Abstract
Background
The aim of this study was to investigate the effects of ozone treatment on the color stability and surface roughness of three different restorative materials aged with various beverages.
Methods
The restorative materials used in this study were alkasite (Cention N-CN), polyacid-modified composite resin (compomer) and resin modified glass ionomer cement (RMGIC), and the aging solutions were orange juice, chocolate milk, cola and control group (distilled water). In this study, 24 groups were determined with n = 10. Vita Easyshade Advance was used for color change measurement and Marsurf M300 profilometer was used for surface roughness measurement on all samples. Half of the groups were applied with ozonated water with Ozonette Dent ozone generator and the other half was not applied. After the ozone procedure, the samples were immersed in the solutions and this process was repeated every day. Color stability and surface roughness measurements were made on days 0, 1, 7 and 14. IBM SPSS V23 programme was used for statistical analyses. Statistically p < 0.05 was considered statistically significant.
Results
A significant difference was obtained between the 7th day values of the compomer material immersed in orange juice in color change according to ozone application (p = 0.029). There was no statistically significant difference in ∆Ra between ozonated and non-ozonated groups after 14 days.
Conclusions
The color change of the compomer material immersed in orange juice on the 7th day of ozonated water application was higher than the compomer material without ozonated water application. CN showed the most color change in distilled water, compomer in chocolate milk and RMGIC in cola. CN showed the most color change on the 7th day, while compomer and RMGIC showed the most color change on the 14th day. Ozone treatment had no significant effect on the surface roughness of the restorative materials.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12903-025-06585-z.
Keywords: Ozone, Color, Surface properties, Pedodontics, Dental materials
Background
In pediatric dentistry, many factors such as fluoride release, esthetics, ease of application and physical stability are taken into consideration when selecting restorative materials. Compomer and resin modified glass ionomer cements (RMGIC) are considered to be ideal restorative materials for use in children [1]. The only example of an alkasite material, which is a subclass of composites, is Cention N [2]. Cention N releases fluoride, calcium and hydroxide ions and is radiopaque. It can be used as a bulk fill material thanks to its dual-cure option [3].
Color stability is a fundamental esthetic requirement for restorations. Color changes can lead to patient dissatisfaction and require premature restoration replacement, which increases treatment costs and time [4]. Resin-based restorative materials may deteriorate in color stability when exposed to low-pH beverages. Surface roughness is another critical factor affecting the clinical performance of restorations. A smooth surface not only contributes to esthetic appearance but also minimizes plaque accumulation, reducing the risk of periodontal disease and secondary caries [5]. The oral environment, characterized by fluctuating pH levels, changing temperatures, and mechanical abrasion from chewing and brushing, can significantly affect the surface texture of restorative materials [6]. The effect of beverages consumed by children on the color stability and surface roughness of restorative materials has been frequently emphasized in the literature [7–9].
Ozone (O3) is a triatomic molecule consisting of three oxygen atoms [10]. Due to its antimicrobial, virucidal, disinfectant and biocompatible properties, ozone has long been recommended for therapeutic use in general and pediatric dentistry [11]. Ozone has areas of use in general dentistry such as endodontic treatment, prosthetic dental treatment, tooth whitening, orthodontic treatment, periodontics, jaw surgery, and restorative dentistry [12]. In pedodontics, it has areas of use such as deep dental caries, early childhood caries management, pulpectomy, wound healing, initial lesions and remineralization, dental anxiety, and traumatic dental injuries [13, 14]. Ozone also has anti-inflammatory, analgesic, and immunostimulating properties and supports tissue regeneration [15].
Recent studies have shown that ozone application as a prophylactic antimicrobial treatment before the application of restoration is beneficial and that ozone can be an alternative to traditional prophylactic treatments [14]. However, this prophylactic approach aims to prevent caries formation by utilizing the antimicrobial effect of ozone [16]. In the literature, it has been observed that the effect of the prophylactic use of ozonated water gargle on color change and surface roughness on resin-containing dental materials has not been investigated. The aim of this study is to investigate the effect of the use of ozonated water gargle on color stability and surface roughness of resin-containing materials used in pedodontics. The null hypothesis of this study is that the application of ozonated water does not have effect on the color stability and surface roughness of restorative materials aged in different solutions.
Method
Sample size calculation
The main hypotheses of the study were planned to investigate the differences between three or more independent groups. In this study, the sample size was calculated at a 95% confidence level using the “G. Power-3.1.9.2” program. As a result of the analysis, α = 0.05, standardized effect size was obtained as 0.9158 from a similar study conducted previously, and the minimum sample size for each group was calculated as 6 with a theoretical power of 0.95. Considering possible data losses, n = 10 was accepted [9].
Sample group
This study was conducted at the Department of Pedodontics, Faculty of Dentistry, Bolu Abant İzzet Baysal University. In the study, a total of 240 disc-shaped samples were prepared from three different restorative materials, namely alkasite (Cention N, İvoclar Vivadent AG FL-9494 schaan/Liechtenstein), compomer, (Glasiosite, VOCO GmbH, Cuxhaven, Germany) and RMGIC (İonolux, VOCO GmbH, Cuxhaven, Germany), with a width of 6 mm and a thickness of 2 mm (Table 1). The 240 samples were divided into three main groups according to the restorative material used (n = 80): alkasite, compomer and resin modified glass ionomer cement. Each group was divided into four subgroups (n = 20) according to the aging solution used: Orange juice (Cappy Orange Juice, Turkey), chocolate milk (Pınar Kido Cacao Milk), cola (Coca Cola Classic, Turkey) and distilled water (n = 20) (Table 2). Half of the groups were ozonated while the other half were left without ozonation (n = 10).
Table 1.
Manufacturer companies and content information of restorative materials used in the study
| Material | Brand | Manufacturer | Content | Filler |
|---|---|---|---|---|
| Alkasite |
Cention N A2 Lot: ZP5PM2 |
Ivoclar Vivadent AG FL-9494 schaan/Liechtenstein | Liquid: UDMA, DCP, tetramethyl-xylene diurethane dimethacrylate, PEGDMA 400, ivocerin, hydroxypiperoxide. Powder: Barium aluminum silicate glass, ytterbium trifluoride, isophylls, calcium barium aluminum, Inorganic fillers 78.4% fluorosilicate glass, calcium fluorosilicate glass | 0.1–7 μm |
| Compomer |
Glasiosite A3 Lot:2,345,478 |
VOCO GmbH, Cuxhaven, Germany | BIS-GMA, Diurethane-dimethylacrylate, TEGDMA and BHT (2,6-bis(1,1-dimethylethyl)-4-methylpheno), Inorganic fillers 77.5 | 3 μm |
| Resin Modified Glass Ionomer Cement |
Ionolux A3 Lot:2,345,091 |
VOCO GmbH, Cuxhaven, Germany | Bis-GMA, polyacrylic acid (2.5–10%), UDMA (2.5–10%), HEMA (2.5–10%), fluoro-alumino-silicate glass |
Table 2.
Beverage groups used in the study
| Beverage | Brand and manufacturer information | Composition | Storage conditions | Purchase date | pH |
|---|---|---|---|---|---|
| Orange juice | Cappy, Coca-Cola İçecek A.Ş., Istanbul, Turkey | Water, sugar or fructose-glucose syrup, orange pulp (5%), orange juice concentrate, acidity regulators (citric acid, sodium citrate), thickeners (acacia gum, glycerol esters of wood resin), flavourings, antioxidant (ascorbic acid), colour (beta-carotene). | Store in a cool, dry place; refrigerate after opening and consume within 1–2 days. | 17.01.2024 | 3.62–3.86 |
| Chocolate milk | Pınar Kido, Pınar Süt Mamülleri San. A.Ş., Izmir, Turkey | Pasteurized cow’s milk (1.3% fat), sugar, cocoa (1.1%), thickeners (microcrystalline cellulose, carrageenan), vanilla flavouring. | Store at room temperature; refrigerate after opening. | 17.01.2024 | 6.47–6.9 |
| Cola | Coca-Cola Classic, Coca-Cola İçecek A.Ş., Istanbul, Turkey | Water, sugar, carbon dioxide, colour (caramel), acidity regulator (phosphoric acid), natural flavourings, caffeine (max. 0.150 g/L). | Store in a cool, dry place (optimal: 5–20 °C); avoid direct sunlight. Refrigerate after opening; consume within 24 h if bottled. | 17.01.2024 | 2.47–2.55 |
Preparation of samples
A total of 240 disc-shaped specimens were prepared using a metal mold (6 mm wide, 2 mm thick). Before the restorative materials were placed in the mold, vaseline was applied to the mold with an applicator to make the disc easy to remove. The compomer group was placed in the mold with the help of a compomer gun after placing a glass plate and mylar strip under the mold and leveled with a mouth spatula. The CN group was mixed with a 1–1 powder-liquid ratio according to the manufacturer’s instructions. The material was placed in a single layer with a plastic mouth spatula. The RMGIC group was mixed in a capsule form in an amalgamator for 10 s and applied directly to the mold. Excess material for all of the discs was removed by applying pressure with two glass plates covered with mylar strips. The discs were then polymerized for 20 s in direct contact with the glass using an LED light (LED F, Woodpecker, China) with a wavelength of 430–480 nm and a light intensity of 1200 mW/cm2 according to the manufacturer’s instructions. The light intensity of the light device was repeatedly checked with a radiometer during the process. The polishing process of the prepared samples was limited to only one surface of the samples and was performed in accordance with the manufacturer’s instructions. The polishing process was performed with the Super Snap Rainbow (Shofu, USA) disc set. Coarse, medium, fine and very fine-grained discs were applied in the same direction with a handpiece at a speed of 10,000–20,000 rpm for 15 s each. The samples were washed under running water for 20 s before switching to each new size disc. The discs were renewed every 5 samples. Prepared samples were kept in distilled water at 37 °C for 24 h to complete polymerization. All samples were placed in different containers according to the active substance and the samples were kept in the oven at 37 °C throughout the study. Images of the prepared disks are given in Fig. 1. All samples were randomly selected and divided into study subgroups and numbered by one of the researchers (DSÖÇ). The aging, color stability and surface roughness measurement procedures of the samples were carried out by the other researcher (TA). In addition, no other group information was provided to the researcher except for the ozone applied group. In this way, the risk of bias was minimized.
Fig. 1.
Resin Modified Glass Ionomer Cement (a), Alkasite (b), Compomer (c)
Aging of materials and ozone application procedure
After the initial color and surface roughness measurements of the samples, the ozone procedure was applied. Easy Shade Advance Vita (VitaZahnfabrik, BadSackingen, Germany) was used as a spectrophotometer for color analysis, and Marsurf M300 (MAHR, Germany) was used as a roughness measuring device. Ozonated water was prepared with an Ozonettte Dent ozone generator (Ozonettte Dent, Sedecal, Spain) for 10 min, at a flow rate of 30 l/h, and at a concentration of 50 µg/ml [17, 18]. This procedure was repeated every day for 14 days. Before the aging procedure, restorative materials in the ozonated water group were applied with ozonated water for 2 min. Ozone application to the materials was done by manual shaking to simulate the use of mouthwash. After the ozone procedure, the samples were subjected to the aging procedure with beverages. Each group of restorative materials was immersed in 4 different solutions. The samples were immersed in the solutions for 3 h per day [19, 20]. The samples were stored in distilled water at all times other than immersion in the solutions. The solutions were renewed daily throughout the entire staining procedure. Before the measurements, the discs were dried with a paper towel. Figure 2a shows the aging solutions placed in separate containers for each disk. Figure 2b shows the freshly prepared orange juice, cocoa milk, cola and distilled water before the aging procedure, respectively.
Fig. 2.
(a) Application of solutions to resin modified glass ionomer, compomer and alkasite disks, (b) Orange juice, chocolate milk, cola and distilled water used in the study
Measurement of color change and surface roughness
Color measurements were performed on a plain matte white standardised background using a spectrophotometer (Easy Shade Advance Vita; Vita Zahnfabrik, Bad Säckingen, Baden-Württemberg, Germany). Three measurements were taken from each sample and the average of the resulting L*, a* and b* values was recorded. The spectrophotometer was calibrated before each measurement. Color measurements of the samples were initially calculated on days 1, 7 and 14. According to the CIEDE2000 (ΔE 00) color system, the total color difference ΔE00 for each sample was calculated using the formula:
ΔE00= [(ΔL’/KLSL) 2 + (ΔC’/KCSC) 2 + (ΔH’/KHSH) 2 + RT(ΔC’/KCSC) (ΔH’/KHSH)] ½ [21].
KL, KC and KH values were accepted as 1. In this study, the ΔE00 values corresponding to acceptability and detectability were accepted as 2.23 and 1.25, respectively [22].
In this study, the average basic surface roughness (Ra, µm) of the tested materials was measured with the Marsurf M300 mobile profilometer device. Before the surface roughness value measurement, the profilometer was calibrated using a standard calibration sample. Surface roughness measurements were made with the measuring distance of the device being 4 mm and the cutting value being 0.8 mm. The average Ra value was determined in micrometers (µm). The measurements were repeated four times and the average values of the test results were used. Surface roughness measurements were made at the beginning, 1, 7 and 14. days and recorded as ΔRa1, ΔRa7 and ΔRa14.
Statistical analysis
Data was analyzed with IBM SPSS V23. Compliance with normal distribution was examined with the Shapiro-Wilk test. The independent two-sample t-test was used for comparing normally distributed data according to two groups, and Mann-Whitney U test was used for comparing non-normally distributed data. One-way ANOVA was used for comparing normally distributed data according to three or more groups, and multiple comparisons were examined with the Tukey test and Tamhane’s T2 tests. Kruskal Wallis test was used to compare data that were not normally distributed according to three or more groups and multiple comparisons were examined with Dunn test. Repeated analysis of variance was used to compare data that were normally distributed according to three or more time periods within groups and multiple comparisons were examined with Bonferroni test. Friedman test was used to compare data that were not normally distributed according to three or more time periods within groups and multiple comparisons were examined with Dunn test. Analysis results were presented as mean ± standard deviation and median (minimum-maximum). Significance level was taken as p < 0.050.
Results
Color stability results
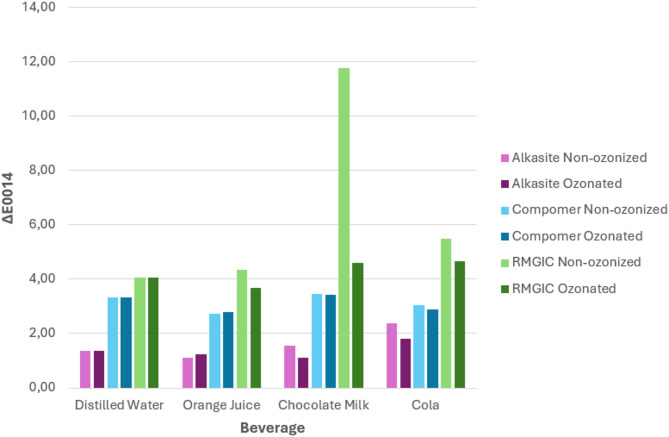
A significant difference was obtained between the mean ∆E007 values of the compomer material immersed in orange juice and the ozonated water application in terms of color change according to ozone application (p = 0.029). The color change of the compomer material applied with ozonated water on the 7th day was higher than the compomer without ozonated water. However, at the end of the 14th day, no significant difference was observed between the groups that were and were not applied with ozonated water (p > 0.05) (Table 3). In Additional Table 1 provides detailed statistical results regarding the color change results according to ozonated and non-ozonized groups.
Table 3.
Color change results according to non-ozonized and ozonated groups
| Material | Beverage | Parameters | Non-ozonized | Ozonated | p |
|---|---|---|---|---|---|
| Alkasite | Distilled Water | ∆E0014 | 1.38 ± 0.65 | 1.37 ± 0.61 | 0.983** |
| Orange Juice | ∆E0014 | 1.11 ± 0.42 | 1.23 ± 0.70 | 0.651** | |
| Chocolate Milk | ∆E0014 | 1.56 ± 0.57 | 1.10 ± 0.90 | 0.191** | |
| Cola | ∆E0014 | 1.96 (1.36–5.57) | 0.94 (0.51–5.32) | 0.075* | |
| Compomer | Distilled Water | ∆E0014 | 3.34 ± 0.41 | 3.33 ± 0.50 | 0.973** |
| Orange Juice | ∆E0014 | 2.73 ± 0.52 | 2.80 ± 0.39 | 0.763** | |
| Chocolate Milk | ∆E0014 | 3.47 ± 0.43 | 3.44 ± 0.54 | 0.879** | |
| Cola | ∆E0014 | 3.04 ± 0.47 | 2.88 ± 0.57 | 0.518** | |
| Resin Modified Glass Ionomer Cement | Distilled Water | ∆E0014 | 4.07 ± 0.74 | 4.06 ± 0.92 | 0.996** |
| Orange Juice | ∆E0014 | 3.44 (2.43–12.06) | 3.65 (2.97–4.28) | 0.684* | |
| Chocolate Milk | ∆E0014 | 4.89 (3.62–75.42) | 4.48 (4.04–5.59) | 0.481* | |
| Cola | ∆E0014 | 5.49 ± 0.78 | 4.67 ± 0.97 | 0.05** |
*Kruskal-Wallis test, **One-way ANOVA, ***Friedman test, ****Repeated analysis of variance
A significant difference was obtained in the color changes of the materials immersed in the aging solutions in both the groups applied with and without ozonated water at the end of the 14th day (p < 0.001). While the least color change was observed in CN among the materials, unacceptable color changes were observed in the other materials. The most and unacceptable color change was observed in the RMGIC material (Fig. 3). In Additional Table 2 provides detailed statistical results regarding color change results according to restorative materials.
Fig. 3.
Results of ∆E values according to restorative materials
A significant difference was obtained in the color changes of the CN, compomer and RMGIC groups without ozonated water at the end of the 14th day compared to the beverages (p < 0.001). In CN, compomer and RMGIC materials to which ozonated water was applied, the least color change was seen in orange juice, while CN and RMGIC were colored the most in cola, and compomer was colored in chocolate milk. A significant difference was obtained in color changes in compomer and RMGIC materials to which ozonated water was applied according to beverages at the end of the 14th day (p < 0.001). While the least color change was seen in orange juice in compomer and RMGIC materials, chocolate milk was the most coloring solution in compomer and cola was the most coloring solution in RMGIC (Table 4).
Table 4.
Color change results according to beverages
| Ozone | Material | Parameters | Distilled Water | Orange Juice | Chocolate Milk | Cola | p |
|---|---|---|---|---|---|---|---|
| Non-ozonized | Alkasite | ∆E0014 | 1.21 (0.37–2.60)ab | 1.11 (0.35–1.67)a | 1.51 (0.80–2.87)ab | 1.96 (1.36–5.57)b | 0.005** |
| Compomer | ∆E0014 | 3.34 ± 0.41b | 2.73 ± 0.52a | 3.47 ± 0.43b | 3.04 ± 0.47ab | 0.005* | |
| Resin Modified Glass Ionomer Cement | ∆E0014 | 4.11 (2.57–5.11)a | 3.44 (2.43–12.06)a | 4.89 (3.62–75.42)ab | 5.32 (4.36–6.84)b | 0.001** | |
| Ozonated | Alkasite | ∆E0014 | 1.40 (0.69–2.75) | 1.04 (0.59–2.72) | 0.95 (0.11–3.20) | 0.94 (0.51–5.32) | 0.572** |
| Compomer | ∆E0014 | 3.33 ± 0.50ab | 2.80 ± 0.39a | 3.44 ± 0.54b | 2.88 ± 0.57ab | 0.015* | |
| Resin Modified Glass Ionomer Cement | ∆E0014 | 4.06 ± 0.92ab | 3.68 ± 0.41a | 4.61 ± 0.53b | 4.67 ± 0.97b | 0.016* |
* Kruskal Wallis test, **One-way ANOVA. a-b: There is no significant difference between materials with the same letter
Surface roughness change results
No statistically significant difference was obtained in ∆Ra results between the groups to which ozonated water was applied and the groups to which no ozonated water was applied (Table 5). In Additional Table 3 provides detailed statistical results regarding the surface roughness results according to ozonated and non-ozonized groups.
Table 5.
∆Ra comparison results according to ozonated and Non-ozonized
| Restorative Material | Beverage | Parameters | Non-ozonized | Ozonated | p |
|---|---|---|---|---|---|
| Alkasite | Distilled Water | ∆Ra14 | 0.41 ± 0.16 | 0.43 ± 0.24 | 0.487** |
| Orange Juice | ∆Ra14 | 0.71 ± 0.30 | 0.66 ± 0.14 | 0.810** | |
| Chocolate Milk | ∆Ra14 | 0.50 ± 0.49 | 0.43 ± 0.28 | 0.146** | |
| Cola | ∆Ra14 | 0.82 ± 0.62 | 0.44 ± 0.33 | 0.074** | |
| Compomer | Distilled Water | ∆Ra14 | 0.07 ± 0.18 | 0.05 ± 0.11 | 0.460** |
| Orange Juice | ∆Ra14 | 0.004 | 0.13 | 0.112* | |
| Chocolate Milk | ∆Ra14 | 0.18 | 0.02 | 0.406* | |
| Cola | ∆Ra14 | 0.49 ± 0.29 | 0.19 ± 0.08 | 0.050** | |
| Resin Modified Glass Ionomer Cement | Distilled Water | ∆Ra14 | 0.01 ± 0.66 | 0.12 ± 0.24 | 0.685** |
| Orange Juice | ∆Ra14 | 0.85 ± 0.63 | 0.34 ± 0.48 | 0.134** | |
| Chocolate Milk | ∆Ra14 | 0.13 ± 0.57 | 0.19 ± 0.58 | 0.782** | |
| Cola | ∆Ra14 | 0.70 ± 0.41 | 0.49 ± 0.62 | 0.666** |
*One-way ANOVA, **Kruskal Wallis test
A significant difference was obtained between compomer and CN in ∆Ra values at the end of the 14th day according to the materials from the samples immersed in distilled water without ozonated water and orange juice with ozonated water (p = 0.008), (p = 0). CN had the highest ∆Ra value. A significant difference was obtained between compomer and other materials in ∆Ra values at the end of the 14th day from the samples immersed in orange juice without ozonated water (p = 0). Compomer had the lowest ∆Ra value. A significant difference was obtained between CN and other materials in ∆Ra values at the end of the 14th day from the samples immersed in distilled water with ozonated water (p = 0.003). CN had the highest ∆Ra value (Fig. 4). In Additional Table 4, detailed statistical results about the surface roughness change results according to the restorative materials are given.
Fig. 4.
Results of ∆Ra Values According to Restorative Materials
A significant difference was obtained between the ∆Ra14 mean values of compomer and CN group without ozonated water according to the beverages (p = 0). A significant difference was obtained between cola and other beverages. Cola had the highest ∆Ra value. A significant difference was obtained between the ∆Ra14 mean values of the RMGIC group according to beverages (p = 0.005). A significant difference was obtained between orange juice and distilled water and chocolate milk. Orange juice had the highest ∆Ra value (Table 6).
Table 6.
Comparison results of ∆ra values according to beverages
| Ozone | Material | Parameters | Distilled Water | Orange Juice | Chocolate Milk | Cola | p |
|---|---|---|---|---|---|---|---|
| Non-ozonized | Alkasite | ∆Ra14 | 0.41 ± 0.16 | 0.71 ± 0.30 | 0.50 ± 0.49 | 0.82 ± 0.62 | 0.046* |
| Compomer | ∆Ra14 | 0.07 ± 0.18 a | 0.005 ± 0.18 a | 0.18 ± 0.28 a | 0.49 ± 0.29b | < 0.001* | |
| Resin Modified Glass Ionomer Cement | ∆R14 | 0.01 ± 0.66 b | 0.85 ± 0.63 a | 0.13 ± 0.57 b | 0.70 ± 0.41 ab | 0.005* | |
| Ozonated | Alkasite | ∆Ra14 | 0.43 ± 0.24 | 0.66 ± 0.14 | 0.43 ± 0.28 | 0.44 ± 0.33 | 0.149* |
| Compomer | ∆Ra14 | 0.05 (-0.10–0.31) | 0.13 (-0.82–0.33) | 0.02 (-0.17–0.90) | 0.18 (0.07–0.35) | 0.056** | |
| Resin Modified Glass Ionomer Cement | ∆Ra14 | 0.12 ± 0.24 | 0.34 ± 0.48 | 0.19 ± 0.58 | 0.49 ± 0.62 | 0.394* |
*One-way ANOVA, **Kruskal Wallis test. a-b: There is no significant difference between beverages with the same letter
Discussion
Aesthetic appearance is the main concern of children as well as adults. In order to provide perfect aesthetics, the tooth-colored material must maintain its unique color stability and surface properties. Beverage consumption is very common, especially among children. Teeth and restorations on teeth can be affected by intraoral pH changes caused by beverages [19]. The aim of this study was to investigate the effects of ozonated water application on color change and surface roughness of restorative materials aged with different solutions in vitro.
Foods and beverages affect the pH in the oral cavity; therefore, teeth and dental restorations may be affected [20]. In many studies, cola, fruit juice, and chocolate milk were used because they are among the most commonly consumed beverages and have excellent staining capabilities; thus, the solutions mentioned in this study were selected for this reason [19, 23, 24]. Previous research indicates that keeping composites in beverages for 48 h at 37 °C corresponds to approximately 2 months in vivo [25, 26]. The beverages were renewed daily for 14 days and the exposure was immersed for 3 h/day. This corresponds to a period of approximately 52 days. This simulates clinically intensive consumption of acidic beverages over a prolonged period. The exposure time used is frequently provided in similar studies accepted in the literatüre [27, 28].
In medical ozone applications, optimum dose ranges to be applied for different diseases and systemic conditions have been determined, and thus systemic ozone applications have achieved international standardization [29]. On the other hand, in the Madrid Declaration, the first guideline written on the use of ozone in dentistry, it was stated that it was difficult to narrow the therapeutic range and give optimum dose range based on previous studies in dentistry, and that the information provided could be updated with new studies [29]. Nicolini et al. evaluated the effect of ozonated water gargle on early plaque formation and gingivitis. In the in vivo study, ozonated water gargle was used once a day for 10 min at 70 µg/ml [18]. Cosola et al. compared the clinical effectiveness of chlorhexidine and ozonated water in maintaining oral hygiene in orthodontic patients. In the study, ozonated water obtained with a flow rate of 50 mg/h (20 °C) and 0.2 64 l/min was used as a gargle twice a day [17]. In a clinical study conducted by Mon et al. with 100 people aged 10–12 years, the antimicrobial activity of ozonated water, herbal gargle and chlorhexidine gargle was investigated. Fresh ozonated water was prepared every day by ozonating the water for 1 min using an ozone generator (Ozone Engineers, Coimbatore). Ozonated water was produced at a concentration of 2.4 mg/L (> 2 ppm) per minute. Subjects were asked to gargle for 1 min every day for 15 days [30]. Ozone was used in water form because it has a strong oxidizing power in the gas or aqueous phase and has a reliable microbicidal effect [31]. In this study, Ozonette Dent, a newly launched ozone generator, was used. There is no study in the literature with the Ozonette Dent ozone generator. Considering the ozone concentrations used in the current literature studies, ozonated water was obtained at a dose of 50 µg/ml for 10 min, with a flow rate of 30 l/h. The ozone dose (50 µg/mL) and manual shaking method used in this study are comparable to those employed in previous clinical investigations. Based on the protocols reported by existing studies, the ozone dosage and application technique chosen in this study appear to have clinical significance and applicability [17, 18, 30]. Furthermore, the manual shaking method was adopted to mimic the swishing action commonly performed by patients during mouth rinsing at home, thereby serving as a practical in vitro simulation of real-life clinical use. Supporting this approach, microfluidic and viscosity-based simulation studies have demonstrated that such dynamic mixing closely approximates oral lavage conditions [32]. Therefore, the protocol implemented in this study is considered a reasonable and clinically relevant simulation of actual use.
Ozone is a highly oxidizing agent that can participate in various chemical reactions with organic and inorganic substances [33]. As a result of the decomposition of ozone, peroxide and hydroxyl radicals with strong oxidizing properties are formed. These radicals are highly electrophilic because they have unpaired electrons and react with the double bonds formed by chromophore molecules between inorganic salts in the enamel structure and organic pigment molecules in order to stabilize. Accordingly, the energy absorption of chromophore molecules changes and simple molecules that reflect less light are formed. In summary, free oxygen radicals formed as a result of ozone decomposition react with colored organic molecules and provide bleaching on the enamel surface [34]. In a study investigating the use of ozone as a preservative in orange juices, it was determined that increasing the gas flow rate, ozone concentration and ozonization time caused an increase in the color tone of orange juices [35]. The reason for the color change was explained by the fact that ozone and hydroxyl radicals (OH−) formed in the aqueous solution opened the aromatic rings of carotenoid pigments responsible for the color in orange juice and could cause partial oxidation of products such as organic acids, aldehydes and ketones. In this study, when the groups that were applied and not applied ozonated water were compared, more color change was observed in the 7th day values of the compomer group immersed in orange juice due to ozone application. It is thought that the reason for this may be that the discs were pre-ozonized and the oxidizing effect of ozone dissolved the organic structures of the resin materials and the acidic orange juice applied afterwards increased the coloration by penetrating more.
Resin-based materials can absorb water due to their hydrophilic properties. Due to this structure, they can also absorb pigments and other liquids; accordingly, discoloration can be observed in restorations [36]. In particular, the extreme color change of the RMGIC material is associated with the HEMA content, a hydrophilic monomer [24]. It has also been reported in the literature that water acts as a carrier for coloring agents during the water absorption process [37]. The CN material contains high fillers as well as organic liquids containing UDMA, DCP, an aromatic aliphatic-UDMA and PEG-400 DMA. Such a combination allows low water absorption, which allows less penetration of chromophores to the surface and less color change [38]. Most of the studies evaluating the color change of CN in the literature have stated that the color change of CN is high [39–41]. Apart from this, CN was found to be the material that showed the least color change with thermal cycling when compared to Fuji IX GP and Fuji IX GP Extra [38]. According to this study results, all restorative materials showed color change in all solutions regardless of ozonated water application. All compomer and RMGIC groups experienced unacceptable color change after 14 days. Similar to the study by Bhattacharya et al. [38] it is thought that since compomer and RMGIC used in this study contain polyacid, it may have been more effective in their coloration compared to CN.
Studies have shown that color stability depends on the materials and the types of beverages consumed by patients also affect the color stability of restorative materials [42, 43]. Low pH due to the presence of orthophosphoric and carbonic acids in cola can cause discoloration and coloration of resin-based restorations. Mohan et al. [44], reported that RMGIC was the most colored material in the study in which they investigated the effects of cola on the color stability of composite, RMGIC and compomer. They stated that the caramel color in the cola solution may be effective in the color change ranging from the palest yellow to the darkest brown. There are many studies showing that cola causes more coloration than other beverages [24, 45]. In this study, cola caused the most color change in RMGIC material among the materials. In some studies, the most pronounced color change occurred in the groups dipped in chocolate milk [19, 23]. In this study, the most color change in compomer was seen in the chocolate milk group. As reported in the studies of Habib et al. [46] the coloring effect of chocolate milk may be due to its organic components and oily nature. Therefore, chocolate milk easily adsorbs on the resin surface and interacts with the organic matrix, causing its color deterioration.
Different results were found when ozone was applied to different restorative materials. Afifi et al. [31], evaluated the surface roughness and marginal sealing of teeth restored with composite and GIS by applying ozonated water at a concentration of 4 mg/l for 20, 40, and 60 s. They observed that surface roughness increased as the ozone application time increased in all groups. Nishikiori et al. [47] ound that ozonated water caused significantly less surface roughness than sodium hypochlorite. In a study conducted on composites, it was shown that the average surface roughness of ozonated gel was lower [48]. hey explained that this effect may be due to the strong oxidizing effect of free active radicals. It has been stated that the effect of such free radicals may not be limited to the resin matrix, but can also be extended to affect filler particles, whether glass or ceramic [48]. In this study, it was observed that the effect of ozone application on the surface roughness of resin-containing materials was not significantly different between the groups. The reason for this difference may be due to the difference in the ozone application procedure and the method of ozone application.
In the study investigating the ion release of Fuji II LC, Activa BioActive, Cention N and Z250 materials, CN showed higher phosphate ion release. It was explained that the higher release of phosphate ions from CN compared to Activa BioActive material in 24 h may be related to the formation of voids and pores in CN during the mixing of powder and liquid and the presence of alkaline fillers of calcium fluorosilicate [49]. The presence of voids in the material structure leads to water absorption and dissolution of the material [50]. In addition, the presence of voids inhibits polymerization reactions and increases the amount of unpolymerized material, leading to more ion release [49]. It has been stated that the presence of hydrophilic liquid monomer (PEG-400 DMA) in CN and its ability to release fluoride, calcium, and hydroxide ions may be the reason for the significant roughness observed in CN groups [51, 52]. The resin structure of CN mainly contains dual-cure initiators and requires manual mixing. They stated that the more porous resin structure of CN may be responsible for the higher solubility compared to single-tube composite resin systems [52]. In addition, glass fillers in compomer and RMGIC materials do not dissolve in acidic environments due to their embeddedness in a polymer resin. This provides resistance to surface roughness [53]. The surface roughness results are similar to the results of this study.
The pH value of the aging solution can affect the surface roughness as well as the color changes, which can cause changes in water absorption [54]. The main organic acid found in citrus juices (such as orange and pomegranate juice) is citric acid. This dominant acid can chelate calcium at higher pH levels, making it corrosive [55]. There are studies that found that fruit juices have a higher corrosive potential than carbonated beverages [56, 57]. In studies comparing the roughness values caused by orange juice and other beverages, it was observed that orange juice caused more surface roughness [9, 23, 58]. Similarly, the RMGIC group without ozonated water in this study had significantly higher ∆Ra values.
Cola is a popular soft drink with the lowest pH (3.8) among the beverages used in this study and is mostly made of phosphoric acid. Citrus fruits contain several different types of acids, including citric, malic, tartaric, benzoic, oxalic and succinic acids [59]. Zajkani et al. [45], reported that cola is a yellow-brown carbonated beverage and has a coloring effect as well as an abrasive effect on resin materials. There are studies evaluating the surface roughness of cola, orange juice and artificial saliva on restorative materials [59, 60]. In these studies, cola was found to have higher roughness than orange juice. In this study, compomer cola without ozonated water showed the highest surface roughness value.
When the findings of this study were evaluated, the null hypothesis that ozonated water application would not have a significant effect on the color stability and surface roughness of restorative materials was partially rejected. Although ozone application did not create a significant difference in terms of surface roughness, a significant color change was detected in the compomer group immersed in orange juice on the 7th day. The fact that this effect decreased on the 14th day indicates that the oxidative effect of ozone may have a temporary effect on some restorative materials, especially in acidic environments. Therefore, the null hypothesis was not fully supported, especially in terms of color stability.
This study has some important clinical limitations. The first of these is that this study was conducted completely under in vitro conditions. Chewing forces, salivary flow, oral microbiota, intraoral temperature and pH changes could not be simulated. In addition, the limited number of studies in the literature on ozone application in dentistry in the field of pedodontics and the lack of a standard for ozone application procedure and dose determination are important issues that limit this study. Another limitation of this study is that it was not possible to apply the blinding method completely because it was designed in vitro. However, all samples were randomly divided into groups and numbered. In addition, only the group that was exposed to ozone was given to the researcher who performed the procedure, and the other group information was hidden. These measures were aimed to increase the impartiality and reliability of the study.
Conclusion
While ozone application caused more color change in the compomer material aged with orange juice, no effect of ozone application on surface roughness was observed among the materials. Further studies with different ozone doses are needed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Special acknowledgment of this work goes to VOCO company (VOCO GmbH, Cuxhaven, Germany) for supplying the resin modified glass ionomer cement and compomer materials used and Ozonettte Dent (Ozonettte Dent, Sedecal, Spain) for the ozone generator in this study.
Abbreviations
- CN
Alkasite
- Compomer
Polyacid Modified Composite Resin
- RMGIC
Resin Modified Glass Ionomer Cement
- ΔE00
CIEDE2000
Author contributions
DSÖÇ, Conceptualization, Methodology, Data Acquisition and Interpretation, Analysis, Critically Revised the Manuscript. TA, Conceptualization, Methodology, Supervision, Data Acquisition and Interpretation, Analysis, Writing-Original Draft Preparation. All authors have read and approved the final article.
Funding
No funding was obtained for this study.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Buerkle V, Kuehnisch J, Guelmann M, Hickel R. Restoration materials for primary molars—results from a European survey. J Dent. 2005;33(4):275–81. [DOI] [PubMed] [Google Scholar]
- 2.Abdallah RM, Aref NS. Development of newly formulated Nanoalumina-/Alkasite-Based restorative material. Int J Dent. 2021;2021(1):9944909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar SA, Ajitha P. Evaluation of compressive strength between cention N and high copper amalgam-An in vitro study. Drug Invent Today. 2019;12(2):255–57.
- 4.Song S-Y, Shin Y-H, Lee J-Y, Shin S-W. Color stability of provisional restorative materials with different fabrication methods. J Adv Prosthodont. 2020;12(5):259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giti R, Dabiri S, Motamedifar M, Derafshi R. Surface roughness, plaque accumulation, and cytotoxicity of provisional restorative materials fabricated by different methods. PLoS ONE. 2021;16(4):e0249551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alnasser M, Finkelman M, Papathanasiou A, Suzuki M, Ghaffari R, Ali A. Effect of acidic pH on surface roughness of esthetic dental materials. J Prosthet Dent. 2019;122(6):567.e1-567.e8. [DOI] [PubMed] [Google Scholar]
- 7.Das K, Murthy CS, Naganath M, Mehta D, Anitha Kumari R, Karobari MI, et al. Insights into the effects and implications of acidic beverages on resin composite materials in dental restorations: an in vitro study. J Esthet Restor Dent. 2025;37(4):1013–23. [DOI] [PubMed] [Google Scholar]
- 8.Ipek İ, Bilge K. The effect of different liquids on the surface roughness and color stability of single shade and nanohybrid resin composites: an AFM and SEM analysis. Microsc Res Tech. 2024;87(9):2063–71. [DOI] [PubMed] [Google Scholar]
- 9.Guler S, Unal M. The evaluation of color and surface roughness changes in resin based restorative materials with different contents after waiting in various liquids: an SEM and AFM study. Microsc Res Tech. 2018;81(12):1422–33. [DOI] [PubMed] [Google Scholar]
- 10.Burns DT. Early problems in the analysis and the determination of Ozone. Fresenius J Anal Chem. 1997;357(2):178–83. [Google Scholar]
- 11.D’Amario M, Di Carlo M, Natale SM, Memè L, Marzo G, Matarazzo G, et al. Application of Ozone therapy in paediatric dentistry. Appl Sci. 2022;12(21):11100. [Google Scholar]
- 12.Gallo S, Scribante A. Ozone therapy in dentistry: from traditional applications towards innovative ones. A review of the literature. IOP Conf Ser Earth Environ Sci. 2021;707(1):012001. [Google Scholar]
- 13.Öter B, Topçuog̃lu N, Tank MK, Çehreli SB. Evaluation of antibacterial efficiency of different root Canal disinfection techniques in primary teeth. Photomed Laser Surg. 2018;36(4):179–84. [DOI] [PubMed] [Google Scholar]
- 14.Dähnhardt JE, Jaeggi T, Lussi A. Treating open carious lesions in anxious children with ozone. A prospective controlled clinical study. Am J Dent. 2006;19(5):267–70. [PubMed] [Google Scholar]
- 15.Mampieri G, Alushi A, Di Girolamo M, Rasicci P, Capogreco M, Marino R et al. Ozone therapy new protocols and innovations: a systematic review. J Biol Regul Homeost Agents. 2022;36(2(S1)):117–21.
- 16.Aktaş N, Atabek D, Akça G, Öztaş N. Antibacterial effectiveness of prophylactic Ozone application with Full-Mouth-Tray in orthodontic patients with high caries activity. J Gazi Univ Health Sci Inst. 2019;1(1):10–20. [Google Scholar]
- 17.Cosola S, Giammarinaro E, Genovesi AM, Pisante R, Poli G, Covani U, et al. A short-term study of the effects of Ozone irrigation in an orthodontic population with fixed appliances. Eur J Paediatr Dent. 2019;20(1):15–8. [DOI] [PubMed] [Google Scholar]
- 18.Nicolini AC, Rotta I dos, Langa S, Friedrich GPJ, Arroyo-Bonilla SA, Wagner DA. Efficacy of ozonated water mouthwash on early plaque formation and gingival inflammation: a randomized controlled crossover clinical trial. Clin Oral Investig. 2021;25(3):1337–44. [DOI] [PubMed] [Google Scholar]
- 19.Bezgin T, Özer L, Tulga Öz F, Özkan P. Effect of toothbrushing on color changes of esthetic restorative materials. J Esthet Restor Dent. 2015;27(S1):S65–73. [DOI] [PubMed] [Google Scholar]
- 20.Shalan HM, Alagami RA, Hasan MA. Effect of coloring beverages on different esthetic restorative materials in primary teeth. Acta Sci Dent Sci. 2019;3:64–8. [Google Scholar]
- 21.Dedijer S, Tomić I, Spiridonov I, Boeva R, Jurič I, Milić N, et al. Ink-jet imprints in just noticeable color difference evaluation. Bulg Chem Commun. 2017;49:140–7. [Google Scholar]
- 22.Ghinea R, Pérez MM, Herrera LJ, Rivas MJ, Yebra A, Paravina RD. Color difference thresholds in dental ceramics. J Dent. 2010;38:57–64. [DOI] [PubMed] [Google Scholar]
- 23.Savas S, Colgecen O, Yasa B, Kucukyilmaz E. Color stability, roughness, and water sorption/solubility of glass Ionomer–Based restorative materials. Niger J Clin Pract. 2019;22(6):824. [DOI] [PubMed] [Google Scholar]
- 24.Tunc ES, Bayrak S, Guler AU, Tuloglu N. The effects of children’s drinks on the color stability of various restorative materials. J Clin Pediatr Dent. 2009;34(2):147–50. [DOI] [PubMed] [Google Scholar]
- 25.Gönülol N, Yılmaz F. The effects of finishing and Polishing techniques on surface roughness and color stability of nanocomposites. J Dent. 2012;40:e64–70. [DOI] [PubMed] [Google Scholar]
- 26.Bilmez ZY, Şeker O, Köse HD, Aslan BG. Evaluation of liquid sorption and color stability of dental composites after exposure to common lactation teas. Int Dent Res. 2021;11(Suppl 1):228–33. [Google Scholar]
- 27.Beltrami R, Colombo M, Bitonti G, Chiesa M, Poggio C, Pietrocola G. Restorative materials exposed to acid challenge: influence of temperature on in vitro weight loss. Biomimetics. 2022;7(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Podgórski F, Musyt W, Nijakowski K. The impact of sports drink exposure on the surface roughness of restorative materials: A systematic review. J Compos Sci. 2025;9(5):234. [Google Scholar]
- 29.Schwartz A, Sánchez GM, Sabah F. Madrid declaration on ozone therapy. Fac Cent Oeste Paul Madr. 2010 [cited 2025 Jun 3]. Available from: https://ozonewithoutborders.ngo/wp-content/uploads/2021/04/2020-Madrid-Declaration.pdf
- 30.Mon J, Asokan S, Priya PR, Kumar TD, Balasubramaniam MG. Effect of herbal water, ozonated water, water, and chlorhexidine mouthrinses on oral health status of children: A randomized controlled trial. Int J Clin Pediatr Dent. 2019;12(6):514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Afifi R, Mosallam RS, Abi Al Hassan MH. Effect of ozonated water on marginal integrity and surface topography of class 5 restorative system. Future. 2013;1:1. [Google Scholar]
- 32.Hinic S, Petrovic B, Kojic S, Omerovic N, Jevremov J, Jelenciakova N, et al. Viscosity and mixing properties of artificial saliva and four different mouthwashes. Biorheology. 2020;57(2–4):87–100. [DOI] [PubMed] [Google Scholar]
- 33.Boztaş G, Ömürlü H. Restoratif diş Hekimliğinde Ozon tedavileri. Atatürk Üniversitesi diş. Hekim Fakültesi Derg. 2014;24(3):158–68. [Google Scholar]
- 34.Perincek SD, Duran,Kerim K,,Aysegul E, Bahtiyari İM. An investigation in the use of Ozone gas in the bleaching of cotton fabrics. Ozone Sci Eng. 2007;29(5):325–33. [Google Scholar]
- 35.Tiwari BK, Muthukumarappan K, O’ Donnell CP, Cullen PJ. Modelling color degradation of orange juice by Ozone treatment using response surface methodology. J Food Eng. 2008;88(4):553–60. [Google Scholar]
- 36.Kaizer Mda, Diesel R, Mallmann PG, Jacques A. Ageing of silorane-based and methacrylate-based composite resins: effects on translucency. J Dent. 2012;40:64–71. [DOI] [PubMed] [Google Scholar]
- 37.Catelan A, Briso ALF, Sundfeld RH, Goiato MC, dos Santos PH. Color stability of sealed composite resin restorative materials after ultraviolet artificial aging and immersion in staining solutions. J Prosthet Dent. 2011;105(4):236–41. [DOI] [PubMed] [Google Scholar]
- 38.Bhattacharya S, Purayil TP, Ginjupalli K, Kini S, Pai S. Effect of thermocycling on the colour stability of aesthetic restorative materials: an in-vitro spectrophotometric analysis. Pesqui Bras Em Odontopediatria E Clínica Integrada. 2020;20:5174. [Google Scholar]
- 39.Lastre CCM, Pedraza MCC, Visbal JHW. Efecto de Las Bebidas pigmentantes sobre materiales restaurativos Directos Del sector posterior. Rev NOVA. 2023;21(40):165–80. [Google Scholar]
- 40.Amalavathy RK, Sahoo HS, Shivanna S, Lingaraj J, Aravinthan S. Staining effect of various beverages on and surface nano-hardness of a resin coated and a non-coated fluoride releasing tooth-coloured restorative material: an in-vitro study. Heliyon. 2020;6(6):04345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Majeti C, Ravi R, Kambhampati B, Borugadda R, Athkuri S, Kakani AK. Evaluation of the color stability of two different posterior tooth colored restorative materials. F1000Res. 2020;9(1251):1251.
- 42.Spina DRF, Grossi JRA, Cunali RS, Baratto Filho F, da Cunha LF, Gonzaga CC, et al. Evaluation of discoloration removal by Polishing resin composites submitted to staining in different drink solutions. Int Sch Res not. 2015;2015(1):853975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meenakshi CM, Sirisha K. Surface quality and color stability of posterior composites in acidic beverages. J Conserv Dent Endod. 2020;23(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohan M, Shey Z, Vaidyanathan J, Vaidyanathan TK, Munisamy S, Janal M. Color changes of restorative materials exposed in vitro to Cola beverage. Pediatr Dent. 2008;30(4):309–16. [PubMed] [Google Scholar]
- 45.Zajkani E, Abdoh Tabrizi M, Ghasemi A, Torabzade H, Kharazifard M. Effect of staining solutions and repolishing on composite resin color change. J Iran Dent Assoc. 2013;25(3):139–46. [Google Scholar]
- 46.Habib ANEA, Abdelmoniem SA, Mahmoud SA. Effect of children’s drinks on color stability of different dental composites: an in vitro study. J Clin Pediatr Dent. 2017;41(2):120–5. [DOI] [PubMed] [Google Scholar]
- 47.Nishikiori R, Sawajiri M, Okuda T, Otoshi A, Watanabe K, Hirata I, et al. Effect of ozonated water on the surface roughness of dental stone casts. Dent Mater J. 2018;37(5):740–5. [DOI] [PubMed] [Google Scholar]
- 48.Elhamid MA, Mosallam R. Effect of bleaching versus repolishing on colour and surface topography of stained resin composite. Aust Dent J. 2010;55(4):390–8. [DOI] [PubMed] [Google Scholar]
- 49.Kasraei S, Haghi S, Valizadeh S, Panahandeh N, Nejadkarimi S. Phosphate ion release and alkalizing potential of three bioactive dental materials in comparison with composite resin. Int J Dent. 2021;2021(1):5572569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Braga RR. Calcium phosphates as ion-releasing fillers in restorative resin-based materials. Dent Mater. 2019;35(1):3–14. [DOI] [PubMed] [Google Scholar]
- 51.Yazkan B, Celik E, Recen D. Effect of aging on surface roughness and color stability of a novel Alkasite in comparison with current direct restorative materials. Oper Dent. 2022;46(5):240–50. [DOI] [PubMed] [Google Scholar]
- 52.Hatırlı H, Tonga G, Boyraz Ş. Water sorption, solubility and color stability of different Bulk-Fill restorative materials. Cumhur Dent J. 2022;25(4):293–301. [Google Scholar]
- 53.Yap AUJ, Mok BYY. Surface finish of a new hybrid aesthetic restorative material. Oper Dent. 2002;27(2):161–6. [PubMed] [Google Scholar]
- 54.Sulaiman TA, Rodgers B, Suliman AA, Johnston WM. Color and translucency stability of contemporary resin-based restorative materials. J Esthet Restor Dent. 2021;33(6):899–905. [DOI] [PubMed] [Google Scholar]
- 55.Behrendt A, Oberste V, Wetzel WE. Fluoride concentration and pH of iced tea products. Caries Res. 2002;36(6):405–10. [DOI] [PubMed] [Google Scholar]
- 56.Bamise CT, Ogunbodede EO, Olusile AO, Esan TA. Erosive Potential Soft Drinks Nigeria. 2007;2(2):115–9. [Google Scholar]
- 57.Jensdottir T, Bardow A, Holbrook P. Properties and modification of soft drinks in relation to their erosive potential in vitro. J Dent. 2005;33(7):569–75. [DOI] [PubMed] [Google Scholar]
- 58.Hamouda IM. Effects of various beverages on hardness, roughness, and solubility of esthetic restorative materials. J Esthet Restor Dent. 2011;23(5):315–22. [DOI] [PubMed] [Google Scholar]
- 59.Singh T, Mahalakshmi V, Sahu S, Chatterjee S, Khan AM, Haqh MF, et al. A comparative evaluation of the effect of different beverages on colour stability and surface micromorphology of nanocomposite restorative material. Cureus. 2023;15(7):2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rajavardhan K, Sankar AJS, Kumar MGM, Kumar KR, Pranitha K, Kishore KK. Erosive potential of cola and orange fruit juice on tooth colored restorative materials. Ann Med Health Sci Res. 2014;4(3):208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.