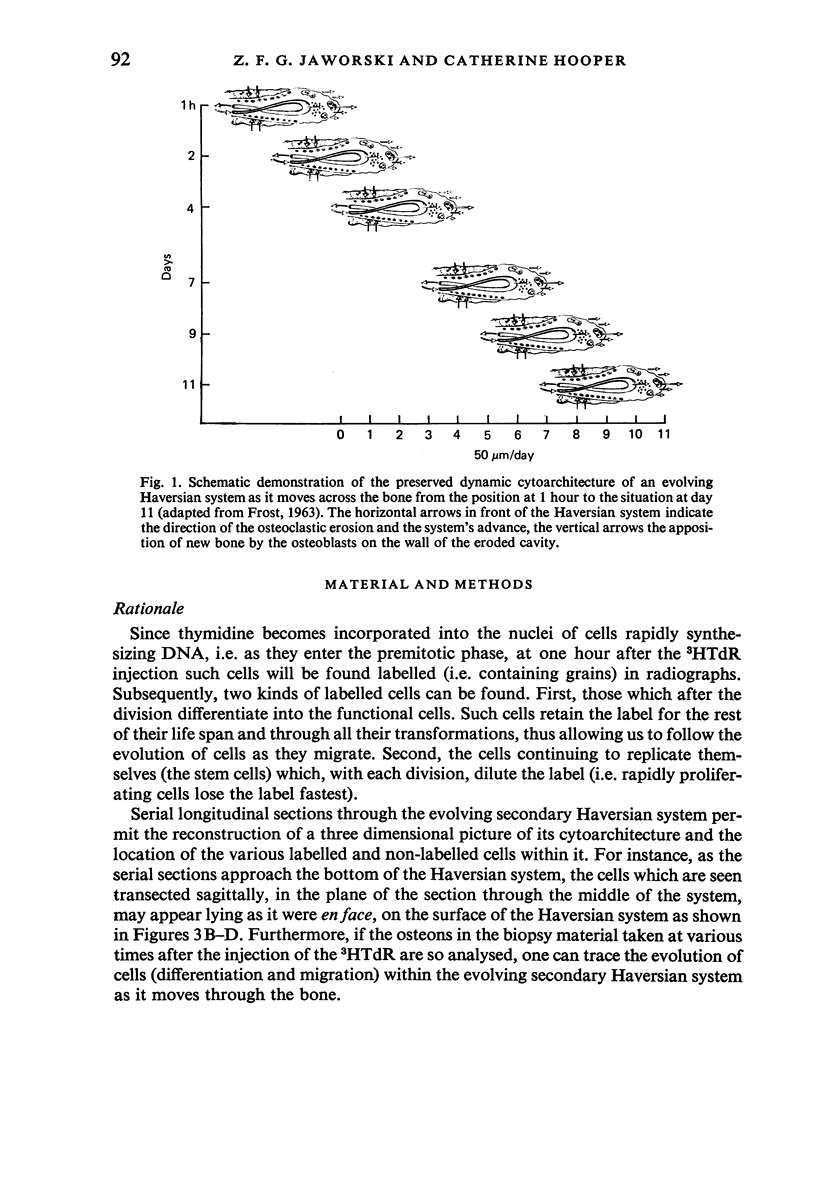
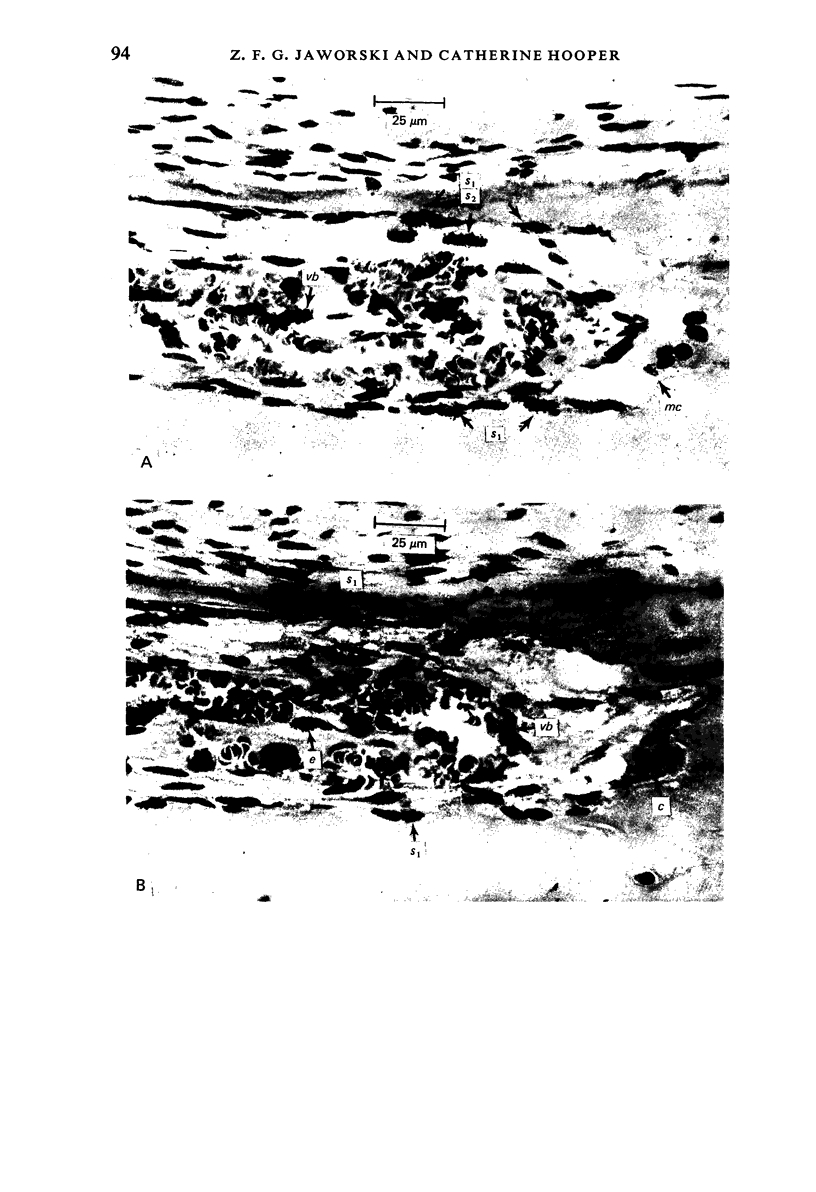
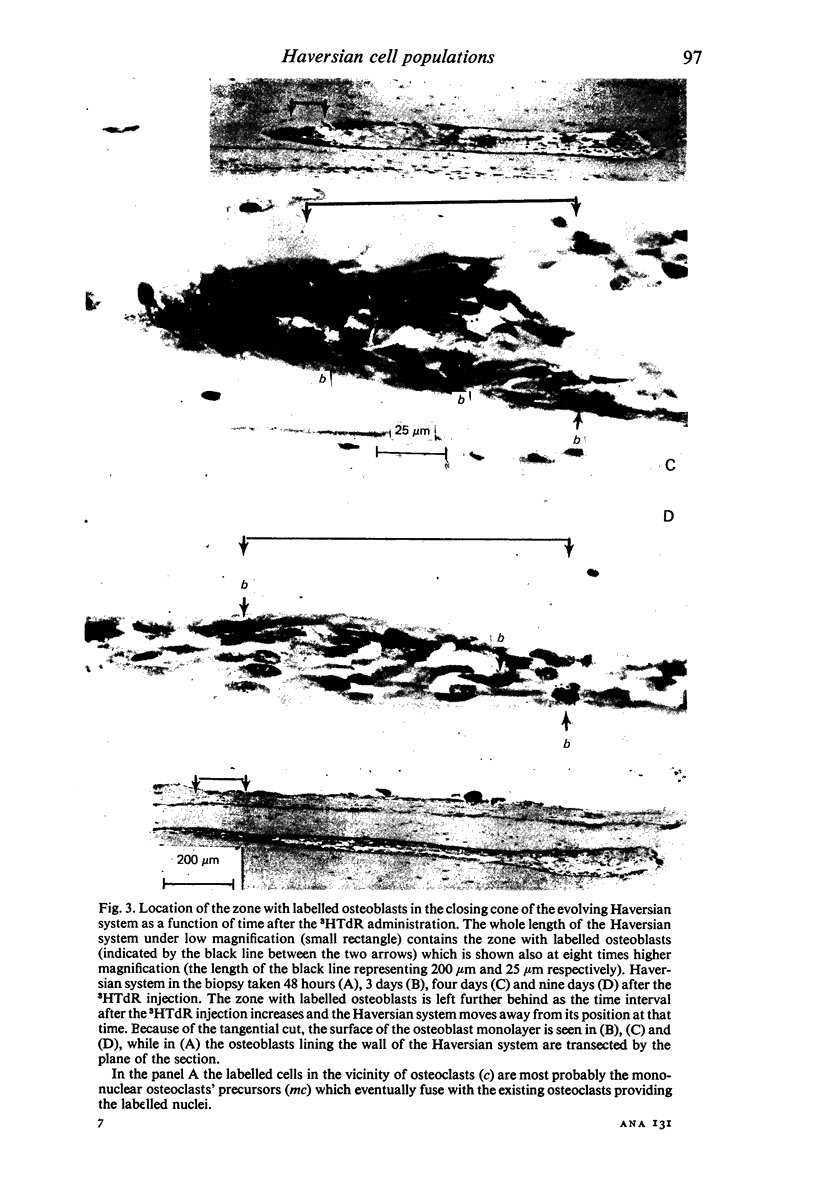
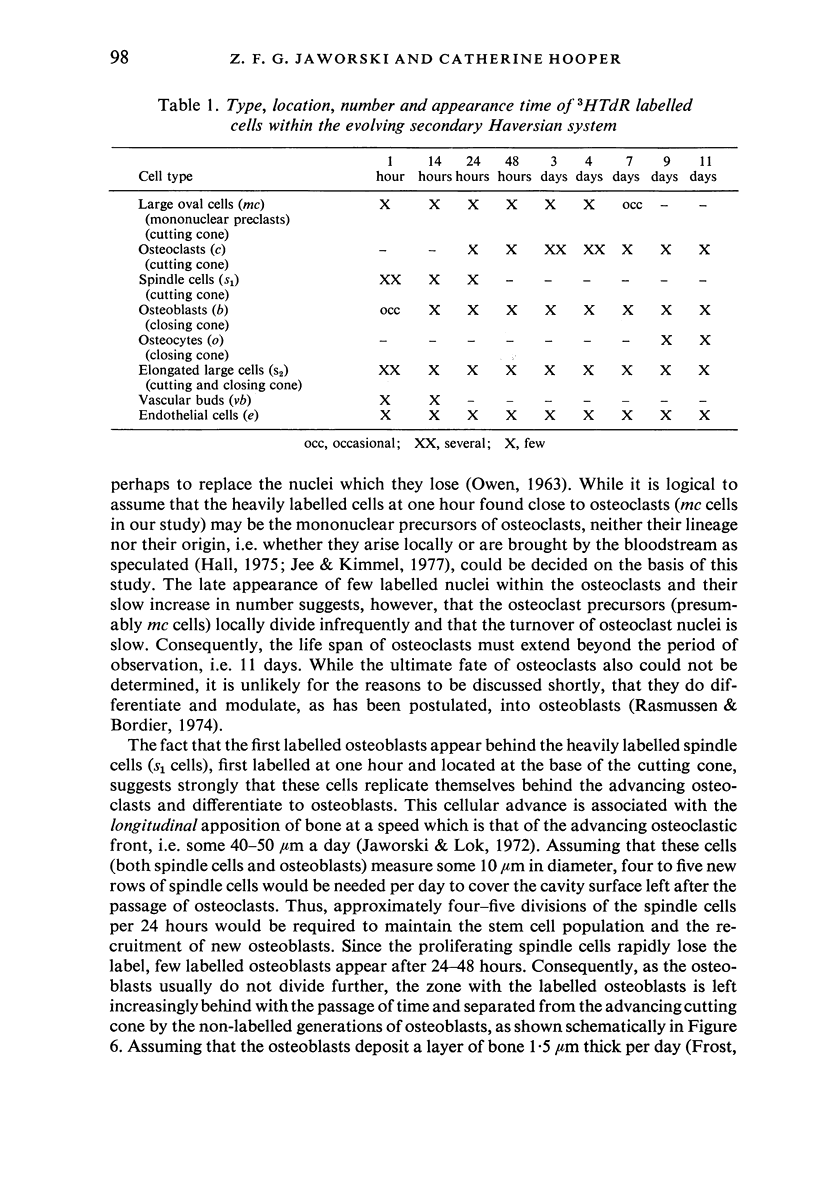
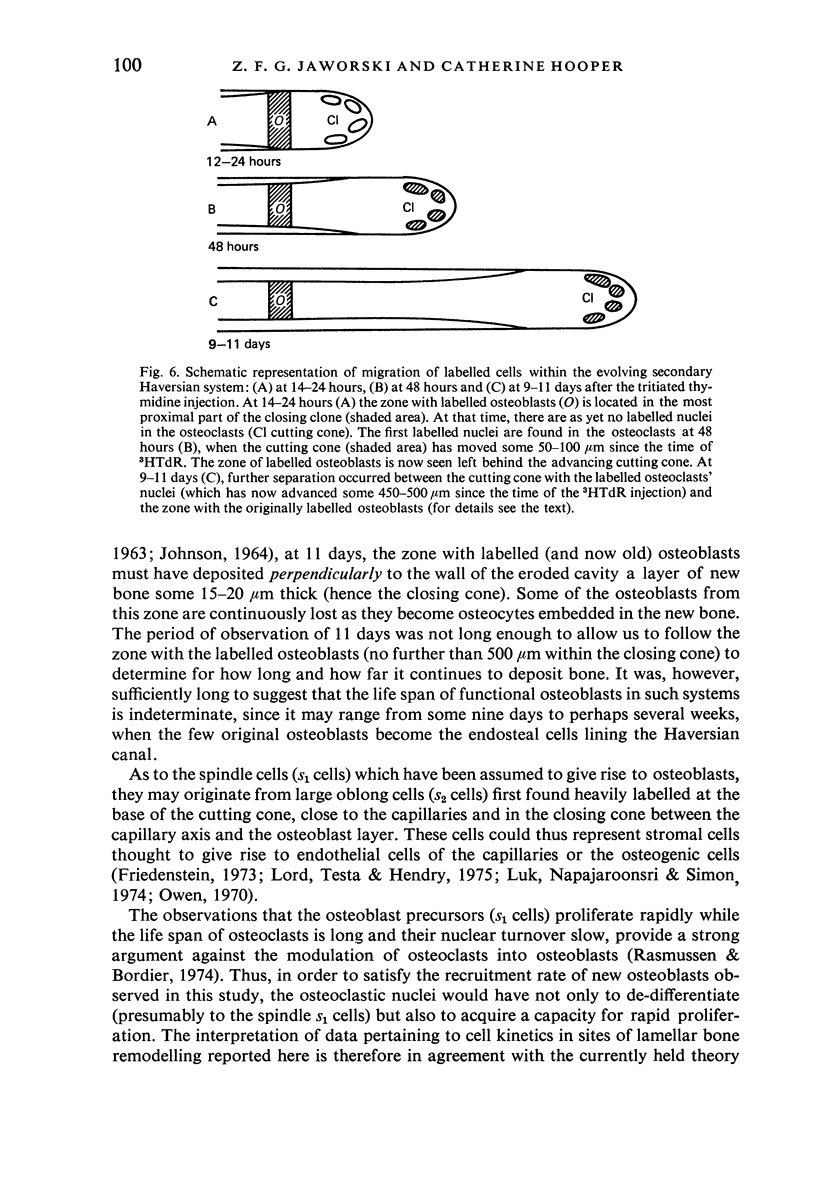
A study of the origin, proliferation rate and migration of cells within the secondary evolving Haversian systems was undertaken in young adult Beagle dogs. Autoradiographs of serial longitudinal sections prepared from rib biopsies taken from one hour to eleven days after the injection of tritiated thymidine were subjected to semiquantitative analysis as to the time of appearance, number, location and transformation of various labelled cells. Numerous labelled osteoblasts appeared early (at 14-24 hours) in the most proximal closing cone. With time, this zone was seen to have been left behind the advancing cutting cone and the successive generations of osteoblasts. The first labelled osteocytes were seen at nine days after injection, in the distal closing cone. Labelled nuclei within the osteoclasts were few and appeared late (none before 24 hours). It is apparent that each self renewing cell population within these systems (i.e. osteoclasts, osteoblasts and endothelial cells) derives from its own immediate precursor and evolves at its own speed. The mononuclear osteoclasts' precursors divide locally and infrequently and the turnover of osteoclastic nuclei appears to be slow; consequently their life span and that of the osteoclasts appears to be longer than the time of the observation, i.e. 11 days. The proliferation of osteoblasts' precursors and osteoblasts recruitment is rapid. The life span of osteoblasts was found to be indeterminate; some osteoblasts may become osteocytes within a few days while others may continue to deposit bone for several weeks. Since the recruitment of osteoclastic nuclei is slow while that of the osteoblasts is fast, it is unlikely that the osteoclasts in the sites of lamellar bone remodelling modulate into osteoblasts.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ENLOW D. H. Functions of the Haversian system. Am J Anat. 1962 May;110:269–305. doi: 10.1002/aja.1001100305. [DOI] [PubMed] [Google Scholar]
- FISCHMAN D. A., HAY E. D. Origin of osteoclasts from mononuclear leucocytes in regenerating newt limbs. Anat Rec. 1962 Aug;143:329–337. doi: 10.1002/ar.1091430402. [DOI] [PubMed] [Google Scholar]
- Frost H. M. Relation between bone tissue and cell population dynamics, histology and tetracycline labeling. Clin Orthop Relat Res. 1966 Nov-Dec;49:65–75. [PubMed] [Google Scholar]
- Hall B. K. The origin and fate of osteoclasts. Anat Rec. 1975 Sep;183(1):1–11. doi: 10.1002/ar.1091830102. [DOI] [PubMed] [Google Scholar]
- Hattner R., Epker B. N., Frost H. M. Suggested sequential mode of control of changes in cell behaviour in adult bone remodelling. Nature. 1965 May 1;206(983):489–490. doi: 10.1038/206489a0. [DOI] [PubMed] [Google Scholar]
- JEE W. S., NOLAN P. D. ORIGIN OF OSTEOCLASTS FROM THE FUSION OF PHAGOCYTES. Nature. 1963 Oct 19;200:225–226. doi: 10.1038/200225a0. [DOI] [PubMed] [Google Scholar]
- Jaworski Z. F., Lok E. The rate of osteoclastic bone erosion in Haversian remodeling sites of adult dog's rib. Calcif Tissue Res. 1972;10(2):103–112. doi: 10.1007/BF02012540. [DOI] [PubMed] [Google Scholar]
- LEBLOND C. P. CLASSIFICATION OF CELL POPULATIONS ON THE BASIS OF THEIR PROLIFERATIVE BEHAVIOR. Natl Cancer Inst Monogr. 1964 May;14:119–150. [PubMed] [Google Scholar]
- Lord B. I., Testa N. G., Hendry J. H. The relative spatial distributions of CFUs and CFUc in the normal mouse femur. Blood. 1975 Jul;46(1):65–72. [PubMed] [Google Scholar]
- Luk S. C., Nopajaroonsri C., Simon G. T. The ultrastructure of endosteum: a topographic study in young adult rabbits. J Ultrastruct Res. 1974 Feb;46(2):165–183. doi: 10.1016/s0022-5320(74)80054-7. [DOI] [PubMed] [Google Scholar]
- OWEN M. CELL POPULATION KINETICS OF AN OSTEOGENIC TISSUE. I. J Cell Biol. 1963 Oct;19:19–32. doi: 10.1083/jcb.19.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen M. The origin of bone cells. Int Rev Cytol. 1970;28:213–238. doi: 10.1016/s0074-7696(08)62544-9. [DOI] [PubMed] [Google Scholar]
- Walker D. G. Osteopetrosis cured by temporary parabiosis. Science. 1973 May 25;180(4088):875–875. doi: 10.1126/science.180.4088.875. [DOI] [PubMed] [Google Scholar]
- van Furth R. Origin and kinetics of monocytes and macrophages. Semin Hematol. 1970 Apr;7(2):125–141. [PubMed] [Google Scholar]