Abstract
Objective
The COVID-19 pandemic has profoundly disrupted global mental health. Medical students, already vulnerable to high stress, experience heightened psychological distress. This study aimed to investigate the association between acute COVID-19 infection and the development of psychological symptoms in medical students, exploring the role of specific COVID-19 symptoms and lifestyle factors.
Methods
A longitudinal study of 2359 medical students assessed psychological symptoms (depression, anxiety, insomnia) before and after COVID-19 infection. Logistic regression models, adjusted for confounders, examined the relationship between COVID-19 status (with or without infection), and the onset of psychological symptoms. The study also explored acute COVID-19 symptoms and lifestyle factors associated with psychological symptoms in the COVID-19 infected (COVID+) group.
Findings
The COVID + group exhibited significantly greater increases in depression and insomnia compared to non-infected group. COVID-19 infection was significantly associated with the onset of insomnia post-infection (OR [95 % CI] 1.83 [1.05–3.21]). Moderate to severe acute symptoms, including sleep disturbances (2.20 [1.17–4.14]), decreased attention/memory (2.47 [1.02–6.00]), and breathlessness (2.05 [1.08–3.85]), were identified as risk factors for onset of insomnia. Regular exercise was found to be negatively associated with the onset of insomnia (0.54 [0.33–0.88]).
Conclusion
Early intervention targeting these acute-phase risk symptoms is crucial for preventing long-term insomnia. Regular exercise is effective in preventing the development of insomnia in medical students following COVID-19 infection. These findings will assist in formulating targeted interventions to address recurrent infections in the post-pandemic era.
Keywords: Long-COVID, Insomnia, Acute COVID-19 symptoms
1. Introduction
As a major global public health crisis of the 21st century, COVID-19 pandemic not only threatens human lives but also has a profound and unparalleled impact on mental health (Alwan, 2020; Weich, 2022). Following the outbreak of COVID-19, college students have faced a surge in the prevalence of depression, anxiety, and insomnia (Liu et al., 2022; Chen and Lucock, 2022). Especially medical students, given their occupational duties and academic obligations, exhibit heightened susceptibility to virus exposure, thereby experiencing increased depression, anxiety, and insomnia (Obradovic et al., 2024; Li et al., 2022). These mental health challenges also significantly affect academic performance, well-being, and future career prospects, and future career prospects (Barbosa-Camacho et al., 2022; Perotta et al., 2021), making it necessary to monitor changes in psychological symptoms among this vulnerable population.
Given the unique trajectory of the pandemic in China, the prolonged restrictions and life disruption were followed by a surge in infections after the nationwide lockdown was lifted in December 2022 (Liang et al., 2023; Fu et al., 2023). The pandemic represents a substantial biopsychosocial stressor, which makes it an ideal context for investigating the psychological impact of COVID-19. COVID-19 is a multisystem disease that, in addition to respiratory symptoms, often presents with neurological manifestations in acute patients, with headache and fatigue being commonly reported (Wesselingh, 2023). The neurological syndromes observed in COVID-19 survivors may stem from the immune response elicited by the body against the virus (Radke et al., 2024). This neuroinflammatory response contributes to neurological syndromes; furthermore, these neurological sequelae are expected to persist for two years (Bowe et al., 2023). COVID-19 survivors face long-term post-acute neuropsychiatric symptoms (Awadalla et al., 2022), with insomnia being one of the most common complications among long-term COVID-19 patients (Akbarialiabad et al., 2021; Alkodaymi et al., 2022). The emergence of post-COVID neuropsychiatric sequelae underscores the need to investigate the relationship between acute COVID-19 symptoms and subsequent mental health outcomes.
COVID-19 survivors who experienced more acute physical symptoms during hospitalization are more likely to develop more severe emotional symptoms and sleep disturbances three months after discharge (Tracy et al., 2024). Furthermore, cognitive impairment and shortness of breath during the acute phase in children and adolescents with pre-existing insomnia predict worsening symptoms after the infection (Zhu et al., 2024). However, there is insufficient awareness of the impact of acute-phase symptoms on the neuropsychiatric sequelae of COVID-19 among medical students. Medical students faced significant academic and psychosocial stressors during COVID-19, including campus closures and career uncertainty, while some also served on pandemic frontlines, raising their infection risk. This dual burden of academic pressures and frontline exposures may render them particularly susceptible to long-term psychiatric sequelae of COVID-1919−21. Investigating how acute symptoms affect the risk of long-term psychiatric sequelae can inform targeted interventions for this vulnerable population, especially in the context of recurrent infections in the post-pandemic era.
This longitudinal study involved two assessments of depression, anxiety, and insomnia among medical students, conducted before and after restrictions were lifted. The objectives of this study are, first, to determine whether COVID-19 infection is a risk factor for the onset of depression, anxiety, and insomnia after balancing for confounding factors such as lifestyle behaviors; and second, to identify the risk factors of acute COVID-19 symptoms and lifestyle behaviors for post-acute psychiatric sequelae in the infected students. In the post-pandemic era, especially medical students who are prone to recurrent COVID-19 infections, identifying the risk of acute-phase symptoms is crucial for preventing long-term psychiatric sequelae.
2. Material and methods
2.1. Study design and participants
This study is part of a mixed longitudinal cohort at Xinxiang Medical University, with six psychological screenings at different time points, including five screenings under COVID-19 restrictions (March 2020 [n = 28584], October 2020 [n = 18545], March 2021 [n = 23019], October 2021 [n = 6829], and February 2022 [n = 4995]), and one screening after the lifting of restrictions in August 2023 (n = 5885). This study included participants from the sixth psychological screening who had completed at least one screening before the lifting of restrictions. The most recent screening record before the sixth screening was used as T1 in this study. Therefore, a total of 2359 medical students aged 18–24 years from Xinxiang Medical University were finally included in this study, covering T1 (between March 2020 and February 2022) and T2 (August 2023). All participants completed the questionnaires using the WeChat official account platform and provided informed consent before participation. The study was approved by the Biomedical Ethics Committee of Xinxiang Medical University (XYLL-2020235).
2.2. Study measures
2.2.1. The characterization of demographic and lifestyle factors
The participants’ age, sex, ethnicity, and educational levels were collected in the study. The lifestyle behaviors consisted of cigarette consumption over the week (yes/no); alcohol consumption over the past week (yes/no), and physical exercise before the outbreak of the pandemic [frequency (infrequent, 1–3 days per week, or 4–7 days per week); intensity of exercise (no exercise, mild (e.g., walking), moderate (e.g., cycling, tai chi, table tennis, and badminton), or vigorous (e.g., running, swimming, soccer, basketball, and jumping rope); duration of exercise (<30 min, 30–60 min, or > 60 min)].
2.2.2. Measurements of psychological symptoms
The severity of psychological symptoms was assessed using the Patient Health Questionnaire-9 (PHQ-9), the Generalized Anxiety Disorder Questionnaire-7 (GAD-7), and the Insomnia Severity Index (ISI). PHQ-9 measures depressive over the past 2 weeks, total score greater than 5 defined as the presence of depression. The GAD-7 assesses anxiety symptoms over the past two weeks, with a total score greater than 5 defining the presence of anxiety disorder. ISI measures sleep problems over the past 2 weeks, and total score greater than 7 defined as the presence of insomnia. Depression, anxiety, and insomnia symptoms were used in subsequent analyses.
2.2.3. COVID-19 infection
Prior COVID-19 infection was confirmed and characterized using self-report questionnaires. Questions included: 1) Was COVID-19 infection confirmed through the polymerase chain reaction (PCR), antibody, or lateral flow test, or the presence of COVID-19 positive symptoms? 2) Have there been two or more times of COVID-19 infection till the completion of the questionnaire? 3) Did you experience any of the acute-phase symptoms of COVID-19 infection and their severity? We assessed for 13 acute-phase symptoms (fatigue, sleep disturbances, decreased attention/memory, difficulty thinking, pharyngeal symptoms, nasal congestion, breathlessness, fever, cough/phlegm, olfactory impairment, gastrointestinal symptoms, headache, fear of COVID-19 virus)? 4) What is the overall severity of acute COVID-19 infection symptoms? 5) What is the cumulative extent of psychological distress experienced during the COVID-19 epidemic? 6) What is the level of concern about the virus before COVID-19 infection? (Appendix p1).
This scale rates the severity of 13 acute-phase symptoms as none, mild, moderate, or severe, and assesses the overall severity of symptoms, the extent of psychological distress during the pandemic, and the level of concern about the virus before infection on a scale from 0 to 10, where 0 represents no symptoms and 10 represents extreme severity.
2.2.4. Patient and public involvement
All participants were recruited through notifications issued by the School of Psychology of Xinxiang Medical University via academic counselors, and the participants voluntarily participated in and completed the psychological assessments.
2.3. Statistical analysis
All statistical analyses were performed using R Studio software (4.3.1). All statistical significance was defined as a two-sided P-value <0.05 level.
2.3.1. Changes (Δ) in symptom scores between COVID + group and COVID-group
The changes (Δ) of PHQ-9, GAD-7, and ISI scores from T1 to T2 were calculated and compared between participants with (COVID+) and without (COVID-) COVID-19 infection using T-tests.
2.3.2. Effects of COVID-19 infection on psychological symptoms
To further investigate the impact of COVID-19 infection on the onset of psychological symptoms, participants were stratified into two groups based on their psychological status change from T1 to T2: healthy controls (HC; asymptomatic at T1 and T2) and symptom onset group (ONS; asymptomatic at T1 and symptomatic at T2). The HC group consisted of those who were asymptomatic at T1 and T2 for twice psychological surveys. Sample sizes for the HC and ONS groups for each psychological symptom are listed in Appendix p2.
Propensity score-based weighting was used to control for demographic and lifestyle factors, including age, gender, ethnic group, education levels, cigarette consumption, alcohol consumption, physical exercise, and the intervals between survey response periods We constructed separate logistic regression models for depression, anxiety, and insomnia symptoms to estimate the propensity scores for group membership after considering the covariates. We then computed the inverse probability weights for each participant and employed logistic regression models to assess whether COVID-19 infection posed a risk for changes in the mental well-being of medical students.
2.3.3. Risk and protective factors for psychological symptoms in COVID + group
To address collinearity and overfitting, we employed lasso techniques for symptom selection during the acute phase. Logistic regression models were performed to evaluate the effects of acute COVID-19 symptoms, and lifestyle factors associated with ONS of psychological symptoms, in terms of the odds ratio (OR) and 95 % confidence interval (CI).
3. Results
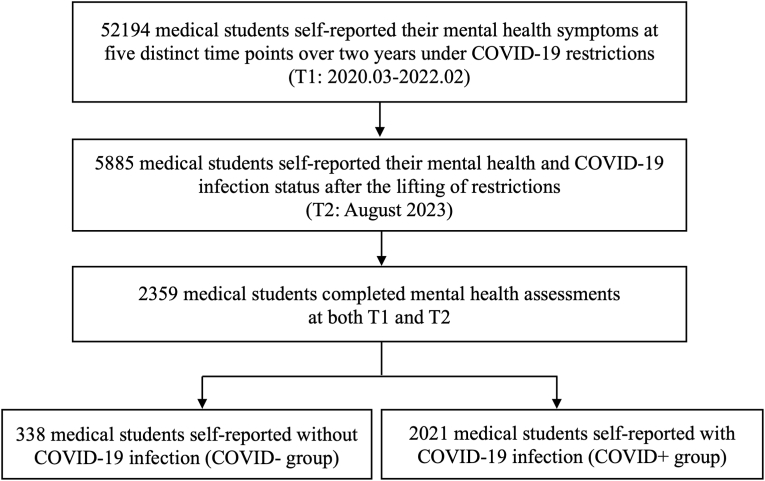
The study flowchart is shown in Fig. 1. The COVID+ and COVID-groups consisted of 2021 (85.67 %) and 338 (14.33 %) medical students, respectively (Table 1).
Fig. 1.
Study flowchart.
Table 1.
Demographic characteristics of participants.
| Characteristics | All participants (N = 2359) | COVID-group (N = 338) | COVID + group (N = 2021) | T/X2 valuea | P valuea |
|---|---|---|---|---|---|
| Age (Mean, SD) | 22.23 (2.49) | 21.72 (2.69) | 22.32 (2.45) | −4.09 | <0.001 |
| Sex (n, %) | 12.26 | 0.001 | |||
| Female | 1485 (62.95) | 184 (54.44) | 1301 (64.37) | ||
| Male | 874 (37.05) | 154 (45.56) | 720 (35.63) | ||
| Ethnic (n, %) | |||||
| Han | 2320 (98.35) | 331 (97.93) | 1989 (98.42) | 0.42 | 0.674 |
| Minority | 39 (1.65) | 7 (2.07) | 32 (1.58) | ||
| Educational level (n, %) | |||||
| Bachelor degree | 1871 (79.31) | 276 (81.66) | 1595 (78.92) | 1.32 | 0.282 |
| Master degree | 488 (20.69) | 62 (18.34) | 426 (21.08) | ||
| Cigarette consumption (n, %) | |||||
| No | 2233 (94.66) | 315 (93.20) | 1918 (94.90) | 1.67 | 0.245 |
| Yes | 126 (5.34) | 23 (6.80) | 103 (5.10) | ||
| Alcohol consumption (n, %) | |||||
| No | 1587 (67.27) | 248 (73.37) | 1339 (66.25) | 6.67 | 0.012 |
| Yes | 772 (32.73) | 90 (26.63) | 682 (33.75) | ||
| Physical exercise (n, %) | |||||
| No | 118 (5.00) | 16 (4.73) | 102 (5.05) | 0.60 | 0.807 |
| Yes | 2241 (95.00) | 322 (95.27) | 1919 (94.95) | ||
| Survey response periods at T1b (n, %) | |||||
| March 2020 to October 2021 | 601 (25.48) | 97 (28.70) | 504 (24.94) | 2.16 | 0.161 |
| February 2022 | 1758 (74.52) | 241 (71.30) | 1517 (75.06) | ||
NOTE.
the T test and X2 test were preformed between COVID-group and COVID + group.
according to the psychological survey at T2, the most recent historical psychological survey records of the participants before the lifting of restrictions were selected for analysis.
3.1. Changes (Δ) in psychological symptoms with COVID-19 infection
The Δ psychological symptoms were significantly different between COVID+ and COVID-groups for depression and insomnia scores (Fig. 2). Participants in the COVID + group demonstrated greater increases in depression (T = 2.07; P = .038) and insomnia (T = 2.89; P = .004) than those in the COVID-group.
Fig. 2.
The differences in symptom changes (T1-T2) between the COVID-group and COVID + group.
NOTE: ∗ indicates significance at p < .05; ∗∗ indicates significance at p < .01; COVID-group: participants who self-reported COVID-19 infection; COVID + group: participants who self-reported no COVID-19 infection.
3.2. Onset of psychological symptom associated with COVID-19 infection
After inverse probability weighting, the confounding factors (including demographic and lifestyle factors) were well balanced between COVID+ and COVID-groups at T1 (see Appendix p6). Fig. 3 depicts the odds ratios of ONS of psychological symptoms between the COVID+ and COVID-groups. Compared to the COVID-group, COVID + group had increased risk of insomnia ONS (OR [95 % CI] 1·83 [1.05–3.21]).
Fig. 3.
The odds ratio (OR) of onset of psychological symptoms in COVID + group compared to COVID-group.
NOTE: ONS: asymptomatic at T1 and symptomatic at T2.
3.3. Effects of acute COVID-19 symptoms and lifestyle factors on insomnia status in COVID + group
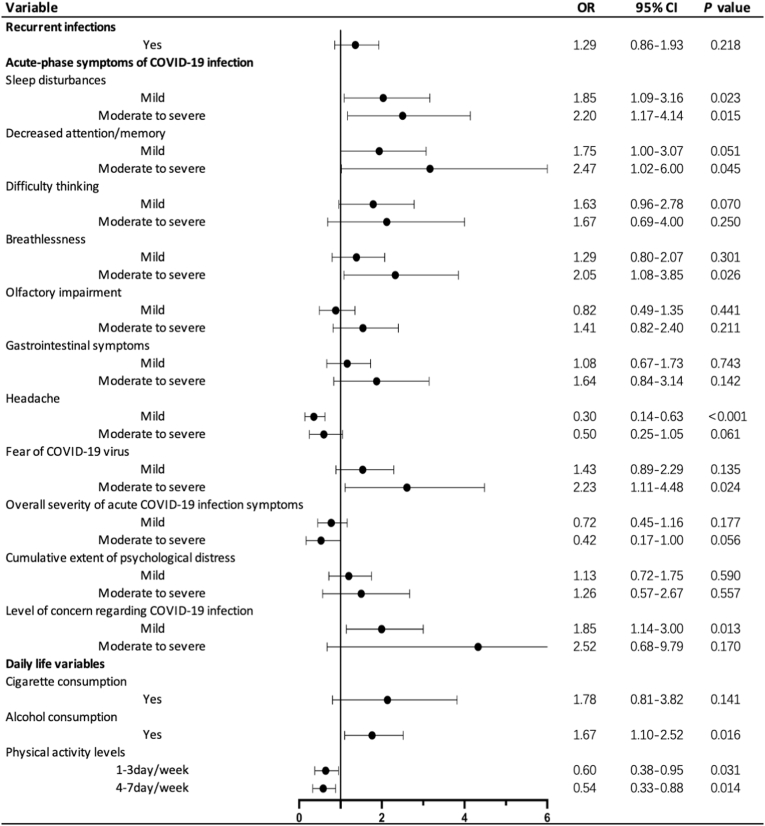
Severity levels for all acute COVID-19 are presented in Appendix p5. After conducting lasso regressions, sleep disturbances, decreased attention/memory, difficulty thinking, breathlessness, olfactory impairment, gastrointestinal symptoms, headache, fear of COVID-19 virus during acute-phase, overall severity of acute COVID-19 infection symptoms, cumulative extent of psychological distress during the COVID-19 pandemic, and level of concern regarding COVID-19 infection were included for subsequent logistic regression analyses assessing the effect of acute COVID-19 symptoms on onset of insomnia.
Mild and moderate to severe sleep disturbances (1.85 [1.09–3.16], 2.20 [1.17–4.14], respectively), moderate to severe decreased attention/memory (2.47 [1.02–6.00]), moderate to severe breathlessness (2.05 [1.08–3.85]), moderate to severe fear of COVID-19 virus (2.23 [1.11–4.48]), and mild level of concern regarding COVID-19 infection (1.85 [1.14–3.00]) was positively associated with insomnia ONS in COVID + group (Fig. 4). Alcohol consumption was positively associated with insomnia ONS (1.67 [1.10–2.52]) in COVID + group. Physical activity, whether 1–3 days/week (0.60 [0.38–0.95]) or 4–7 days/week (0.54 [0.33–0.88]), was negatively correlated with insomnia ONS in COVID + group (Fig. 4).
Fig. 4.
The risk and protective factors related to acute COVID-19 symptoms and daily lifestyle for insomnia in the COVID + group.
NOTE: The healthy controls as reference. Recurrent infections: not recurrent infection as reference; acute-phase symptoms of COVID-19 infection: no acute symptom as reference; physical activity levels: infrequent exercise as reference.
4. Discussion
To our knowledge, this is the first longitudinal study to examine how COVID-19 infection affects the onset of psychological symptoms in medical students and to identify specific acute COVID-19 symptoms that pose a risk for these psychological outcomes. Depression and insomnia worsened significantly more in the COVID + group compared to the COVID-group. However, following the lifting of COVID-19 restrictions, the COVID-group exhibited improvements in depression, anxiety, and insomnia. Additionally, compared to the COVID-group, the COVID + group was positively associated with the onset of insomnia, indicating that COVID-19 infection poses a higher risk of insomnia. Persistence of sleep problems have been observed even two years post-COVID-19 infection in general population (Fernandez-de-Las-Peñas et al., 2024). Moreover, individuals with pre-existing insomnia symptoms are more prone to experiencing prolonged COVID-19 symptoms (Vargas et al., 2023). To minimize confounding effects of pre-existing insomnia symptoms on post-COVID-19 infection outcomes, this study only included participants without insomnia pre-infection. At the same time, this study found that the onset of insomnia was associated with COVID-19 infection, even after balancing of lifestyle factors. In individuals without pre-existing insomnia symptoms before infection, the incidence of long-COVID insomnia post-infection was 60.6 % (Chen et al., 2023). Therefore, COVID-19 as a risk factor for long-term insomnia underscores the critical need for early intervention for insomnia following infection.
Our study also indicates that in the COVID + group, acute symptoms such as moderate to severe decreased attention/memory, breathlessness, and mild to severe sleep disturbances were associated with the onset of insomnia post-COVID-19 infection. Cognitive dysfunction, such as brain fog, memory deterioration, and attention decline, appears to be key features of post-COVID-19 syndrome (Premraj et al., 2022). There is a significant correlation between continuous attention dysfunction and C-reactive protein levels at admission in COVID-19 patients, with the inflammatory process contributing to long-term cognitive impairment (Zhou et al., 2020). Despite the lack of persistent virus in the central nervous system, ongoing peripheral inflammation contributes to neurological sequelae of COVID-19. This study finds that reduced attention/memory during the acute stage predicts long-term insomnia, suggesting that cognitive impairment and sustained inflammation increase the risk of long-COVID insomnia.
Another interesting finding of the study is the significant correlation between acute-phase insomnia and long-term insomnia post-acute. Among adolescents with long COVID-19, 32.1 % reported insomnia as the primary reason for their school absences (Sakurada et al., 2023). Long-term COVID-19 insomnia is highly prevalent and significantly impacts academic performance, yet most affected individuals in China do not receive treatment. (Lu et al., 2024). Digital sleep interventions have been confirmed as effective tools for improving sleep quality and addressing insomnia during the COVID-19 pandemic (Zhang et al., 2023). Insomnia, as an undeniable mental health challenge impacting both physical and mental well-being, as well as academic performance, of students. This issue is particularly critical among medical students, given the demanding nature of their academic pursuits; therefore, timely intervention following infection is imperative.
Maintaining regular exercise habits before COVID-19 infection was found as a protective factor against developing insomnia post-infection in the study. In the post-pandemic era, recurrent infections are unavoidable, and promoting regular exercise may be an effective measure for preventing and treating long-COVID insomnia. Various intensities and types of exercise have been shown to significantly enhance sleep duration and quality, and mitigate the severity of insomnia (Tian et al., 2024; Riedel et al., 2024). Increased exercise frequency can alleviate insomnia in individuals concerned about COVID-19, potentially due to the anxiety-reducing effects of physical activity (Yu et al., 2024). Exercise offers a cost-effective, safe, and easily accessible strategy for the prevention and treatment of insomnia. Therefore, in the post-pandemic era, college administrators are advocating for increased physical activity among students as a potential measure to mitigate long-COVID insomnia.
The strengths of our study include that the participants comprised students from a single medical school, providing a relatively homogeneous living environment that effectively controls for potential confounding factors. As future healthcare professionals, these medical students represent a critical reserve force for the healthcare system. Therefore, it is important to investigate the impact of COVID-19 on their mental health, which not only pertains to their academic performance but also has significant implications for the entire healthcare system. The study has several limitations, as detailed below. Firstly, the COVID-19 infection status among students was determined through self-reports without validation by laboratory results, which could lead to misclassification bias due to underreporting of asymptomatic infections, thereby weakening group differences. Although this study focuses on the impact of acute COVID-19 symptoms on long-term mental health, this bias may still underestimate the true psychological impact of COVID-19 infection. Secondly, there was variability in the response periods of individuals prior to their COVID-19 infection (T1). More than 25 % of participants responded between March 2020 to October 2021. In the study, the intervals between survey response periods were used in propensity score-based weighting to balance their confounding effects. Thirdly, to control for the influence of prior mental health conditions, we included only students without a history of mental health problems in the logistic regression analyses assessing the impact of COVID-19 infection. However, we did not account for participants’ pre-existing physical health conditions, which could potentially amplify their physiological stress responses, leading to an overestimation of the severity of acute COVID-19 symptoms and, consequently, an exaggeration of the psychological impact of COVID-19 infection.
5. Conclusion
Medical students infected with COVID-19 experienced more severe depression and insomnia symptoms post-infection. COVID-19 infection is a risk factor for the onset of insomnia. Furthermore, acute symptoms such as sleep disturbances, decreased attention/memory, and breathlessness are also risk factors for insomnia. In the post-pandemic era, recurrent and persistent COVID-19 infections are inevitable. Early intervention targeting theses risk acute-phase symptoms is essential for preventing long-term insomnia. Additionally, college administrators should advocate for and encourage students to develop regular exercise habits, as this is an effective method for preventing insomnia.
CRediT authorship contribution statement
Yue Zhu: Writing – review & editing, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Conceptualization. Xiaoyang Qin: Writing – original draft, Visualization, Methodology. Yuhong Lu: Writing – original draft. Yuenan Yu: Visualization, Software, Methodology. Rongxun Liu: Resources, Project administration, Data curation. Rongxin Zhu: Resources, Project administration. Kuan-Pin Su: Resources, Project administration. Fei Wang: Supervision, Resources, Project administration, Data curation, Conceptualization.
Data availability statement
Data are available upon reasonable request. Deidentified original transcript data will be available exclusively for academic purposes. For reasonable data requests, please contact the corresponding author (fei.wang@yale.edu).
Ethics statements
All participants completed the questionnaires using the WeChat official account platform and provided informed consent before participation. The study was approved by the Biomedical Ethics Committee of Xinxiang Medical University (XYLL-2020235).
Financial support
This work was supported by National Science Fund for Distinguished Young Scholars (81725005 to Fei Wang), NSFC-Guangdong Joint Fund (U20A6005 to Fei Wang), Jiangsu Provincial Key Research and Development Program (BE2021617 to Fei Wang), Henan Provincial Research and Practice Project for Higher Education Teaching Reform (2021SJGLX189Y to Rongxun Liu), National Natural Science Foundation of China (82151315 to Rongxin Zhu), and Natural Science Foundation of Jiangsu Province (Yue Zhu to BK20240273).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We would like to thank all participants and study personnel who worked on the parent study.
Footnotes
This article is part of a special issue entitled: Post-COVID19 condition published in Brain, Behavior, & Immunity - Health.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2025.101067.
Contributor Information
Kuan-Pin Su, Email: cobolsu@gmail.com.
Fei Wang, Email: fei.wang@yale.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Akbarialiabad H., Taghrir M.H., Abdollahi A., et al. Long COVID, a comprehensive systematic scoping review. Infection. 2021;49(6):1163–1186. doi: 10.1007/s15010-021-01666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkodaymi M.S., Omrani O.A., Fawzy N.A., et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: a systematic review and meta-analysis. Clin. Microbiol. Infection.Official Pub. Euro. Soc. Clin. Microbiol. Infectious Dis. 2022;28(5):657–666. doi: 10.1016/j.cmi.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwan N.A. Surveillance is underestimating the burden of the COVID-19 pandemic. Lancet. 2020;396(10252):e24. doi: 10.1016/s0140-6736(20)31823-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awadalla N.J., Alsabaani A.A., Alsaleem M.A., et al. Increased mental stress among undergraduate medical students in south-western Saudi Arabia during the COVID-19 pandemic. PeerJ. 2022;10 doi: 10.7717/peerj.13900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa-Camacho F.J., Romero-Limón O.M., Ibarrola-Peña J.C., et al. Depression, anxiety, and academic performance in COVID-19: a cross-sectional study. BMC Psychiatry. 2022;22(1):443. doi: 10.1186/s12888-022-04062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe B., Xie Y., Al-Aly Z. Postacute sequelae of COVID-19 at 2 years. Nat. Med. 2023;29(9):2347–2357. doi: 10.1038/s41591-023-02521-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Lucock M. The mental health of university students during the COVID-19 pandemic: an online survey in the UK. PLoS One. 2022;17(1) doi: 10.1371/journal.pone.0262562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.J., Morin C.M., Ivers H., et al. The association of insomnia with long COVID: an international collaborative study (ICOSS-II) Sleep Med. 2023;112:216–222. doi: 10.1016/j.sleep.2023.09.034. [DOI] [PubMed] [Google Scholar]
- Fernandez-de-Las-Peñas C., Notarte K.I., Macasaet R., et al. Persistence of post-COVID symptoms in the general population two years after SARS-CoV-2 infection: a systematic review and meta-analysis. J. Infect. 2024;88(2):77–88. doi: 10.1016/j.jinf.2023.12.004. [DOI] [PubMed] [Google Scholar]
- Fu D., He G., Li H., et al. Effectiveness of COVID-19 vaccination against SARS-CoV-2 Omicron variant infection and symptoms - China, december 2022-february 2023. China CDC Wkly. 2023;5(17):369–373. doi: 10.46234/ccdcw2023.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Zhao Z., Chen D., Peng Y., Lu Z. Prevalence and associated factors of depression and anxiety symptoms among college students: a systematic review and meta-analysis. J Child Psychol Psychiatry. 2022;63(11):1222–1230. doi: 10.1111/jcpp.13606. [DOI] [PubMed] [Google Scholar]
- Liang J., Liu R., He W., et al. Infection rates of 70% of the population observed within 3 weeks after release of COVID-19 restrictions in Macao, China. J. Infect. 2023;86(4):402–404. doi: 10.1016/j.jinf.2023.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Dai L., Cai Y., Chen X., Li J., Shi L. Psychological state and its correlates of local college students in Wuhan during COVID-19 pandemic. Psychol. Sch. 2022 doi: 10.1002/pits.22699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M.L., Zhu J.W., Wu J.L., et al. Insomnia among coronavirus disease 2019 survivors: a single-center cross-sectional study. Medicine. 2024;103(7) doi: 10.1097/md.0000000000037311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradovic A., Toubat O., Chen N.W., et al. Impact of COVID-19 pandemic on physician-scientist trainees to faculty one year into the pandemic. BMC Med. Educ. 2024;24(1):587. doi: 10.1186/s12909-024-05541-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perotta B., Arantes-Costa F.M., Enns S.C., et al. Sleepiness, sleep deprivation, quality of life, mental symptoms and perception of academic environment in medical students. BMC Med. Educ. 2021;21(1):111. doi: 10.1186/s12909-021-02544-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premraj L., Kannapadi N.V., Briggs J., et al. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: a meta-analysis. J. Neurol. Sci. 2022;434 doi: 10.1016/j.jns.2022.120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke J., Meinhardt J., Aschman T., et al. Proteomic and transcriptomic profiling of brainstem, cerebellum and olfactory tissues in early- and late-phase COVID-19. Nat. Neurosci. 2024;27(3):409–420. doi: 10.1038/s41593-024-01573-y. [DOI] [PubMed] [Google Scholar]
- Riedel A., Benz F., Deibert P., et al. The effect of physical exercise interventions on insomnia: a systematic review and meta-analysis. Sleep Med. Rev. 2024;76 doi: 10.1016/j.smrv.2024.101948. [DOI] [PubMed] [Google Scholar]
- Sakurada Y., Otsuka Y., Tokumasu K., et al. Trends in long COVID symptoms in Japanese teenage patients. Medicina (Kaunas, Lithuania) 2023;59(2) doi: 10.3390/medicina59020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C., Wei Y., Xu M., et al. The effects of exercise on insomnia disorders: an umbrella review and network meta-analysis. Sleep Med. 2024;115:66–75. doi: 10.1016/j.sleep.2024.02.002. [DOI] [PubMed] [Google Scholar]
- Tracy M.F., Hagstrom S., Mathiason M., Wente S., Lindquist R. Emotional, mental health and physical symptom experience of patients hospitalized with COVID-19 up to 3 months post-hospitalization: a longitudinal study. J. Clin. Nurs. 2024;33(2):591–605. doi: 10.1111/jocn.16880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas I., Muench A., Grandner M.A., Irwin M.R., Perlis M.L. Insomnia symptoms predict longer COVID-19 symptom duration. Sleep Med. 2023;101:365–372. doi: 10.1016/j.sleep.2022.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weich S. Mental health after covid-19. Bmj. 2022;376 doi: 10.1136/bmj.o326. [DOI] [PubMed] [Google Scholar]
- Wesselingh R. Prevalence, pathogenesis and spectrum of neurological symptoms in COVID-19 and post-COVID-19 syndrome: a narrative review. Med. J. Aust. 2023;219(5):230–236. doi: 10.5694/mja2.52063. [DOI] [PubMed] [Google Scholar]
- Yu B.Y., Lam C.S., Tam K.Y.Y., Cheung D.S.T., Chen S.C., Yeung W.F. The role of insomnia and exercise in COVID-19 worries for psychological distress in Hong Kong Chinese: a moderated mediation model. Behav. Sleep Med. 2024;22(3):378–392. doi: 10.1080/15402002.2023.2270095. [DOI] [PubMed] [Google Scholar]
- Zhang H., Yang Y., Hao X., Qin Y., Li K. Effects of digital sleep interventions on sleep and psychological health during the COVID-19 pandemic: a systematic review and meta-analysis. Sleep Med. 2023;110:190–200. doi: 10.1016/j.sleep.2023.07.036. [DOI] [PubMed] [Google Scholar]
- Zhou H., Lu S., Chen J., et al. The landscape of cognitive function in recovered COVID-19 patients. J. Psychiatr. Res. 2020;129:98–102. doi: 10.1016/j.jpsychires.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Yu Y., Wang Y., et al. Unmasking the mental health scars of COVID-19: a longitudinal investigation of children and adolescents in post-lockdown China. Brain Behav. Immun. 2024;119:275–285. doi: 10.1016/j.bbi.2024.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. Deidentified original transcript data will be available exclusively for academic purposes. For reasonable data requests, please contact the corresponding author (fei.wang@yale.edu).
Data will be made available on request.