Abstract
Medications contribute to about a quarter of acute kidney injury (AKI) cases among patients in hospitals. The impact of AKI is substantial on both families and society, and it has become a worldwide public health concern. Recently, a new framework for drug-induced acute kidney injury (DI-AKI) classification has been proposed. According to this new framework, drugs are divided into four categories. Thus, we explain the mechanism thoroughly and give examples of drugs or drug categories linked to the classes in the new framework. Furthermore, a patient’s condition may dynamically shift between categories. At the same time, we also took into account some susceptibility factors. These susceptibility factors may drive inter-class variation. The new classification system may shed new light on the mechanism of DI-AKI for clinicians and researchers.
Keywords: Drug-induced kidney injury, mechanisms, acute kidney injury, nephrotoxicity
1. Introduction
Drug-induced kidney disease (DIKD) refers to newly developed kidney injury or exacerbation of preexisting kidney disease following drug exposure [1]. DIKD encompasses a wide spectrum of clinical phenotypes, including acute kidney injury (AKI), glomerular disease, tubular dysfunction, and nephrolithiasis/crystalluria [2]. No universally accepted criteria currently exist for defining or classifying DIKD [3], due to the heterogeneity of causative drugs, variability in clinical manifestations, overlap between phenotypes, and challenges in distinguishing DIKD from kidney injury due to other etiologies in patients with complex comorbidities or polypharmacy. DIKD is a significant contributor to AKI, acute and chronic kidney disease (CKD). Among these, AKI may account for a significant proportion.
AKI is identified by a quick rise in serum creatinine (SCr) levels of 1.5 times of the baseline, a decrease in urine output, or both, occurring within a 48-h period, according to the Kidney Disease: Improving Global Outcomes (KDIGO) criteria [4]. Around 10%–15% of patients admitted to hospitals and up to 50% of those in ICUs are affected by it [5]. The causes and mechanisms of AKI are diverse and complex, which significantly heightens the likelihood of progressing to CKD, uremia, cardiovascular disease, and increased mortality [6]. The medical and financial costs associated with AKI are substantial. AKI is a major challenge for families and society, becoming a global public health issue [7–9]. Drug-induced AKI (DI-AKI) makes up about a quarter of all AKI cases in patients admitted to hospitals [3].
Different drugs induce renal injury through various mechanisms, including pre-renal, renal, and post-renal AKI. However, this strategy neglects the intersecting and fluid nature of DI-AKI [3]. Recently, a novel framework—a 2 × 2 table—was proposed at the 23rd Acute Dialysis Quality Initiative (ADQI) conference [10]. This new framework addresses the previously neglected intersecting and fluid nature of DI-AKI. It is advisable to categorize drugs based on biomarkers into four main classifications (neither dysfunction nor damage, damage without dysfunction, dysfunction without damage, and both dysfunction and damage). Furthermore, the incidence of DI-AKI can be effectively reduced or mitigated through early intervention and prevention. Therefore, we need to pay more attention to DI-AKI. This article explores the mechanisms, biomarkers, and susceptibility factors behind common types of DI-AKI based on this new framework.
2. Mechanisms, biomarkers and susceptible factors of DI-AKI
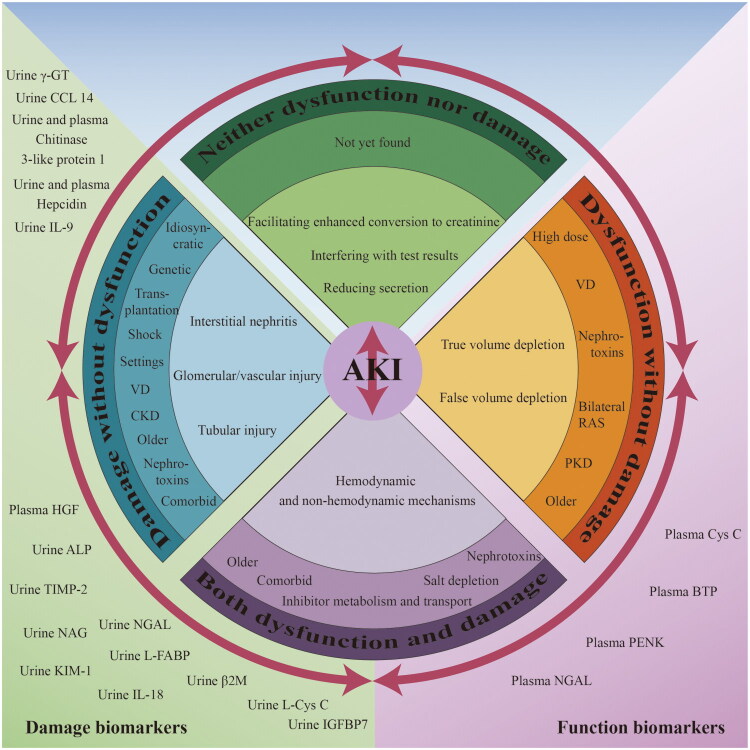
AKI is typically caused by one or more mechanisms. Additionally, certain drugs have specific mechanisms that can lead to kidney damage. The categorization of these drugs is done according to functional and damage biomarkers, along with the primary mechanisms of nephrotoxicity, as follows: neither dysfunction nor damage, damage without dysfunction, dysfunction without damage, and both dysfunction and damage (Figure 1) [10]. Moreover, transitions between different categories may occur as circumstances change. Common medications that can induce AKI can be broadly categorized into four groups.
Figure 1.
Mechanisms, risk factors and biomarkers of drug-induced kidney injury based on the new category. VD: Volume depletion; bilateral RAS: Bilateral renal artery stenosis or stenosis in a single kidney; PKD: Preexisting kidney disease; genetic: Genetic vulnerability; transplantation: Solid organ transplantation and stem cell transplantation; CKD: Chronic kidney disease; γ-GT: γ-glutamyl transpeptidase; CCL14: C-C motif chemokine ligand 14; IL-9: Interleukin-9; HGF: Hepatocyte growth factor; ALP: Alkaline phosphatase; TIMP-2: Tissue inhibitor of metalloproteinases-2; NGAL: Neutrophil gelatinase-associated lipocalin; NAG: N-acetyl-β-D-glucosaminidase; L-FABP: Liver-type fatty acid-binding protein; KIM-1: Kidney injury molecule-1; β2M: β2-microglobulin; IL-18: Interleukin-18; Cys C: Cystatin C; BTP: Beta-trace protein; PENK: Proenkephalin.
The use of drugs, both individually and in combination, should be discussed separately. For example, the concurrent use of sulfamethoxazole and trimethoprim should be distinguished from the use of trimethoprim alone. Cases of interstitial nephritis or crystalline nephropathy induced by combination use should be considered as a separate disease. This can be interpreted as a risk factor that exacerbates each other. This article mainly discusses the use of drugs alone.
2.1. Neither dysfunction nor damage
Some medicines may elevate SCr, but they do not induce renal impairment or injury (Table 1). Since alterations in SCr do not correlate with notable changes in glomerular filtration rate (GFR) or injury to the kidney’s glomeruli, tubules, or interstitial tissue. Consequently, they are classified in this category based on current guidelines and understanding of AKI diagnosis. Nevertheless, they may be considered nephrotoxic drug according to current guidelines, which identify kidney injury by SCr and urine.
Table 1.
List of classer and drugs/drug classes associated with pseudo-AKI and their possible mechanism(s).
| Classes | Mechanism(s) | Drugs/drug classes |
|---|---|---|
| Extrinsic creatinine administration or increased metabolism | Increasing the precursor of creatinine |
|
| Increasing catabolic state is associated with the release of creatine from muscle, spontaneously converted to creatinine | ||
| Some formulations of dexamethasone may contain creatinine as a buffer excipient. | ||
| Increasing the metabolic production of creatinine | ||
| Interfere with tubular secretion of creatinine | Competing with and decreasing proximal tubule creatinine secretion in a dose-dependent manner, mediated via the inhibition of drug efflux transporters such as organic cation transporter |
Dronedarone
Cimetidine
Tyrosine kinase inhibitors (e.g., imatinib, bosutinib, sorafenib, sunitinib, crizotinib, gefitinib, and pazopanib) Poly-ADP-ribose polymerase inhibitors (e.g., olaparib, niraparib talazoparib) Cyclin-dependent kinase 4/6 inhibitors (e.g., palbociclib, abemaciclib, ribociclib) Anaplastic lymphoma kinase inhibitors (e.g., crizotinib, ceritinib, alectinib, brigatinib, lorlatinib)
Probenecid |
| Interference with analytical measurement of creatinine | Interfering with the analytical measurement of creatinine (Jaffe method) |
|
| Interfering with the analytical measurement of creatinine (Ektachem enzymatic system) |
|
|
| IVIG (intravenous immune globulin) has proline stabilizer. The effect of sarcosine oxidase on proline interferes with the determination of creatinine by enzymatic method. |
|
|
| Unknown | Unknown |
|
Some medications may influence creatinine levels [11–13]. Creatine supplements, classified as nutritional supplements, provide energy to muscle and nerve cells. Creatine is stored in the muscles, which increases the formation of phosphocreatine and adenosine triphosphate (ATP). In the body, creatine is converted to creatinine. However, creatinine is often used as a key indicator for AKI diagnosis. Creatine supplementation, whether short-term or long-term, has the potential to elevate serum creatinine level [12]. The mechanism may involve an increase in creatine, facilitating enhanced conversion to creatinine through skeletal muscle and the liver [12].
Some drugs compete with and reduce proximal tubular creatinine secretion in a dose-dependent manner by inhibiting drug efflux transporters, such as organic cation transporters [11,14–16]. For instance, cimetidine is excreted into the urine by reducing creatinine secretion through proximal tubular cells [17].
Additionally, certain medications may interfere with test results, leading to pseudo-AKI [18–21]. For instance, cefoxitin may interfere with the Jaffe method for determining creatinine levels [21]. Examples relevant are shown in Table 1 [11,12,22–25].
In case of pseudo-AKI caused by these drugs, an increase in SCr does notindicate a true decline in true GFR. Therefore, it is essential to introduce novel biomarkers for assessing renal function. Cystatin C (Cys C), a type of biomarkers of renal tubular filtration, is theoretically normal in pseudo-AKI because it is not affected by muscle mass, sex, and age [3,26]. At the same time, it is evident that these drugs do not induce kidney damage, and therefore, the levels of kidney injury markers are anticipated to remain within normal ranges.
2.2. Dysfunction without damage
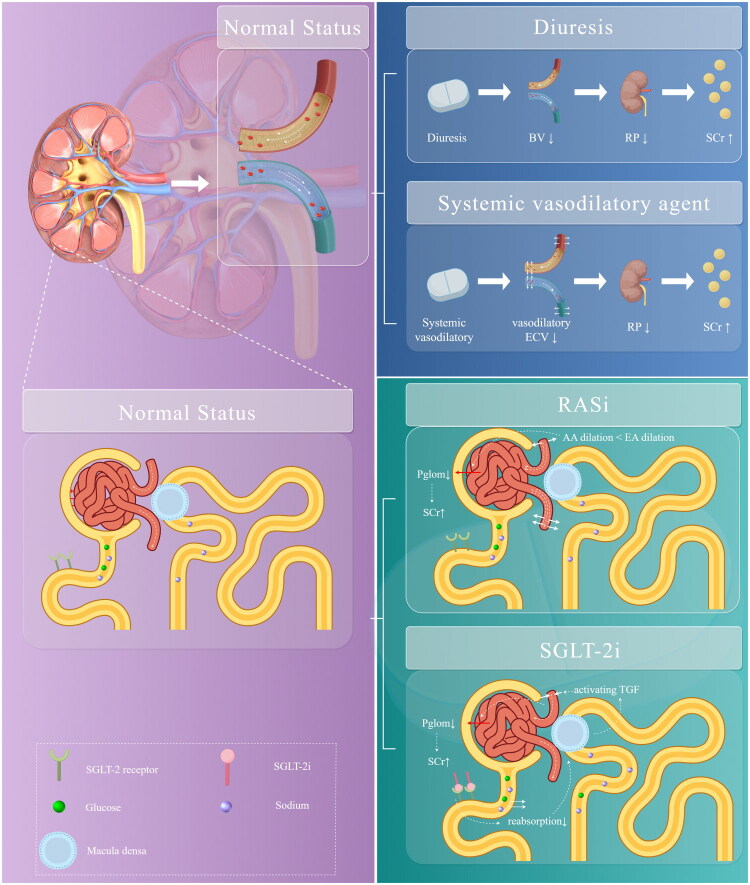
A series of medications may not directly affect glomerular or tubular damage, but they can influence kidney function. These medications are related to systemic or intraglomerular hemodynamics [27]. In the presence of impaired autoregulation, these medications can excessively constrict afferent arterioles, leading to reduced blood flow in the glomerular and peritubular capillaries. This decline impacts GFR, exacerbating tubular ischemic damage and increasing SCr levels [10,28]. However, it can treat certain diseases; on the other hand, it can lead to others. This class of drugs mainly consists of vasodilators, diuretics, sodium-dependent glucose transporter 2 inhibitor (SGLT-2i) and renin-angiotensin-aldosterone system inhibitors (RASi) (Figure 2). Additionally, volume depletion and exposure to concomitant nephrotoxins, among others, may be significantly associated with the occurrence of this type of mechanism.
Figure 2.
Medications associated with hemodynamically mediated AKI (developed by Figdraw). Diuretics may result in a decrease in effective arterial blood volume, which may lead to a decrease in renal perfusion, resulting in an increase in SCr. Systemic vasodilatory decreases the effective circulating volume and may lead to renal hypoperfusion, resulting in an increase in SCr. RASi dilates incoming and outgoing glomerular arterioles, however, has a stronger dilating effect on outflow of glomerular arterioles, lowering intraglomerular pressure and lowering GFR. SGLT-2i binds to receptors, increases glucose reabsorption, and induces vasoconstriction of afferent arterioles due to glomerular feedback. BV: blood volume; RP: renal perfusion; SCr: serum creatinine; ECV: effective circulating volume; RASi: renin-angiotensin-aldosterone system inhibitors; AA: affective arterial; EA: effective arterial; SGLT-2i: sodium-dependent glucose transporters 2 inhibitors; pglom: intraglomerular pressure; TGF: tubuloglomerular feedback.
2.2.1. True volume depletion
The use of systemic vasodilators induces to visceral vasodilation, which is crucial for reducing effective circulating volume; however, this can lead to inadequate renal perfusion [29]. Vasoactive drugs, such as norepinephrine and vasopressin, may cause excessive constriction of blood vessels throughout the body, potentially reducing renal perfusion and leading to an increase in SCr levels [30].
Diuretics may cause true volume depletion through diuresis, which further reduces renal perfusion and results in elevated SCr level [31,32]. The use of vasoactive drugs in specific clinical situations—such as fluid resuscitation and hyperdynamic sepsis—maintains renal blood flow and GFR. However, achieving optimal fluid balance and hemodynamic optimization in critically ill patients necessitates meticulous and ongoing monitoring, as well as adjustments based on individual responses to therapy, dynamic changes in the patient’s situation, and the potential inadvertent medication errors [3].
2.2.2. False volume depletion
Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs), which belong to the class of RASi, have a dilating effect on both the afferent and efferent arterioles of the glomerulus. However, their effect on the efferent arterioles is more potent, lowering intraglomerular pressure and the GFR [33]. Patients with acute volume loss due to vomiting and/or diarrhea may be especially vulnerable [34].
Interestingly, SGLT-2i have also been observed to cause elevated SCr level [35]. Its mechanism may be attributed vasoconstriction of the afferent small arteries induced by tubulo-glomerular feedback [36].
2.2.3. Diagnostic biomarkers
SCr and urine output, the classic markers of renal impairment, are often recommended by guidelines as identification criteria for the diagnosis of AKI. These conventional markers may have limitations, suggesting the need to incorporate new functional markers [26]. Drugs that cause functional damage may necessitate greater focus on alternative markers, such as CysC or proenkephalin (PENK). CysC is a cysteine protease inhibitor produced by all nucleated cells, freely filtered by the glomeruli, and subsequently reabsorbed and metabolized in the proximal renal tubules without being secreted. Because it is unaffected by muscle mass, sex, or age, CysC provides a more stable and reliable assessment of renal function, particularly in elderly patients or those with muscle-wasting conditions [37]. However, it can be affected by factors such as smoking and alcohol consumption [37]. PENK is a member of the enkephalin peptide family and is freely filtered in the glomerulus [37,38]. Furthermore, β-trace protein and Neutrophil gelatinase-associated lipocalin are used as functional markers to enhance classification accuracy [3].
The risk factors for AKI caused by true volume depletion and pseudo volume depletion primarily due to lacking of volume itself, which can manifest in different ways, such as volume depletion, bilateral renal artery stenosis, stenosis in a single kidney, and the effects of aging [39]. Additionally, underlying diseases are as well one of the risk factors [39].
2.3. Damage without dysfunction
AKI within this class is typically caused by one or more aspects of damage, including injury to the renal tubules, glomeruli, and interstitium. Some medications may lead to tubular damage either directly or indirectly, such as crystals and casts in the distal part of the tubule. Meanwhile, oxidative stress, inflammation, and mitochondrial damage play crucial roles in causing injury without immediate dysfunction [40,41]. Drugs and their metabolites can induce tubulointerstitial nephritis, which is primarily mediated by an immune response [40,42]. This damage can be classified as either intrinsic (predictable and dose-dependent) or idiosyncratic (unpredictable and dose-independent) [3]. This requires analysis on a case-by-case basis. In this drug classification, based on the primary mechanisms of renal toxicity, those categorized as ‘dysfunction without damage’, if not detected and managed early, may shift to ‘Both dysfunction and damage’. Under the influence of susceptibility factors, it is more likely to shift toward the category of ‘Both dysfunction and damage’.
2.3.1. Acute tubular injury
Acute tubular injury occurs when the proximal tubules’ role in concentrating and reabsorbing glomerular filtrate makes them susceptible to toxic damage. Various classes of drugs, including antibiotics, chemotherapeutics, and contrast agents, can lead to acute tubular injury [43]. In addition, there are drug-related injuries such as Fanconi syndrome, obstructive nephropathy, rhabdomyolysis, tumor lysis syndrome, osmotic nephrosis, nephrogenic diabetes insipidus and nephrogenic syndrome of inappropriate antidiuresis, which can occur secondary to AKI [3].
Aminoglycosides are endocytosed into proximal renal tubular cells of the S1/S2 segment via megalin-cubilin receptors [23,44]. The drug accumulates in lysosomes, leading to structural damage and myeloid formation, as well as in the Golgi apparatus and endoplasmic reticulum, leading to cellular damage. Following intracellular membrane destabilization, the drug enters the cytoplasm and promotes mitochondrial damage and consequently activates the intrinsic pathway of apoptosis/necrosis and generation of oxidative stress [45]. However, vancomycin may increase mitochondrial stress, leading to cellular stress and apoptosis [46]. Ultimately, this process contributes to AKI through acute tubular injury and necrosis (Figure 3).
Figure 3.
Mechanisms of tubular damage to the kidney caused by medications (developed by Figdraw). A detailed description of the mechanism is provided in the text. ROS: reactive oxygen species; AKI: acute kidney injury; AG: aminoglycosides; MCR: megalin-cubilin receptors; TF: tenofovir; OAT: organic anion transport proteins; MRP: multidrug resistance proteins; Ctp-B: cathepsin-B; RIPK: receptor-interacting protein kinase; TNF: tumor necrosis factor; MLKL: mixed lineage kinase domain–like protein; NLRP3: NACHT-, LRR-, and PYD-domains-containing protein-3; TLR: toll-like receptor; DAMPs: damage-associated molecular patterns; AQP: aquaporin; ENaC: epithelial sodium channel; V2R: vasopressin V2 receptor; ADH: antidiuretic hormone.
Tenofovir is actively transported into proximal tubular cells through organic anion transport proteins (hOAT 1> OAT 3) and secreted into the renal tubular lumen via multidrug resistance proteins (MRP 2 and MRP 4) [47]. The drug reduces mitochondrial DNA content by inhibiting mitochondrial DNA polymerase-γ, which result to structural changes in the mitochondria and apoptosis [48]. This process leads to proximal renal tubular injury (Figure 3), accompanied by urinary abnormalities similar observed in Fanconi’s syndrome [49].
In addition, any level of anatomical obstruction of the urinary system caused by a drug can lead to AKI (Figure 3). For example, sulfanilamide crystalline deposits that occlude the renal tubules, while atazanavir can lead to crystalluria, kidney stones, and AKI [50–52]. Crystals can activate intracellular signaling pathways, inducing necrosis and destabilizing lysosomes, leading to the release of cathepsin B [23]. This process deregulates cell death pathways, ultimately resulting in autophagy and necroptosis, thereby promoting cell necrosis [23]. Moreover, crystal-induced necrosis triggers the release of damage-associated molecular patterns (DAMPs), histones, demethylated DNA and RNA, as well as mitochondrial DNA [53,54]. These factors may further activate death receptors on neighboring cells, leading to secondary cell necrosis [53,54]. Additionally, crystal deposition exacerbates kidney injury by triggering inflammation [53,54]. This process is characterized by crystal-induced inflammation, which enhances necroinflammation through toll-like receptor-mediated signaling pathways, as well as by complement activation and leukocyte infiltration, which further damage renal tubular cells [53,54]. Furthermore, crystals activate the NLRP3 inflammasome, leading to IL-1β secretion. By binding to toll-like receptors 4 (TLR4), DAMPs activate the nuclear factor kappa-B (NF-κB) pathway, thereby promoting the transcription and expression of pro-inflammatory cytokines and chemokines [23]. Crystals may also exacerbate inflammation by inducing cell membrane lipid redistribution and activating the tyrosine-protein kinase Syk, which subsequently stimulates B cell activation [23]. Inflammatory cell death associated with crystals may occur indirectly through NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasome activation, leading to pyroptosis [23]. Additionally, TNF-α and other inflammatory cytokines contribute to crystal-induced necroptosis, further amplifying tissue injury [23].
Statins may cause rhabdomyolysis (Figure 3). Following rhabdomyolysis, myoglobin is released, which can result in kidneys injury through renal tubular obstruction, direct damage to proximal tubular epithelial cells and vasoconstriction leading to decreased blood flow to the outer medulla [55,56].
Tumor lysis syndrome (Figure 3) is an oncologic emergency that occurs due to the rapid breakdown of tumor cells, which releases large quantities of potassium, phosphate and nucleic acids into the circulation. The catabolism of nucleic acids produces uric acid, leads to hyperuricemia; this significant increase in uric acid excretion can result in the deposition of leads to uric acid in the renal tubules, as well as renal vasoconstriction, impaired autoregulation, decreased renal blood flow, oxidation, and inflammation, ultimately resulting in AKI [57]. Tumor lysis syndrome is typically caused by the initiation of cytotoxic chemotherapy [58].
The high concentration of sucrose in immunoglobulin preparations leads to osmotic damage to the renal tubules (Figure 3). In this process the sugar is absorbed by the tubular cells, resulting in increased solute load that leads to cellular vacuolization, swelling, and obstruction of the tubules [59].
After the administration of lithium (Figure 3), some patients may develop nephrogenic diabetes insipidus, and uncontrolled nephrogenic diabetes insipidus can lead to fluid and electrolyte disturbances such as hypovolemia, hypernatremia, hyperchloremic metabolic acidosis, and distal renal tubular acidosis [60].
Drugs such as carbamazepine, haloperidol, cyclophosphamide, selective serotonin reuptake inhibitors may cause Nephrogenic syndrome of Inappropriate Antidiuresis (NSIAD) (Figure 3) may lead to impaired water excretion, which is manifested as AKI with decreased urine output [61]. NSIAD is produced by intrarenal activation for water reabsorption and characterized by suppressed plasma AVP levels [61]. Its clinical manifestations include hypotonic hyponatremia, euvolemia, and concentrated urine with increased urinary sodium excretion. Hypotonic stress induces disruption of the renal tubular epithelial tight junction barrier through a dense protein-mediated pathway [62].
Exposure to multiple nephrotoxins and potentially co-morbid medical conditions may increase the likelihood of renal tubular injury. Advanced age, preexisting renal disease, and insufficient true or effective intravascular volume are important risk factors [23].
2.3.2. Acute glomerular/vascular injury
The kidney maintains or automatically regulates intraglomerular pressure by modulating afferent and efferent arteriolar tone to maintain GFR and urine output. However, it may be directly or indirectly injured by drugs (Table 2).
Table 2.
Interstitial and glomerular damage to the kidney caused by medications.
| Mechanisms | Classes | Medications |
| Acute Interstitial Nephritis | Acute tubulointerstitial nephritis |
|
| Acute Glomerular/vascular Injury | Minimal change disease |
|
| Focal segmental glomerulosclerosis |
|
|
| Membranous nephropathy |
|
|
| Vasculitis |
|
|
| Thrombotic microangiopathy |
|
|
| Cholesterol emboli |
|
Mechanisms of renal injury secondary to drug-induced thrombotic microangiopathy include immune-mediated responses or direct endothelial toxicity. Drugs most commonly associated with this toxic mechanism of nephrotoxicity include antiplatelet agents [63]. Mitomycin, cyclosporine, tacrolimus, OKT3, interferon, cocaine, indinavir, and can quinine all cause acute renal tubule injury through vascular lesions [64–66].
However, the mechanisms by which bisphosphonates are associated with nephrotoxicity are not fully understood. Some studies have hypothesized from that pamidronate-associated focal segmental glomerulosclerosis may be caused by drug-induced mitochondrial toxicity targeting visceral epithelial cells and renal tubular epithelial cells [67].
Exposures to agents such as penicillamine, anti-tumor necrosis factor agents, and immune checkpoint inhibitors have all been associated with membranous nephropathy, which causes AKI [68]. However, the mechanisms by which drugs induce membranous nephropathy remain unclear.
ANCA-associated vasculitis (AAV) is a necrotizing form of vasculitis that predominantly affects small vessels (including capillaries, venules, arterioles, and small arteries). It is characterized by the absence or minimal presence of immune deposits in the vessel walls and the presence of autoantibodies to the neutrophil protein’s leukocyte proteinase 3 or myeloperoxidase, although not all patients with AAV have a positive ANCA [69]. Numerous reports indicate that AAV may be a complication of prior exposure to certain drugs, most commonly hydralazine, propylthiouracil, and cocaine [69].
Furthermore, lithium treatment may also cause minimal change disease which is characterized by pedunculated fusions of renal epithelial cells [70]. In patients with severe atherosclerosis, the use of thrombolytic drugs may allow atherosclerotic emboli to embolize in the glomerulus, resulting in kidney injury [71].
2.3.3. Acute interstitial nephritis (AIN)
AIN is an immune-mediated renal injury characterized by the infiltration of immune cells into tubular epithelial cells [72] (Table 2). It occurs on an allergic basis in an idiosyncratic and non-dose-dependent manner. The onset of illness can last several weeks following the first exposure and 3 to 5 days after the second exposure [33]. AIN has been observed in approximately 15% of biopsies assessed as AKI [72–74]. However, these figures may underestimate the true incidence of AIN. Many patients, particularly those in the ICU, are often incorrectly presumed to have tubular injury because they lack features suggesting an allergic response and are not biopsied [23]. Drugs are the most common cause of AIN, estimated to account for over 70% of AIN cases observed in high-income countries [72]. AIN is associated with antibiotics (beta-lactams, quinolones, especially ciprofloxacin, rifampicin, macrolides, sulfonamides, tetracycline), diuretics (thiazides, loop diuretics, and amphenopteridine), anticonvulsants (phenytoin), cimetidine, ranitidine, allopurinol, antiviral drugs (acyclovir, indinavir), and cocaine [23].
Neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), N-acetyl-β-D-glucosaminidase (NAG), and liver-type fatty acid-binding protein (L-FABP) are emerging as valuable biomarkers for assessing kidney damage. These markers can provide critical insights into the specific location of injury within the kidney, aiding in more accurate diagnosis and management of renal conditions [26]. The release of biomarkers of kidney injury is usually more rapid than that of elevated SCr, which may require a certain amount of time for kidney damage to begin. At the same time, proteinuria and albuminuria have some performance in detecting drug-induced glomerular and/or tubular injury (e.g., cisplatin) [75]. The phenomenon of elevated biomarkers of injury without an increase in SCr is referred to as ‘subclinical AKI’. Recognition of subclinical AKI may help reduce the rate of missed diagnoses of renal injury.
2.4. Both dysfunction and damage
This category includes drugs associated with both hemodynamic and non-hemodynamic mechanisms of AKI [10]. The non-hemodynamic sites of injury in AKI include the glomeruli, tubules, and interstitium, which may be injured partially or entirely simultaneously. Notably, a drug may induce dysfunction through and damage through difference mechanism, or both may arise through the same mechanism [3]. Nevertheless, the progression of dysfunction and damage must be assessed on a case-by-case basis.
The typical drugs in this category include nonsteroidal anti-inflammatory drugs (NSAIDs). These drugs can reduce renal blood flow and glomerular pressure by constricting the afferent arterioles. In addition, they may cause acute tubular injury, AIN, glomerular injury (including minimal degenerative disease or membranous glomerulonephritis), and renal papillary necrosis [2,76]. Calcineurin inhibitors represent another typical class of drugs whose nephrotoxicity is associated with afferent arteriole vasoconstriction, resulting in decreased renal blood flow and glomerular pressure. They can also lead to thrombotic microangiopathy, as well as focal segmental glomerulosclerosis and chronic tubular interstitial nephritis or fibrosis [3,77].
Risk factors for this condition include advanced age, concomitant nephrotoxic exposure, high dosages, comorbidities (including CKD, liver disease with ascites, congestive heart failure, and volume depletion), as well as potential drug interactions [39].
AKI in this classification may be elevated in both functional and impairment markers. Biomarkers may deserve more attention in prediction, diagnosis, and prognosis.
3. Movement between categories
The new classification focuses on DI-AKI based on the predominant mechanism of injury or functional impairment in an individual. However, the different categories are not fixed and may shift from one category to another as events progress or resolve. This classification comprehensively integrates the traditional prerenal/renal/postrenal classification system. Specific factors such as insufficient blood volume, advanced age, underlying kidney disease, hypertension, and diabetes may delay or accelerate transitions between classes [10]. For example, treatment with ACEIs or ARBs can reduce glomerular pressure, leading to decreased blood volume associated with dehydration and resulting in insufficient renal blood flow, which can precipitate AKI. Additionally, diabetes appears to increase the risk of nephrotoxicity induced by aminoglycosides, NSAIDs, and ACEIs [78–80].
Interestingly, the classification operates bidirectionally, shifting in either direction as the specific situation changes. For example, the use of ACEIs or ARBs in the context of dehydration may cause DI-AKD to progress from the ‘dysfunction without damage’ category to the ‘both dysfunction and damage’ category. However, when hydration is administered, this trend may be reversed.
The pathogenesis and severity of DI-AKD are typically influenced by multiple factors, including the combined effects of nephrotoxic drugs, risk factors, and protective factors. For instance, in the case of drugs categorized as ‘damage without dysfunction,’ the complete spectrum and various aspects of nephrotoxicity may not be fully observed unless other drug-related and non-drug-related risk factors are present. Recent studies have found that among patients adjudicated with DI-AKI, there is a lower rate of cardiac surgery, an increased vascular volume, and a higher incidence of hyperglycemia [81]. Regarding NSAIDs, factors such as age over 60 years, true blood volume deficit, effective arterial blood volume deficit, and the concurrent use of renal hemodynamic-altering medications increase the risk of AKI [82,83]. The presence or absence of concomitant risk factors may significantly influence the determination and classification of DI-AKI.
4. Conclusions
We integrated dysfunction and risk factors into the assessment of DI-AKI within a novel and modern classification framework. This system aligns DI-AKI with contemporary AKI classifications, emphasizing mechanisms of injury while advancing the field through the incorporation of risk factors. Compared with older classifications—which predominantly focused on anatomical sites of injury (e.g., glomerular, tubular or interstitial damage) or specific histopathological changes—our approach offers a more dynamic and clinically relevant perspective. It facilitates risk stratification, guides therapeutic decision-making, and supports personalized patient care. Importantly, understanding the underlying mechanisms helps clinicians explain to patients and caregivers why certain medications should be continued despite a declining GFR and why others can be safely reintroduced even when AKI persists. We believe this classification will aid in improving diagnostic accuracy and optimizing drug management strategies in patients at risk for or experiencing DI-AKI.
Acknowledgements
Figdraw (www.figdraw.com) was acknowledged since the main images and elements used to draw the figures were downloaded from this website.
Funding Statement
This study was supported by the General Project Funds from the Health Department of Zhejiang Province [2022KY063], Zhejiang Yangtze River Delta Health Research Fund Project [2022CSJ-A003], and the Outstanding Research Initiation Fund within the Institute [C-2022-YYQD26].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
No data was used for the research described in the article.
References
- 1.Usui J, Yamagata K, Imai E, et al. Clinical practice guideline for drug-induced kidney injury in Japan 2016: digest version. Clin Exp Nephrol. 2016;20(6):827–831. doi: 10.1007/s10157-016-1334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta RL, Awdishu L, Davenport A, et al. Phenotype standardization for drug-induced kidney disease. Kidney Int. 2015;88(2):226–234. doi: 10.1038/ki.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karimzadeh I, Barreto EF, Kellum JA, et al. Moving toward a contemporary classification of drug-induced kidney disease. Crit Care. 2023;27(1):435. doi: 10.1186/s13054-023-04720-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):C179–C184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 5.Ronco C, Bellomo R, Kellum JA.. Acute kidney injury. Lancet. 2019;394(10212):1949–1964. doi: 10.1016/S0140-6736(19)32563-2. [DOI] [PubMed] [Google Scholar]
- 6.See EJ, Jayasinghe K, Glassford N, et al. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. 2019;95(1):160–172. doi: 10.1016/j.kint.2018.08.036. [DOI] [PubMed] [Google Scholar]
- 7.Monard C, Rimmelé T, Blanc E, et al. Economic burden of in-hospital AKI: a one-year analysis of the nationwide French hospital discharge database. BMC Nephrol. 2023;24(1):343. doi: 10.1186/s12882-023-03396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang J, Xu D, Yang L.. Acute kidney injury in Asia: disease burden. Semin Nephrol. 2020;40(5):443–455. doi: 10.1016/j.semnephrol.2020.08.001. [DOI] [PubMed] [Google Scholar]
- 9.James MT, Bhatt M, Pannu N, et al. Long-term outcomes of acute kidney injury and strategies for improved care. Nat Rev Nephrol. 2020;16(4):193–205. doi: 10.1038/s41581-019-0247-z. [DOI] [PubMed] [Google Scholar]
- 10.Ostermann M, Bellomo R, Burdmann EA, et al. Controversies in acute kidney injury: conclusions from a kidney disease: improving global outcomes (KDIGO) conference. Kidney Int. 2020;98(2):294–309. doi: 10.1016/j.kint.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inker L, MSRonald D. Drugs that elevate the serum creatinine concentration. UpToDate; 2023. https://sso.uptodate.com/contents/drugs-that-elevate-the-serum-creatinine-concentration?search=Drugs%20that%20elevate%20the%20serum%20creatinine%20concentration&source=search_result&selectedTitle=1∼150&usage_type=default&display_rank=1;%202023.
- 12.Davani-Davari D, Karimzadeh I, Ezzatzadegan-Jahromi S, et al. Potential adverse effects of creatine supplement on the kidney in athletes and bodybuilders. Iran J Kidney Dis. 2018;12(5):253–260. [PubMed] [Google Scholar]
- 13.Payne RB. Creatinine clearance: a redundant clinical investigation. Ann Clin Biochem. 1986;23(Pt 3):243–250. doi: 10.1177/000456328602300304. [DOI] [PubMed] [Google Scholar]
- 14.Rocci ML, Vlasses PH, Ferguson RK.. Creatinine serum concentrations and H2-receptor antagonists. Clin Nephrol. 1984;22(4):214–215. [PubMed] [Google Scholar]
- 15.Berg KJ, Gjellestad A, Nordby G, et al. Renal effects of trimethoprim in ciclosporin- and azathioprine-treated kidney-allografted patients. Nephron. 1989;53(3):218–222. doi: 10.1159/000185747. [DOI] [PubMed] [Google Scholar]
- 16.Duncker D, Oswald H, Gardiwal A, et al. Stable cystatin C serum levels confirm normal renal function in patients with dronedarone-associated increase in serum creatinine. J Cardiovasc Pharmacol Ther. 2013;18(2):109–112. doi: 10.1177/1074248412453873. [DOI] [PubMed] [Google Scholar]
- 17.Urakami Y, Kimura N, Okuda M, et al. Creatinine transport by basolateral organic cation transporter hOCT2 in the human kidney. Pharm Res. 2004;21(6):976–981. doi: 10.1023/b:pham.0000029286.45788.ad. [DOI] [PubMed] [Google Scholar]
- 18.Smith CH, Landt M, Steelman M, et al. The Kodak Ektachem 400 Analyzer evaluated for automated enzymic determination of plasma creatinine. Clin Chem. 1983;29(7):1422–1425. [PubMed] [Google Scholar]
- 19.Noble MA, Harper B, Grant AG, et al. Rapid determination of 5-fluorocytosine levels in blood. J Clin Microbiol. 1984;20(5):996–997. doi: 10.1128/jcm.20.5.996-997.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrington D, Drusano GL, Smalls U, et al. False elevation in serum creatinine levels. JAMA. 1984;252(21):2962–2962. [PubMed] [Google Scholar]
- 21.Saah AJ, Koch TR, Drusano GL.. Cefoxitin falsely elevates creatinine levels. JAMA. 1982;247(2):205–206. [PubMed] [Google Scholar]
- 22.Ostermann M, Joannidis M.. Acute kidney injury 2016: diagnosis and diagnostic workup. Crit Care. 2016;20(1):299. doi: 10.1186/s13054-016-1478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perazella MA, Rosner MH.. Drug-induced acute kidney injury. Clin J Am Soc Nephrol. 2022;17(8):1220–1233. doi: 10.2215/CJN.11290821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta S, Gudsoorkar P, Jhaveri KD.. Acute kidney injury in critically ill patients with cancer. Clin J Am Soc Nephrol. 2022;17(9):1385–1398. doi: 10.2215/CJN.15681221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nolin TD, Perazella M.. Drug-induced kidney disease. Pharmacotherapy, a pathophysiologic approach. 12th ed. New York: Mc Graw Hill companies Inc; 2023. pp. 795–810. [Google Scholar]
- 26.Ostermann M, Legrand M, Meersch M, et al. Biomarkers in acute kidney injury. Ann Intensive Care. 2024;14(1):145. doi: 10.1186/s13613-024-01360-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mark DO, Daphne K. MEHP. Etiology and diagnosis of prerenal disease and acute tubular necrosis in acute kidney injury in adults; 2024. [Google Scholar]
- 28.Juncos LA, Wieruszewski PM, Kashani K.. Pathophysiology of acute kidney injury in critical illness: a narrative review. Compr Physiol. 2022;12(4):3767–3780. doi: 10.1002/cphy.c210028. [DOI] [PubMed] [Google Scholar]
- 29.Rangaswami J, Bhalla V, Blair JEA, et al. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2019;139(16):e840–e878. doi: 10.1161/CIR.0000000000000664. [DOI] [PubMed] [Google Scholar]
- 30.Thiele RH, Isbell JM, Rosner MH.. AKI associated with cardiac surgery. Clin J Am Soc Nephrol. 2015;10(3):500–514. doi: 10.2215/CJN.07830814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coleman AJ, Arias M, Carter NW, et al. The mechanism of salt wastage in chronic renal disease. J Clin Invest. 1966;45(7):1116–1125. doi: 10.1172/JCI105418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Danovitch GM, Bourgoignie J, Bricker NS.. Reversibility of the “salt-losing” tendency of chronic renal failure. N Engl J Med. 1977;296(1):14–19. doi: 10.1056/NEJM197701062960104. [DOI] [PubMed] [Google Scholar]
- 33.Schetz M, Dasta J, Goldstein S, et al. Drug-induced acute kidney injury. Curr Opin Crit Care. 2005;11(6):555–565. doi: 10.1097/01.ccx.0000184300.68383.95. [DOI] [PubMed] [Google Scholar]
- 34.Stirling C, Houston J, Robertson S, et al. Diarrhoea, vomiting and ACE inhibitors:–an important cause of acute renal failure. J Hum Hypertens. 2003;17(6):419–423. doi: 10.1038/sj.jhh.1001571. [DOI] [PubMed] [Google Scholar]
- 35.Phadke G, Kaushal A, Tolan DR, et al. Osmotic nephrosis and acute kidney injury associated with SGLT2 inhibitor use: a case report. Am J Kidney Dis. 2020;76(1):144–147. doi: 10.1053/j.ajkd.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Copur S, Yildiz A, Basile C, et al. Is there any robust evidence showing that SGLT2 inhibitor use predisposes to acute kidney injury? J Nephrol. 2023;36(1):31–43. doi: 10.1007/s40620-022-01422-w. [DOI] [PubMed] [Google Scholar]
- 37.Yang H, Chen Y, He J, et al. Advances in the diagnosis of early biomarkers for acute kidney injury: a literature review. BMC Nephrol. 2025;26(1):115. doi: 10.1186/s12882-025-04040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khorashadi M, Beunders R, Pickkers P, et al. Proenkephalin: a new biomarker for glomerular filtration rate and acute kidney injury. Nephron. 2020;144(12):655–661. doi: 10.1159/000509352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joseph TD, Gary CY, Stuart TH, et al. DiPiro’s pharmacotherapy: a pathophysiologic approach. New York: McGraw Hill LLC; 2023:699–715. [Google Scholar]
- 40.Kwiatkowska E, Domański L, Dziedziejko V, et al. The mechanism of drug nephrotoxicity and the methods for preventing kidney damage. Int J Mol Sci. 2021;22(11):6109. doi: 10.3390/ijms22116109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barnett LMA, Cummings BS.. Nephrotoxicity and renal pathophysiology: a contemporary perspective. Toxicol Sci. 2018;164(2):379–390. doi: 10.1093/toxsci/kfy159. [DOI] [PubMed] [Google Scholar]
- 42.Kan WC, Chen YC, Wu VC, et al. Vancomycin-associated acute kidney injury: a narrative review from pathophysiology to clinical application. Int J Mol Sci. 2022;23(4):2052. doi: 10.3390/ijms23042052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perazella MA. Pharmacology behind common drug nephrotoxicities. Clin J Am Soc Nephrol. 2018;13(12):1897–1908. doi: 10.2215/CJN.00150118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olbricht CJ, Fink M, Gutjahr E.. Alterations in lysosomal enzymes of the proximal tubule in gentamicin nephrotoxicity. Kidney Int. 1991;39(4):639–646. doi: 10.1038/ki.1991.76. [DOI] [PubMed] [Google Scholar]
- 45.Lopez-Novoa JM, Quiros Y, Vicente L, et al. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int. 2011;79(1):33–45. doi: 10.1038/ki.2010.337. [DOI] [PubMed] [Google Scholar]
- 46.Filippone EJ, Kraft WK, Farber JL.. The nephrotoxicity of vancomycin. Clin Pharmacol Ther. 2017;102(3):459–469. doi: 10.1002/cpt.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ray AS, Cihlar T, Robinson KL, et al. Mechanism of active renal tubular efflux of tenofovir. Antimicrob Agents Chemother. 2006;50(10):3297–3304. doi: 10.1128/AAC.00251-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewis W, Day BJ, Copeland WC.. Mitochondrial toxicity of NRTI antiviral drugs: an integrated cellular perspective. Nat Rev Drug Discov. 2003;2(10):812–822. doi: 10.1038/nrd1201. [DOI] [PubMed] [Google Scholar]
- 49.Joshi M, Clark B, Lee TA.. Fanconi syndrome in patients with human immunodeficiency virus treated with tenofovir-based antiretroviral therapy: a systematic literature review. Ann Pharmacother. 2024;58(8):857–869. doi: 10.1177/10600280231206703. [DOI] [PubMed] [Google Scholar]
- 50.Hein R, Brunkhorst R, Thon WF, et al. Symptomatic sulfadiazine crystalluria in AIDS patients: a report of two cases. Clin Nephrol. 1993;39(5):254–256. [PubMed] [Google Scholar]
- 51.Couzigou C, Daudon M, Meynard JL, et al. Urolithiasis in HIV-positive patients treated with atazanavir. Clin Infect Dis. 2007;45(8):e105-8–e108. doi: 10.1086/521930. [DOI] [PubMed] [Google Scholar]
- 52.Brewster UC, Perazella MA.. Acute interstitial nephritis associated with atazanavir, a new protease inhibitor. Am J Kidney Dis. 2004;44(5):e81–e84. doi: 10.1016/S0272-6386(04)01093-5. [DOI] [PubMed] [Google Scholar]
- 53.Mulay SR, Shi C, Ma X, et al. Novel insights into crystal-induced kidney injury. Kidney Dis. 2018;4(2):49–57. doi: 10.1159/000487671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mulay SR, Evan A, Anders HJ.. Molecular mechanisms of crystal-related kidney inflammation and injury. Implications for cholesterol embolism, crystalline nephropathies and kidney stone disease. Nephrol Dial Transplant. 2014;29(3):507–514. doi: 10.1093/ndt/gft248. [DOI] [PubMed] [Google Scholar]
- 55.Zager RA. Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int. 1996;49(2):314–326. doi: 10.1038/ki.1996.48. [DOI] [PubMed] [Google Scholar]
- 56.Heyman SN, Rosen S, Fuchs S, et al. Myoglobinuric acute renal failure in the rat: a role for medullary hypoperfusion, hypoxia, and tubular obstruction. J Am Soc Nephrol. 1996;7(7):1066–1074. doi: 10.1681/ASN.V771066. [DOI] [PubMed] [Google Scholar]
- 57.Richard AL, Ching-Hon P.. Tumor lysis syndrome: pathogenesis, clinical manifestations, definition, etiology and risk factors. UpToDate. https://sso.uptodate.com/contents/tumor-lysis-syndrome-pathogenesis-clinical-manifestations-definition-etiology-and-risk-factors?search=Tumor%20lysis%20syndrome%3A%20pathogenesis%2C%20clinical%20manifestations%2C%20definition%2C%20etiology%20and%20risk%20factors&source=search_result&selectedTitle=1∼106&usage_type=default&display_rank=1;%202024.
- 58.Adeyinka A, Kaur A, Bashir K.. Tumor lysis syndrome. Treasure Island: StatPearls; 2024. [PubMed] [Google Scholar]
- 59.Ahsan N, Palmer BF, Wheeler D, et al. Intravenous immunoglobulin-induced osmotic nephrosis. Arch Intern Med. 1994;154(17):1985–1987. doi: 10.1001/archinte.154.17.1985. [DOI] [PubMed] [Google Scholar]
- 60.Gong R, Wang P, Dworkin L.. What we need to know about the effect of lithium on the kidney. Am J Physiol Renal Physiol. 2016;311(6):F1168–F1171. doi: 10.1152/ajprenal.00145.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim GH. Pathophysiology of drug-induced hyponatremia. J Clin Med. 2022;11(19):5810. doi: 10.3390/jcm11195810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fujii N, Matsuo Y, Matsunaga T, et al. Hypotonic stress-induced down-regulation of Claudin-1 and -2 mediated by dephosphorylation and clathrin-dependent endocytosis in renal tubular epithelial cells. J Biol Chem. 2016;291(47):24787–24799. doi: 10.1074/jbc.M116.728196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Naughton CA. Drug-induced nephrotoxicity. Am Fam Physician. 2008;78(6):743–750. [PubMed] [Google Scholar]
- 64.Pisoni R, Ruggenenti P, Remuzzi G.. Drug-induced thrombotic microangiopathy: incidence, prevention and management. Drug Saf. 2001;24(7):491–501. doi: 10.2165/00002018-200124070-00002. [DOI] [PubMed] [Google Scholar]
- 65.Medina PJ, Sipols JM, George JN.. Drug-associated thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Curr Opin Hematol. 2001;8(5):286–293. doi: 10.1097/00062752-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 66.Dlott JS, Danielson CF, Blue-Hnidy DE, et al. Drug-induced thrombotic thrombocytopenic purpura/hemolytic uremic syndrome: a concise review. Ther Apher Dial. 2004;8(2):102–111. doi: 10.1111/j.1526-0968.2003.00127.x. [DOI] [PubMed] [Google Scholar]
- 67.Sauter M, Jülg B, Porubsky S, et al. Nephrotic-range proteinuria following pamidronate therapy in a patient with metastatic breast cancer: mitochondrial toxicity as a pathogenetic concept? Am J Kidney Dis. 2006;47(6):1075–1080. doi: 10.1053/j.ajkd.2006.02.189. [DOI] [PubMed] [Google Scholar]
- 68.Laurence HB, David JS.. Membranous nephropathy: pathogenesis and etiology. http://114.255.80.174:8084/contents/membranous-nephropathy-pathogenesis-and-etiology.
- 69.Yaseen K, Nevares A, Tamaki H.. A spotlight on drug-induced vasculitis. Curr Rheumatol Rep. 2022;24(11):323–336. doi: 10.1007/s11926-022-01088-0. [DOI] [PubMed] [Google Scholar]
- 70.Wood IK, Parmelee DX, Foreman JW.. Lithium-induced nephrotic syndrome. Am J Psychiatry. 1989;146(1):84–87. doi: 10.1176/ajp.146.1.84. [DOI] [PubMed] [Google Scholar]
- 71.Munawar T, Ibe U, Jiwa N, et al. Renal cholesterol crystal embolism in the setting of warfarin use. BMJ Case Rep. 2019;12(8):e230314. doi: 10.1136/bcr-2019-230314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perazella MA, Markowitz GS.. Drug-induced acute interstitial nephritis. Nat Rev Nephrol. 2010;6(8):461–470. doi: 10.1038/nrneph.2010.71. [DOI] [PubMed] [Google Scholar]
- 73.Muriithi AK, Nasr SH, Leung N.. Utility of urine eosinophils in the diagnosis of acute interstitial nephritis. Clin J Am Soc Nephrol. 2013;8(11):1857–1862. doi: 10.2215/CJN.01330213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gesualdo L, Di Palma AM, Morrone LF, et al. The Italian experience of the national registry of renal biopsies. Kidney Int. 2004;66(3):890–894. doi: 10.1111/j.1523-1755.2004.00831.x. [DOI] [PubMed] [Google Scholar]
- 75.Nickolas TL, Barasch J, Devarajan P.. Biomarkers in acute and chronic kidney disease. Curr Opin Nephrol Hypertens. 2008;17(2):127–132. doi: 10.1097/MNH.0b013e3282f4e525. [DOI] [PubMed] [Google Scholar]
- 76.Parsels KA, Seabury RW, Darko W, et al. Recurrent renal dysfunction secondary to probable piperacillin-tazobactam-induced acute interstitial nephritis. Ann Pharmacother. 2021;55(1):133–134. doi: 10.1177/1060028020936778. [DOI] [PubMed] [Google Scholar]
- 77.Farouk SS, Rein JL.. The many faces of calcineurin inhibitor toxicity-what the FK? Adv Chronic Kidney Dis. 2020;27(1):56–66. doi: 10.1053/j.ackd.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Knight EL, Glynn RJ, McIntyre KM, et al. Predictors of decreased renal function in patients with heart failure during angiotensin-converting enzyme inhibitor therapy: results from the studies of left ventricular dysfunction (SOLVD). Am Heart J. 1999;138(5 Pt 1):849–855. doi: 10.1016/s0002-8703(99)70009-8. [DOI] [PubMed] [Google Scholar]
- 79.Baciewicz AM, Sokos DR, Cowan RI.. Aminoglycoside-associated nephrotoxicity in the elderly. Ann Pharmacother. 2003;37(2):182–186. doi: 10.1177/106002800303700203. [DOI] [PubMed] [Google Scholar]
- 80.Blackshear JL, Davidman M, Stillman MT.. Identification of risk for renal insufficiency from nonsteroidal anti-inflammatory drugs. Arch Intern Med. 1983;143(6):1130–1134. [PubMed] [Google Scholar]
- 81.Yousif ZK, Koola JD, Macedo E, et al. Clinical characteristics and outcomes of drug-induced acute kidney injury cases. Kidney Int Rep. 2023;8(11):2333–2344. doi: 10.1016/j.ekir.2023.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Parikh CR, Coca SG.. “Permissive AKI” with treatment of heart failure. Kidney Int. 2019;96(5):1066–1068. doi: 10.1016/j.kint.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 83.Randy L, Mark AP.. NSAIDs: acute kidney injury. UpToDate; 2023. https://sso.uptodate.com/contents/nsaids-acute-kidney-injury?search=NSAIDs%3A%20acute%20kidney%20injury&source=search_result&selectedTitle=1∼150&usage_type=default&display_rank=1;%202024. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.