Abstract
INTRODUCTION
Early life bilateral salpingo‐oophorectomy (BSO) leads to immediate estradiol loss and increased risk of late‐life Alzheimer's disease (AD). We investigated risk and resilience (RandR) on the average of 5 years post BSO and their possible impact on subjective cognitive decline (SCD), and brain structure.
METHODS
A cohort in Canada (n = 333) included women with and without BSO of whom some reported SCD. We used multiple factor analysis, logistic regression, and magnetic resonance imaging to assess RandR, odds of SCD, and differences in gray matter volume (GMV).
RESULTS
BSO was a risk for worse neuropsychological performance, a 2‐fold increase in the odds of SCD, and decreases in GMV in regions sensitive to estradiol, and important for mood, memory, and language. However, estradiol therapy (ETBSO), and verbal IQ mitigated this risk.
DISCUSSION
Risk of SCD exists for younger women with BSO, which affects GMV. Importantly, ETBSO and verbal IQ may shift that risk.
Highlights
Oophorectomy in early middle age may be a risk for subjective cognitive decline.
Oophorectomy is associated with less gray matter volume in the prefrontal cortex.
Estradiol therapy and verbal IQ might be resilience factors mitigating such risk.
Keywords: early life bilateral salpingo‐oophorectomy, estradiol, estradiol therapy, risk and resilience, subjective cognitive decline, verbal IQ
1. BACKGROUND
Risk and resilience (RandR) are important concepts for identifying who might be likely to have late‐life Alzheimer's disease (AD) 1 as well as for determining the necessity of intervention. In mixed sex studies, risk factors for late‐life AD include age, sex, metabolic conditions (e.g., hypertension, diabetes), psychosocial factors (e.g., depression, stress), and lifestyle choices (e.g., smoking). 2 Some of the resilience factors 1 that allow some individuals to maintain cognitive ability in the face of normal or pathological aging include verbal IQ, 3 education, 1 and linguistic diversity. 4 , 5
Although global epidemiological data show that more women have AD than men, 6 little is known about what factors might increase or reduce risk and delay AD onset in women. RandR is most often studied in elderly mixed sex cohorts, but there may be cognitive changes in early midlife for women signaling the need for early intervention as well as elucidating who might resist AD even into late age. 7 While there is much focus on the risk of later‐life menopause (spontaneous menopause [SM]) as a cognitive inflection point, 8 , 9 there are many types of menopause 10 with perhaps the riskiest for cognitive and brain health being early‐midlife bilateral salpingo‐oophorectomy ([BSO]; the removal of both the ovaries and fallopian tubes) which often occurs on average 10 to 15 years prior to SM for many reasons including chronic pain, 11 ovarian cancer, 12 and cancer risk‐reduction in women with the BReast CAncer 1 or 2 mutation (BRCA1/2m ). 13
BSO prior to SM with its immediate loss of ovarian 17‐b‐estradiol (E2) synthesis 10 , 13 has already been identified as a risk factor for women's late‐life AD and we have reported cognitive and brain changes already at an average of 5 years past BSO. 14 , 15 Within 2 to 6 months of early BSO, women have decrements in verbal episodic and associative memory; 16 within 5 years, they have decreased verbal episodic and spatial working memory, 17 functional hippocampal activation and connectivity, 18 and volume loss in the dentate gyrus–CA2–CA3 region (DGCA23). 19 Later in life, they show thinner parahippocampal–entorhinal cortical thickness, 20 and increased odds of cognitive impairment. 21 Thus, understanding the earliest RandR factors for women with BSO at early midlife may be an important window into some of the earliest influencers of women's late‐life dementia, encouraging the earliest intervention.
Subjective cognitive decline (SCD)—memory complaints despite standard clinical memory tests not revealing memory deficits— 22 is recognized as one of the earliest potential stages in the long course of AD. 22 SCD has been studied primarily during late middle‐age to observe the first cognitive changes occurring years before the onset of mild cognitive impairment. 23 However, SCD can also occur earlier in life for other reasons such as depression, side effects of medications, or poor sleep; whatever the cause, SCD is viewed as a risk for AD. 24 Importantly, SCD is little studied in younger people with high late‐life dementia risk, understudied in women with E2 loss, and not yet studied with respect to its accompanying RandR factors. 25
Thus, the primary goal of this study was to elucidate in our cohort of younger middle‐aged women RandR factors previously identified in older female cohorts and their association with performance on neuropsychological tasks. In doing so we asked: (1) What are potential RandR factors that influence cognitive function for younger middle‐aged women? (2) Are these factors associated with SCD? (3) What are the differences in gray matter volume (GMV) across SCD in our cohort?
2. METHODS
2.1. Cohort
Table 1 shows the group definitions. BSO and BRCA controls (BRCA‐C) were recruited from familial breast and ovarian cancer clinics in Toronto and Montreal. A comparator group, age‐matched controls (AMCs) and a group of women in SM were recruited from the community via fliers and social media advertisement. In the full study, participants were tested at three different time points 1 year apart; the current study used data from time point 1, which was their first visit. For BSO participants, time since BSO in our cohort was on average 5 years. 17 Magnetic resonance imaging (MRI), cognitive testing, and hormone collection for assessment were done on the same day for most participants (more information about our protocol and cohort can be found in Table S1 in supporting information). Exclusion criteria for everyone included: contraindications for MRI safety; perimenopause; less than 6 months post‐pregnancy, breastfeeding, and chemo/radiation/adjuvant therapies; unmanaged health/psychiatric conditions; endocrine disorders; and history of concussion with loss of consciousness. AMC and SM groups had the added exclusion criteria: no hormone therapy. The BSO group had the added exclusion criterion: BSO after age 49. All participants answered a demographic questionnaire, provided urine for hormone analysis, and saliva for genotyping of apolipoprotein E gene(APOE). 17 Many participants also agreed to MRI.
TABLE 1.
Group definitions.
| Group | Definition |
|---|---|
| Bilateral salpingo‐oophorectomy (BSO) | This category includes women with the BRCA1/2 mutation who have had BSO at average age 44. |
| BRCA controls (BRCA‐C) | This category includes women pre‐BSO, with intact ovaries (no BSO) and with BRCA1/2 mutation. |
| Aged‐matched controls (AMC) | This category includes women pre‐menopause, with intact ovaries and no BRCA1/2 mutation. This group is age matched to the women with BSO. |
| Spontaneous menopause (SM) | This category includes women with spontaneous or natural menopause at average age 55. |
2.2. Hormone and gene analysis
Urinary metabolites of E2 (estrone‐3‐glucuronide [E1G]) and progesterone (pregnanediol‐3 glucuronide [PdG]) and APOE genotype were collected and analyzed as previously described. 17 BRCA genotype was determined at the Familial Cancer Units prior to recruitment.
RESEARCH IN CONTEXT
Systematic review: We undertook a wide search of the literature using traditional sources (e.g., PubMed). Although a few articles have reported early bilateral salpingo‐oophorectomy (BSO) as a risk factor for Alzheimer's disease (AD), none have analyzed other contributing factors during early middle age (e.g., BRCA1, verbal IQ) and their association with BSO to impact cognition. No quantitative study has examined the link between BSO and subjective cognitive decline (SCD), and no imaging study has identified changes in brain structure associated with BSO and SCD.
Interpretation: We provide evidence that early BSO may be a risk for SCD and lead to decreases in gray matter volume in regions important for mood, memory, and language. We also show that estradiol therapy and verbal IQ may mitigate this risk.
Future directions: Future work should include longitudinal approaches to understand the trajectory of these risk and resilience factors toward dementia.
2.3. Cognitive measures
Participants were administered the following tests: logical memory—including delayed paragraph recall (Wechsler Memory Scale Form I 26 ), Rey Auditory Verbal Learning Test (RAVLT, 27 including the sum of RAVLT for list A1 through A5), spatial working memory (SPWM), 28 North American Adult Reading Test (NAART), 29 The Center for Epidemiological Studies ([CESD], Depression Scale), 30 and Perceived Stress Scale (PSS). 31 A full description of the neuropsychological protocol can be found in Gervais et al. 17
2.4. Image acquisition
We acquired images at three sites: Toronto, Siemens 3T MAGNETOM Prisma scanners at Baycrest Health Sciences Centre and the Toronto Neuroimaging Institute at the University of Toronto; and Montreal, 3T MAGNETOM Prisma‐Fit scanner at the Douglas Hospital Research Institute Brain Imaging Center. T1‐weighted anatomical images were acquired using a 3D gradient echo magnetization‐prepared rapid gradient echo sequence with voxel dimensions = 1.0mm3, 160 sagittal slices, field of view = 256mm2, echo time = 2.67 ms, repetition time = 2000 ms, and flip angle = 9°. Acquisition protocols were harmonized across scanning sites. Data from the first scanning session for each participant were used.
2.5. Image processing
After realignment, T1 images were preprocessed using the Computational Anatomy Toolbox (CAT12) (http://www.neuro.uni‐jena.de/cat12/) in SPM12. The preprocessing pipeline consisted of spatially normalizing images to the Montreal Neurological Institute (MNI) space (resampled to a voxel size of 1.5 mm), segmenting into gray matter, white matter, and cerebrospinal fluid, and smoothing with an 8 mm gaussian kernel. Quality control of all scans consisted of visual inspection before data segmentation and assessing the quality of pre‐processing using CAT12 tools.
2.6. Statistical analysis
All statistical analyses were conducted in R version 4.3.0. 32 Bivariate analyses (analysis of variance [ANOVA] or χ2 tests) were used to assess group differences with Tukey post hoc comparisons for ANOVAs and standardized residuals to calculate p values for χ2.
Multiple factor analysis (MFA) was used to assess the multivariate pattern of covariation between demographics/health and neuropsychological performance. MFA was appropriate because it allowed for the comparison and analysis of nominal and quantitative variables. Moreover, MFA can handle multiple covariates and confounders simultaneously. In our analysis, we designed three different matrices: (1) one containing nominal variables either identified in previous literature or that might be particular to our population as RandR factors—BSO, 15 BRCA, ever use of ET for early life BSO (ETBSO), 15 ever use of hormone contraceptives (HC_ever), 33 parity, 34 the APOE ε4 allele of APOE, 15 multilingualism; 35 (2) one containing quantitative variables related to RandR: E1G, 36 PdG, 36 NAART, 3 Education, 37 CESD, 38 PSS; 39 and (3) one containing mean scores from logical memory, RAVLT, and spatial working memory tasks which have been shown in the literature to either be affected specifically in our population 17 , 19 , 40 or in SM. 41 All data were centered and standardized prior to analysis. MFA uses principal component analysis on each matrix, and then normalizes by dividing its elements by the square root of the first eigenvalue from the principal component analysis—importantly, this ensures that none of the variable sets dominate representation in the overall MFA. 42 The normalized data matrices were then aggregated into one overall grand matrix that was analyzed using global principal component analysis. Significant components were determined by examining eigenvalues larger than one. The MFA was run in R using the FactoMineR package. 43
Binomial univariate logistic regression models were used to estimate the odds ratio (OR) and 95% confidence interval (CI) for the association of each factor identified in the MFA analysis with SCD as the outcome. For this analysis only, we included our SM participants because SCD is more likely to be reported in this age group and we wanted to determine whether the odds of SCD factors differed depending on the age group (BSO, mean age = 44 vs. SM, mean age = 55). Missing data were imputed using multiple imputation with the R “mice” package, 44 which uses predictive mean matching.
2.7. Voxel‐based morphometry of brain scans of women with SCD
We conducted voxel‐based morphometry on the smoothed GMV. First, a 4 × 1 full factorial model analysis was conducted to identify significant GMV differences among AMC, BSO, and SM controlling for age, scan site, and intracranial volume. Within this design, we tested for the effect of the main factor group using an F test. We then conducted an exploratory analysis and used visualization techniques to identify patterns or trends related to E1G levels. E1G was operationalized as: (1) low due to surgical menopause (BSO with and without ET, mean = 26.5 pg/mL); (2) low due to spontaneous menopause ([SM], mean = 18.9 pg/mL); (3) average as in the case of pre‐menopausal women (i.e., BRCA‐C and AMC, mean = 40 pg/mL). Post hoc group comparisons and region of interest (ROI) analyses were conducted using t tests. Alpha was set at p < 0.05, and corrections for multiple comparisons were applied using the family‐wise error (I). Clusters < 10 voxels were not considered.
3. RESULTS
3.1. Potential RandR factors for early middle‐aged women
We first conducted MFA and analyzed the following groups: BSO, BRCA‐C, and AMC. Thus, the total N for this study was 288, and the mean age of participants was 41 years.
ANOVAs (Table 2) revealed that there was a statistically significant difference among the three groups in age F (2, 285) = 33.53, p < 0.001; education F (2, 285) = 3.61, p = 0.02; and E1G levels F (2, 285) = 8.83, p = 0.0001. A Tukey honestly significant difference (HSD) test adjusting for multiple comparisons found that the mean value of age was significantly different between BRCA‐C and AMC (p < 0.001, 95% CI = −6.73, −2.89) and between BRCA‐C and BSO (p < 0.001, 95% CI = 4.60, 8.35). Regarding education, the Tukey test showed a significant difference between AMC and BSO (p = 0.02, 95% CI = −2.11, −0.13). The Tukey test for E1G showed a significant difference between BRCA‐C and BSO (p < 0.003, 95% CI = −20.90, −3.49) and between AMC and BSO (p < 0.001, 95% CI = −22.54, −5.51). χ2 tests showed a significant difference in ETBSO χ2 (2, N = 288) = 105.89, p < 0.001 and SCD χ2 (2, N = 288) = 10.54, p = 0.0005; participants with BSO were more likely to have ever taken ET (Table 2).
TABLE 2.
Cohort characteristics across groups for MFA analysis.
|
BRCA‐C (N = 97) |
AMC (N = 91) |
BSO (N = 100) |
p value | |
|---|---|---|---|---|
| Age | 38 ± 5.8 | 42.8 ± 5.0 | 44.4 ± 5.8 | <0.001 |
| Education (years) | 17.5 ± 5.8 | 18.1 ± 3.6 | 17.0 ± 2.4 | 0.02 |
| E1G | 41.3 ± 29.4 | 39.2 ± 26.5 | 26.5 ± 19.9 a | 0.00 |
| PdG | 2.8 ± 4.3 | 3.3 ± 3.7 | 3.9 ± 12.8 | 0.70 |
| ETBSO | – | – | 52 (52%) | <0.001 |
| HC_ever | 77 (79.3%) | 69 (62.7%) | 78 (78%) | 0.63 |
| Parity b | 53 (51.4%) | 40 (36.4%) | 70 (70%) | 0.25 |
| NAART | 107.2 ± 9.1 | 110.4 ± 9.0 | 108.3 ± 9.8 | 0.06 |
| Multilingualism c | 18 (16.3%) | 21 (19.1%) | 19 (19%) | 0.69 |
| PSS | 16.5 ± 6.5 | 15.5 ± 6.5 | 16.4 ± 6.6 | 0.34 |
| CESD | 11.1 ± 8.3 | 9.8 ± 6.9 | 11.0 ± 7.8 | 0.46 |
| APOE ε4 | 24 (23.2%) | 23 (20.9%) | 19 (19%) | 0.36 |
Note: continuous variables are presented as mean ± SD and categorical variables as number (percentage). Significant p values are highlighted in bold.
Abbreviations: AMC, age‐matched control; APOE, apolipoprotein E gene; BRCA, breast cancer gene; BRCA‐C, BRCA controls; BSO, bilateral salpingo‐oophorectomy; CESD, the Center for Epidemiologic Studies Depression Scale; E1G, metabolite of the hormone estradiol; ETBSO, ever use of estradiol therapy for early‐life bilateral salpingo‐oophorectomy; HC_ever, ever use of hormone contraceptives; MFA, multiple factor analysis; NAART, North American Adult Reading Test; PdG, pregnanediol glucuronide; PSS, the Perceived Stress Scale; SCD, subjective cognitive decline; SD, standard deviation.
E1G levels of BSO are higher than expected because the group comprises participants taking estradiol therapy (ET) as well as not taking ET.
Parity includes one or more live births.
Participants reported knowledge and use of at least two languages.
MFA revealed two significant dimensions that explained ≈ 45% of the cumulative variance. Dimension 1 (D1) had an eigenvalue of 1.39, and dimension 2 (D2) of 1.03 (Figure 1A, but see also Table S2 in supporting information).
FIGURE 1.
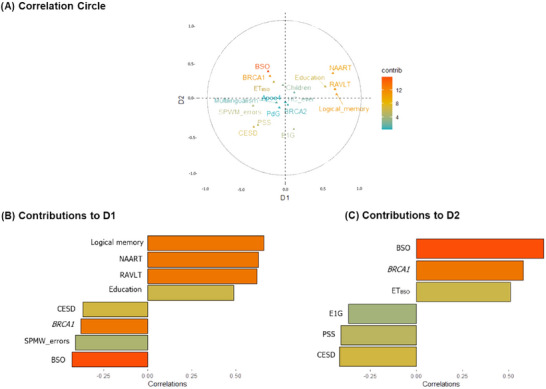
MFA correlation pattern for AMC, BRCA‐C, and BSO. A, Correlation circle showing the topographical influence in the arrangement of the variables on the graph along the abscissa (D1) and the ordinate (D2). Positively correlated variables are grouped together, whereas negative ones are positioned on opposite sides of the plot origin (opposed quadrants). For D1, the variables that were positively associated are grouped together on the right side of the plot, and the variables that were negatively associated with these are on the left side. D2 shows a similar separation with the variables positively associated at the top of the plot and the variables negatively associated at the bottom of the plot. Note that E1G is not grouped together with the other set of variables. The most contributing variables are on a gradient, with orange representing the most contribution to the pattern. The bar plots for (B) and (C) represent the moderate and strong correlations of the variables that contributed the most. Variables in the top of the bar plots show positive correlations, and variables on the bottom show negative correlations. Variables that contribute the most to D1 and D2 (either positive or negative correlations) are the most important in explaining the variability in the data set. APOE, apolipoprotein E gene; BRCA1, BRCA1m , breast cancer gene; BSO, bilateral salpingo‐oophorectomy; CESD, total score of the Center for Epidemiologic Studies Depression Scale; D1, dimension 1; D2, dimension 2; E1G, metabolite of the hormone estradiol; ETBSO, ever use of estradiol therapy for early life BSO; HC_ever, ever use of hormone contraceptives; MFA, multiple factor analysis; NAART, North American Adult Reading Test IQ; PdG, pregnanediol glucuronide; PSS, total score of the Perceived Stress Scale; RAVLT, Rey Auditory Verbal Learning test; SPWM_errors, total of errors in the Spatial Working Memory Test
D1 showed a general pattern for early middle age (mean age 41) and showed a positive correlation among logical memory, verbal IQ (NAART), the RAVLT, and education (Figure 1B; Table S2). All these variables correlated negatively with BSO, increased errors in spatial working memory, BRCA1m , and CESD. Thus, having BSO and BRCA1 were inversely associated with neuropsychological performance but positively associated with depressed mood. Verbal IQ and education, which have been suggested as resilience proxies in past literature, were positively associated with neuropsychological performance and inversely associated with BRCA1, BSO, and depressed mood.
D2 showed a pattern specifically for women with BSO. In D2, the main negative loading factor identified in D1 (i.e., BSO) correlated positively with BRCA1, and ETBSO, but negatively with E1G levels. Thus, BSO was associated with decreased E1G levels in general, as well as with both increased BRCA1 and increased ETBSO. This pattern of covariation was negatively associated with CESD and PSS scores. Overall, D2 extended D1's results revealing a pattern associated with BSO, alone related to ETBSO and ET's possible effects on depressive mood and stress (Figure 1C; Table S2).
3.2. Potential RandR factors associated with SCD
Across all cohort groups, 102 women reported problems with their memory on their demographic questionnaire, which we classified as SCD. Some of the most common memory problems reported were remembering specific words, names of people, names of streets, names of books and movies, where their car was parked, and why they were involved in certain behaviors such as walking into a room or forgetting what they were doing. To study SCD, we included another group; women with SM (n = 45, mean age = 55) because SCD has previously been reported in that age group (Table 3). Thus, including the SM group increased the total N for this sub‐study to 333 and the mean age of the full cohort was 43.5.
TABLE 3.
Cohort characteristics across groups for SCD study.
|
BRCA‐C (N = 97) |
AMC (N = 91) |
BSO (N = 100) |
SM (n = 45) |
p value | |
|---|---|---|---|---|---|
| Age | 38 ± 5.8 | 42.8 ± 5.0 | 44.4 ± 5.8 | 55.0 ± 3.2 | <0.001 |
| Education (years) | 17.5 ± 5.8 | 18.1 ± 3.6 | 17.0 ± 2.4 | 17.2 ± 2.4 | 0.07 |
| E1G | 41.3 ± 29.4 | 39.2 ± 26.5 | 26.5 ± 19.9 a | 18.9 ± 13.9 | <0.001 |
| PdG | 2.8 ± 4.3 | 3.3 ± 3.7 | 3.9 ± 12.8 | 3.5 ± 12.6 | 0.84 |
| ETBSO | 2 (2.0%) | 0 | 52 (52%)0.02 | 8 (17.7%) | <0.001 |
| HC_ever | 75 (77.3%) | 69 (75.8%) | 78 (78%) | 38 (84.4%) | 0.57 |
| Parity b | 53 (54.6%) | 40 (43.9%) | 70 (70%) | 27 (60%) | 0.02 |
| NAART | 107.2 ± 9.1 | 110.4 ± 9.0 | 108.3 ± 9.8 | 111.5 ± 9.2 | 0.02 |
| Multilingualism c | 18 (18.5%) | 21 (23.0%) | 19 (19%) | 10 (22.2%) | 0.84 |
| PSS | 16.5 ± 6.5 | 15.5 ± 6.5 | 16.4 ± 6.6 | 12.5 ± 6.1 | 0.00 |
| CESD | 11.1 ± 8.3 | 9.8 ± 6.9 | 11.0 ± 7.8 | 7.4 ± 6.2 | 0.03 |
| APOE ε4 | 24 (24.7%) | 23 (25.2%) | 19 (19%) | 12 (26.6%) | 0.56 |
Note: Continuous variables are presented as mean ± SD and categorical variables as number (percentage). Significant p values are highlighted in bold.
E1G levels of BSO are higher than expected because the group comprises participants taking estradiol therapy (ET) as well as not taking ET.
Parity includes one or more live births.
Participants reported knowledge and use of at least two languages.
Abbreviations: AMC, age‐matched control; APOE, apolipoprotein E gene; BRCA, BReast CAncer gene; BRCA‐C, BRCA controls; BSO, bilateral salpingo‐oophorectomy; CESD, the Center for Epidemiologic Studies Depression Scale; E1G, metabolite of the hormone estradiol; ETBSO, ever use of estradiol therapy for early life bilateral salpingo‐oophorectomy; HC_ever, ever use of hormone contraceptives; NAART, North American Adult Reading Test; PdG, pregnanediol glucuronide; PSS, the Perceived Stress Scale; SCD, subjective cognitive decline; SD, standard deviation; SM, spontaneous menopause.
ANOVAs for this group comparison (BSO, BRCA‐C, AMC, SM) revealed statistically significant differences in: age F (3, 329) = 106.4, p < 0.001; E1G levels F (3, 329) = 12.9, Pp < 0.001; NAART F (3, 329) = 3.12, p = 0.02; PSS F (3, 329) = 3.431, p = 0.01; and CESD F (3, 329) = 2.958, p = 0.03 (Table 3). The Tukey HSD test for multiple comparisons found that unsurprisingly BRCA‐C participants were significantly younger than AMC (p < 0.001, 95% CI = −2.80, −6.82), BSO (p < 0.001, 95% CI = 4.51, 8.44), and SM (p < 0.001, 95% CI = 14.56, 19.52) participants. AMC participants were also significantly younger than the SM (p < 0.001, 95% CI = 9.72, 14.73) and BSO (p < 0.001, 95% CI = 8.09, 13.03) groups. Post hoc analysis for E1G levels showed that the BRCA‐C group had higher E1G levels than both BSO (p = 0.0001, 95% CI = −23.87, −5.81) and SM (p < 0.001, 95% CI = −33.05, −10.19) groups. AMCs also had higher E1G levels than both BSOs (p = 0.001, 95% CI = −22.30, −3.94), and SMs (p < 0.001, 95% CI = −31.44, −8.34). For NAART, SM participants had significantly higher scores than BRCA‐C (p = 0.03, 95% CI = 0 = 9.03, 0.26) participants. For PSS, the BRCA‐C group had significantly higher scores than SMs (p = 0.01, 95% CI = −6.52, −0.46); and BSO participants also had significantly higher PSS scores than SMs (p = 0.02, 95% CI = −6.37, −0.34). Similarly, for CESD, BRCA‐C and BSO participants had significantly higher scores than the SM group (p = 0.03, 95% CI = −7.42, −0.16; Pp = 0.04, 95% CI = −7.16, −0.11, respectively).
χ2 tests revealed a significant difference in ETBSO χ2 (3, N = 333) = 104.76, p < 0.001; parity χ2 (3, N = 333) = 9.25, p = 0.02; and SCD χ2 (3, N = 333) = 8.28, p = 0.04. Participants with BSO were more likely to have ever taken ETBSO, and both BSO and SM participants were more likely to have children (parity).
Univariate logistic regression revealed factors that significantly predicted SCD: BSO was the main predictor associated with higher odds of SCD (OR = 2.39, 95% CI [1.48–3.89], p = < 0.001), followed by BRCA1 (OR = 1.94, 95% CI [1.21–3.13], p = < 0.001), and older age (OR = 1.03, 95% CI [1.00–1.07], p = 0.02). Regarding resilience proxies, verbal IQ (OR 0.97, 95% CI [0.94–0.99], p = 0.02) was associated with decreased odds of SCD as were higher levels of E1G (OR 0.0.98, 95% CI [0.97–0.99], p = 0.002). There was also a small effect showing that parity might be associated with decreased odds of SCD (OR 0.56, 95% CI [0.31–0.99], p = 0.05; Table 4).
TABLE 4.
Univariate logistic regression analysis for SCD study (n = 333).
| Factors | β | p | OR | Lower CI | Upper CI |
|---|---|---|---|---|---|
| Age | 0.03 | 0.02 | 1.03 | 1.00 | 1.07 |
| BSO | 0.87 | <0.001 | 2.39 | 1.48 | 3.89 |
| SM | 0.07 | 0.82 | 0.93 | 0.49 | 1.75 |
| BRCA1 | 0.66 | <0.001 | 1.94 | 1.21 | 3.13 |
| APOE ε4 | 0.12 | 0.61 | 1.13 | 0.68 | 1.86 |
| E1G | −0.01 | 0.002 | 0.98 | 0.97 | 0.99 |
| ETBSO | 0.09 | 0.79 | 1.09 | 0.63 | 1.87 |
| Parity a | −0.56 | 0.05 | 0.56 | 0.31 | 0.99 |
| Education | 0.08 | 0.17 | 1.08 | 0.96 | 1.22 |
| NAART | −0.03 | 0.02 | 0.97 | 0.94 | 0.99 |
| PSS | 0.05 | <0.001 | 1.06 | 1.02 | 1.09 |
| CESD | 0.05 | 0.001 | 1.05 | 1.02 | 1.09 |
Parity includes one or more live births.
Abbreviations: APOE, apolipoprotein E gene; BRCA1, BReast CAncer gene; BSO, bilateral salpingo‐oophorectomy; CESD, The Center for Epidemiologic Studies Depression Scale; CI, confidence interval; E1G, metabolite of the hormone estradiol; ETBSO, ever use of estradiol therapy for early life bilateral salpingo‐oophorectomy; NAART, North American Adult Reading Test; OR, odds ratio; PSS, The Perceived Stress Scale; SCD, subjective cognitive decline; SM, spontaneous menopause.
Although descriptive statistics for group comparisons revealed that both BSO and SM were more likely to report SCD, the logistic regression showed that only BSO might increase the odds of SCD. SM was not associated with any odds of SCD (OR 0.93, 95% CI [0.49–1.72], p = 0.82).
3.3. GMV differences
3.3.1. General GMV differences across different subgroups
Across all groups, there were 177 participants who completed an MRI scanning session. We first analyzed GMV differences in all the groups that have been previously included in the different analysis controlling for age, scan site, and intracranial volume.
The full factorial analysis comparing the four groups (BSO, BRCA‐C, AMC, SM) showed a significant main group effect that indicated lower GMV in two clusters spanning the left inferior frontal gyrus (MNI coordinates x = −40, y = −21, z = 18, t = 5.28, p < 0.1, family‐wise error [FWE] corrected, 0.000) and the left postcentral gyrus (MNI coordination x = −54, y = −12, z = 46, t = 5.11, p < 0.1, FWE corrected, 0.001; Figure 2A). Exploratory analysis revealed that the groups with low E1G levels (mean = 22pg/mL) regardless of age (i.e., BSO and SM) had less GMV than the other groups (Table 5; Figure 2A). Post hoc tests indicated that only the comparison between BSO and BRCA‐C was statistically significant.
FIGURE 2.
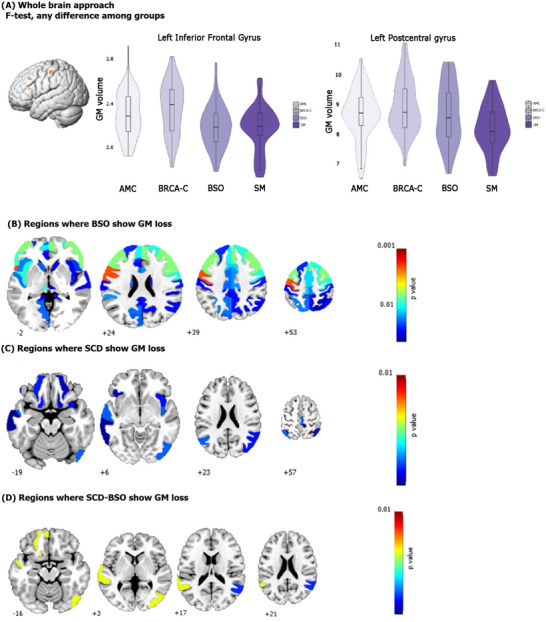
Group comparison of gray matter volume: (A) shows the two clusters found in the whole brain approach and the gray matter differences among the groups, (B) shows the post hoc analysis revealing greater gray matter loss in BSO group, (C) shows the ROI analysis comparing women with and without SCD, (D) shows the ROI analysis for women with BSO and SCD. AMC, age‐matched control; BRCA1, BRCA1m , breast cancer gene; BSO, bilateral salpingo‐oophorectomy; GM, gray matter; ROI, region of interest; SCD, subjective cognitive decline; SM, spontaneous menopause
TABLE 5.
Brain volumes across groups.
| NO‐SCD (n = 118) | SCD (n = 59) | |||||||
|---|---|---|---|---|---|---|---|---|
|
BRCA‐C (n = 34) |
AMC (n = 32) |
BSO (n = 33) |
SM (n = 19) |
BRCA‐C (n = 6) |
AMC (n = 19) |
BSO (n = 23) |
SM (n = 11) |
|
| TIV | 1405.9 ± 133,99 | 1390.5 ± 103,8 | 1376.9 ± 136,6 | 1433.3 ± 95.5 | 1394.7 ± 46.4 | 1410.0 ± 93.6 | 1369.3 ± 123.0 | 1391.8 ± 86.2 |
| GMV_total | 658.3 ± 58.67 | 638.7 ± 48.1 | 629.2 ± 56.7 | 626.1 ± 34.9 | 644.8 ± 24.4 | 638.1 ± 41.4 | 616.7 ± 52.3 | 610,8 ± 46.4 |
Note: Variables are presented as mean ± SD.
Abbreviations: AMC, age‐matched control; BRCA‐C: BRCA controls; BSO, bilateral salpingo‐oophorectomy; GMV_total, total value for gray matter volume; SCD, subjective cognitive decline; SD, standard deviation; SM, spontaneous menopause; TIV, intracranial volume.
BSO participants had lower GMV than BRCA‐C participants in both hemispheres. In the right, there were decreases in the: opercular part of the inferior frontal gyrus (t statistic = 3.47; corrected p = 0.014480), precentral gyrus (t statistic = 3.22; corrected p = 0.011865), middle frontal gyrus (t statistic = 3.58; corrected p = 0.011130), triangular part of the inferior frontal gyrus (t statistic = 3.69; corrected p = 0.019654), supplementary motor cortex (t statistic = 2.98; corrected p = 0.026301), superior frontal gyrus medial segment (t statistic = 3.51; corrected p = 0.031541), medial frontal cortex (t statistic = 2.98; corrected p = 0.037572), basal forebrain (t statistic = 2.35; corrected p = 0.020642), and anterior orbital gyrus (t statistic = 1.98; corrected p = 0.024475). In the left, there were GMV decreases in the: opercular part of the inferior frontal gyrus (t statistic = 4.18; corrected p = 0.004180), precentral gyrus (t statistic = 4.18; corrected p = 0.003647), middle frontal gyrus (t statistic = 3.63; corrected p = 0.001913), triangular part of the inferior frontal gyrus (t statistic = 3.68; corrected p = 0.002870), and the supplementary motor cortex (t statistic = 2.96; corrected p = 0.023776). In addition, there were decreases in the: ventral diencephalon (t statistic = 3.28; corrected p = 0.011067), the posterior insula (t statistic = 3.17; corrected p = 0.047423), and the cerebellum exterior (t statistic = 2.32; corrected p = 0.033355).
The main difference between hemispheres was that the right hemisphere included gray matter decreases in the superior frontal gyrus, basal forebrain, and medial frontal cortex, while the left included the ventral diencephalon, posterior insula, and cerebellum (Figure 2B).
3.3.2. SCD
ROI analysis comparing GMV between participants with and without SCD, revealed GMV decreases in both hemispheres. In the right, decreases were in the precentral gyrus medial segment (t statistic = 3.08; corrected p = 0.035445) and posterior insula (t statistic = 3.02; corrected p = 0.035445). Unique were decreases in the: inferior occipital gyrus (t statistic = 3.03; corrected p = 0.035445), middle occipital gyrus (t statistic = 2.80; corrected p = 0.040423), angular gyrus (t statistic = 2.78; corrected p = 0.040423), anterior insula (t statistic = 2.78; corrected p = 0.040423), medial orbital gyrus (t statistic = 2.67; corrected p = 0.045681), and postcentral gyrus medial segment (t statistic = 2.59; corrected p = 0.048429). The left hemisphere had decreases in the: angular gyrus (t statistic = 3.20; corrected p = 0.035445) and medial orbital gyrus (t statistic = 2.92; corrected p = 0.039439). Also, unique were decreases in the: superior temporal gyrus (t statistic = 3.38; corrected p = 0.035445), orbital part of the inferior frontal gyrus (t statistic = 2.68; corrected p = 0.045681), and middle temporal gyrus (t statistic = 2.60; corrected p = 0.048429).
Thus, these results differed from the whole cohort findings in that there were GMV decreases related specifically to SCD regardless of E2 loss, which included right occipital cortex and left temporal gyri (Figure 2C).
3.3.3. BSO‐SCD
We then analyzed this same comparison (SCD and no SCD) within the BSO group. Participants with both SCD and BSO had GMV loss in the right angular gyrus (t statistic = 3.10; corrected p = 0.038415) and inferior occipital gyrus (t statistic = 3.49; corrected p = 0.018913); in the left, GMV loss in superior temporal gyrus (t statistic = 3.67; corrected p = 0.018913), gyrus rectus (t statistic = 3.64; corrected p = 0.018913), and the medial orbital gyrus (t statistic = 3.42; corrected p = 0.018913; Figure 2D). Thus, the right hemisphere had losses in the posterior part of inferior parietal lobe and the occipital lobe, while the left, in the temporal lobe.
Overall, BSO was related to GMV decreases mainly in the left inferior frontal gyrus and the left postcentral gyrus, which are important for language processing, 45 working memory, 46 and emotional regulation, 47 , 48 and also in regions in which E2 plays a role such as the basal forebrain. 49 SCD alone (irrespective of E2 status) was related to decreases in occipital‐temporal regions, which are important for associative memory, 50 a potential hallmark for SCD as it has been associated with amyloid burden in SCD individuals. 51 Participants with both SCD and BSO showed less GMV in some of the regions that were shown to be relevant for SCD and E2, but that are also important for semantic cognition (e.g., right angular gyrus), 52 and phonological processing (left superior temporal gyrus). 53 The latter pattern of results showing decreases in language areas (Broca's area) coincides with a previous observational qualitative study, 54 and also with the subjective experience that the women in the cohort of the present study report about not being able to retrieve specific words.
4. DISCUSSION
4.1. Summary of findings
RandR has never been studied in any population of younger middle‐aged women, a unique age offering a window of opportunity for preventive strategies against the earliest stages of late‐life dementia. Particularly novel is the identification of both BSO and BRCA1 as main predictors of SCD in early middle age. Also novel is the identification of key resilience factors and GMV patterns associated with SCD, itself a risk for late‐life dementia. 7
4.2. Potential RandR factors for younger middle‐aged women
The first study revealed that verbal IQ, education, and ETBSO were positively associated with performance on verbal episodic and spatial working memory tasks. Performance on these memory tasks was negatively associated with BSO, BRCA1, and depressive mood. Although it is difficult to determine the influence of each proxy separately, MFA analysis allowed the exploration of a composite proxy measure that represented overall RandR patterns while showing the individual contributions of each factor to neuropsychology performance. While cognitive reserve is influenced by many different factors—for example, it is difficult to separate verbal IQ from education— 3 our MFA showed that both factors are related to resilience as indexed by strong performance on neuropsychology tasks, but verbal IQ contributed more than education. This coincides with theories identifying cognitive reserve as a dynamic construct. 3
In the same way, BSO, BRCA1, and depressive mood all relate to cognitive risk among early middle‐aged women, but BSO was the factor that contributed the most to poorer neuropsychological performance on both memory tasks. Other studies, including previous studies from our own lab, have shown poorer performance on verbal and spatial working memory in women with early‐life BSO who are not taking ET. 17 , 19 , 40 However, the present study is the first to examine the association of BSO and cognitive changes with other contributing factors among early middle‐aged women.
After BSO, BRCA1 was an important risk factor in these younger women. There are studies in both non‐human animals and humans 55 , 56 showing that BRCA1, itself, is associated with smaller brains, 55 , 56 decreased hippocampal memory performance, 57 and reduced expression in people with AD and other tauopathies. 58 In the present study, the risk that BRCA1 presents cannot be separated from that of BSO; therefore, we are only able to say that the combined effects of BRCA1 and BSO present a particular risk for women with BRCA1. It is also the case that in our cohort a higher percentage of women with BRCA1 have had cancer and therefore, chemotherapy. Although they were at least 6 months without chemotherapy at the time of testing (average time = 4.6 years) and an exploratory analysis comparing those with and without chemotherapy showed no differences (see 4.1 in supporting information; Table S3), the effects of chemotherapy coupled with BRCA1 cannot be ruled out.
Depressive mood and perceived stress were also risk factors for early middle‐aged women and these loading factors were positively associated with BSO. Previous studies have shown depressive mood and clinically perceived stress after BSO. 59 Our study showed that the previously reported poor mood and perceived stress after BSO has cognitive consequences. Notably, a few RandR factors suggested in previous literature were not relevant factors in our cohort. Specifically, it is notable that APOE ε4 contributed so little risk. In older women with early‐life BSO, APOE ε4 increases the risk of late‐life AD four times that than for women with intact ovaries. 15 However, the lack of early‐life effect is supported by literature showing that the risk of carrying APOE ε4 has its larger effect in older age, 60 and our cohort comprised younger women.
4.3. Potential risks and resiliencies associated with SCD
A substantial subset of the women in our cohort reported subjective cognitive changes, even those without BSO. It's possible that there was a recruitment bias in that women may have joined our study because they had memory concerns. With respect to the BSO participants this is in concert with a small, Australian qualitative study of women who report memory problems such as brain fog, memory and retrieval issues, processing speed decrements, and attention problems within 24 months of BSO. 54 In our full cohort, BSO, BRCA1, older age, depressive mood, and stress predicted higher odds of SCD. Our study is the first to identify BSO and BRCA1 as possible predictors of SCD in younger middle aged women, and it is also in line with previous mixed‐sex studies which have identified mood disorders as possible risk factors for SCD in late middle age. 38 , 39 Moreover, higher levels of E1G, verbal IQ, and parity were associated with reduced odds of SCD, supporting their role as resilience proxies even in younger women. Recently, parity has been associated with a lower risk of AD; 61 our study adds to these results showing reduced odds of SCD in younger women.
4.4. Differences on GMV
The first analysis comparing all groups showed an E2 effect regardless of age. Groups with low E2 (BSO and SM) had less GMV in the left inferior frontal gyrus and the postcentral gyrus. Post hoc tests then showed that only comparison between BSO and BRCA‐C was significant. We expected to see a significant difference also between AMC and BSO because AMC had E2 levels comparable to those of BRCA‐C; however, note that AMC reported a higher percentage of SCD than BRCA‐C. It is possible that the brain structure of some AMC was similar to that of BSO or SM participants. Importantly, the fact that the analysis only revealed differences between BRCA‐C and BSO suggests that the first analysis detected brain differences specifically related to ovarian removal, and that the brain changes related to BSO (and not age, BRCA mutation, or SCD) might occur in the prefrontal cortex.
The second analysis identified a brain pattern specifically related to SCD. Regardless of age, group, or menopause type, participants reporting SCD had less GMV primarily in the left temporal gyri—a neuroimaging pattern that has been reported previously in mixed‐sex studies analyzing SCD 51 and preclinical AD in older adults. 7 This suggests that some GMV loss in SCD might correspond with accelerated aging 8 because mixed‐sex studies in the aging literature show faster left‐hemisphere degeneration followed by decreases in language tasks. 62
For women with BSO‐SCD, GMV decreases were mainly in the medial prefrontal cortex in regions sensitive to semantic cognition, 52 language, 53 and E2 49 and that also change in mood disorders. 47 Previous work in our Swedish cohort of women with BSO substantially aligns with the current findings in that they had reduced GMV in the frontal lobe as well as left temporal structures for both BSO and BRCA‐C. 63 However, whether the changes in the Swedish cohort were commensurate with the SCD changes in our Canadian cohort is yet to be determined.
4.5. Strengths and limitations
One limitation of the current study is that our cohort size is relatively small and only exploratory for the imaging results. While the MFA analysis does show moderate to strong effect sizes and is powered for our early middle‐aged cohort (n = 288), our MRI study (n = 177) should be replicated with larger numbers in the subgroups. One important risk factor that was omitted was poor sleep which, in mixed‐sex studies, is well established as a risk factor for late‐life AD. 64 Indeed, in our own studies, we have previously found that women with BSO have poorer sleep than both AMC and SM, but we have not been able to correlate this finding with decreased memory function due to BSO. 40 , 65 It may be that our cohort is still too young for sleep to have an impact on verbal episodic and spatial working memory performance, or that we were underpowered in the previous study to find this association. In the current study, which includes the full cohort, we did not have information about sleep for all participants; and thus, we were not able to include the variable of sleep in our statistical models. However, sleep is an important variable that might contribute to poorer cognitive outcomes and should be considered in future research with larger cohorts. Another limitation is that our cohort is demographically relatively homogeneous (see supporting information section 2.2), and thus our results may not be generalizable to other female cohorts. However, the uniformly high education of our cohort makes the finding of cognitive issues that much more notable.
5. CONCLUSION
Addressing RandR in women requires a multifaceted approach that considers not only clinical, lifestyle, and psychosocial factors but also biological variables unique to women: ovarian hormone changes, parity, menopause type, and hormone treatments. 66 Our study delineated some of these RandR factors in younger middle‐aged women and identified BSO, depressed mood, and stress as main risks for decrements in verbal episodic and spatial working memory in early middle age with verbal IQ, education, and ETBSO as important resilience proxies. Moreover, our imaging analysis revealed that women with BSO had unique patterns of GMV loss in prefrontal areas. Women with BSO plus SCD had additional GMV loss in areas important for semantic and phonological processing. Except for a small qualitative study on memory complaints, our study is the first to describe SCD and GMV associated to SCD in women with BSO prior to SM. The fact that younger women have SCD underscores the importance of early‐life events such as BSO contributing to later life AD.
CONFLICT OF INTEREST STATEMENT
The authors have no financial or non‐financial interests to disclose.
CONSENT STATEMENT
Written informed consent was obtained from all participants.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The authors thank Dr. William Foulkes from the Department of Human Genetics at McGill University for their help with participant recruitment. They would also like to thank the dedicated research assistants of the Einstein Lab who contributed to data curation. This work was supported by the Wilfred and Joyce Posluns Chair in Women's Brain Health and Aging from the Posluns Family Foundation, Canadian Institutes of Health Research (CIHR), Ontario Brain Institute, and Ontario Brain Institute and the Centre for Aging+ Brain Health Innovation (CABHI) to GE (grant WJP‐150643), the Canadian Consortium on Neurodegeneration in Aging (CCNA) Phase II to GE (grant CCNA 049‐04; CIHR reference number: CNA 163902), Canadian Cancer Society (310336), Canadian Institutes of Health Research (MOP‐130490), the Jacqueline Ford Gender and Health Fund to GE, and the Research Chair in Women's Health—a salary award to Dr. Hampson by the Canadian Institutes of Health Research (2010‐2015).
Calvo N, Gravelsins L, Brown A, et al. Cognitive and brain health in women with early bilateral salpingo‐oophorectomy: Implications for risk, resilience, and subjective cognitive decline. Alzheimer's Dement. 2025;21:e70454. 10.1002/alz.70454
REFERENCES
- 1. Stern Y, Albert M, Barnes CA, Cabeza R, Pascual‐Leone A, Rapp PR. A framework for concepts of reserve and resilience in aging. Neurobiol Aging. 2023;124:100‐103. doi: 10.1016/j.neurobiolaging.2022.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Livingston G, Huntley J, Liu KY, et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet. 2024;404(10452):572‐628. doi: 10.1016/S0140-6736(24)01296-0 [DOI] [PubMed] [Google Scholar]
- 3. Boyle R, Knight SP, De Looze C, et al. Verbal intelligence is a more robust cross‐sectional measure of cognitive reserve than level of education in healthy older adults. Alzheimers Res Ther. 2021;13(1):128. doi: 10.1186/s13195-021-00870-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Calvo N, Ibáñez A, Muñoz E, García AM. A core avenue for transcultural research on dementia: on the cross‐linguistic generalization of language‐related effects in Alzheimer's disease and Parkinson's disease. Int J Geriatr Psychiatry. 2018;33(6):814‐823. doi: 10.1002/gps.4712 [DOI] [PubMed] [Google Scholar]
- 5. Bialystok E, Craik FI, Freedman M. Bilingualism as a protection against the onset of symptoms of dementia. Neuropsychologia. 2007;45(2):459‐464. doi: 10.1016/j.neuropsychologia.2006.10.009 [DOI] [PubMed] [Google Scholar]
- 6. Snyder HM, Asthana S, Bain L, et al. Sex biology contributions to vulnerability to Alzheimer's disease: a think tank convened by the Women's Alzheimer's Research Initiative. Alzheimers Dement. 2016;12(11):1186‐1196. doi: 10.1016/j.jalz.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rabin LA, Smart CM, Amariglio RE. subjective cognitive decline in preclinical Alzheimer's disease. Annu Rev Clin Psychol. 2017;13(1):369‐396. doi: 10.1146/annurev-clinpsy-032816-045136 [DOI] [PubMed] [Google Scholar]
- 8. Crestol A, Rajagopal S, Lissaman R, et al. Menopause status and within‐group differences in chronological age affect the functional neural correlates of spatial context memory in middle‐aged females. J Neurosci. 2023;43(50):8756‐8768. doi: 10.1523/JNEUROSCI.0663-23.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coughlan GT, Betthauser TJ, Boyle R, et al. Association of age at menopause and hormone therapy use with tau and β‐amyloid positron emission tomography. JAMA Neurol. 2023;80(5):462‐473. doi: 10.1001/jamaneurol.2023.0455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Edwards H, Duchesne A, Au AS, Einstein G. The many menopauses: searching the cognitive research literature for menopause types. Menopause. 2019;26(1):45. doi: 10.1097/GME.0000000000001171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beard RW, Kennedy RG, Gangar KF, et al. Bilateral oophorectomy and hysterectomy in the treatment of intractable pelvic pain associated with pelvic congestion. BJOG Int J Obstet Gynaecol. 1991;98(10):988‐992. doi: 10.1111/j.1471-0528.1991.tb15336.x [DOI] [PubMed] [Google Scholar]
- 12. Marchetti C, De Felice F, Palaia I, et al. Risk‐reducing salpingo‐oophorectomy: a meta‐analysis on impact on ovarian cancer risk and all cause mortality in BRCA 1 and BRCA 2 mutation carriers. BMC Womens Health. 2014;14(1):150. doi: 10.1186/s12905-014-0150-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rebbeck TR, Kauff ND, Domchek SM. Meta‐analysis of risk reduction estimates associated with risk‐reducing salpingo‐oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101(2):80‐87. doi: 10.1093/jnci/djn442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69(11):1074‐1083. doi: 10.1212/01.wnl.0000276984.19542.e6 [DOI] [PubMed] [Google Scholar]
- 15. Calvo N, McFall GP, Ramana Shreeyaa, et al. Associated risk and resilience factors of Alzheimer disease in women with early bilateral oophorectomy: data from the UK Biobank. J Alzheimers Dis. 2024;102(1):119‐128. doi: 10.3233/JAD-240646 [DOI] [PubMed] [Google Scholar]
- 16. Sherwin BB. Estrogen and cognitive functioning in women. Endocr Rev. 2003;24(2):133‐151. doi: 10.1210/er.2001-0016 [DOI] [PubMed] [Google Scholar]
- 17. Gervais NJ, Au A, Almey A, et al. Cognitive markers of dementia risk in middle‐aged women with bilateral salpingo‐oophorectomy prior to menopause. Neurobiol Aging. 2020;94:1‐6. doi: 10.1016/j.neurobiolaging.2020.04.019 [DOI] [PubMed] [Google Scholar]
- 18. Brown A, Gervais NJ, Rieck J, et al. Women's brain health: midlife ovarian removal affects associative memory. Mol Neurobiol. 2023;60(11):6145‐6159. doi: 10.1007/s12035-023-03424-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gervais NJ, Gravelsins L, Brown A, et al. Scene memory and hippocampal volume in middle‐aged women with early hormone loss. Neurobiol Aging. 2022;117:97‐106. doi: 10.1016/j.neurobiolaging.2022.05.003 [DOI] [PubMed] [Google Scholar]
- 20. Zeydan B, Tosakulwong N, Schwarz CG, et al. Association of bilateral salpingo‐oophorectomy before menopause onset with medial temporal lobe neurodegeneration. JAMA Neurol. 2019;76(1):95‐100. doi: 10.1001/jamaneurol.2018.3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rocca WA, Lohse CM, Smith CY, Fields JA, Machulda MM, Mielke MM. Association of premenopausal bilateral oophorectomy with cognitive performance and risk of mild cognitive impairment. JAMA Netw Open. 2021;4(11):e2131448‐e2131448. doi: 10.1001/jamanetworkopen.2021.31448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jessen F, Amariglio RE, Van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10(6):844‐852. doi: 10.1016/j.jalz.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nicholas CR, Dowling NM, Racine AM, et al. Longitudinal assessment of self‐and informant‐subjective cognitive complaints in a sample of healthy late‐middle aged adults enriched with a family history of Alzheimer's disease. J Int Neuropsychol Soc. 2017;23(8):617‐626. doi: 10.1017/S1355617717000509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jessen F, Amariglio RE, Buckley RF, et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020;19(3):271‐278. doi: 10.1016/S1474-4422(19)30368-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reuben R, Karkaby L, McNamee C, Phillips NA, Einstein G. Menopause and cognitive complaints: are ovarian hormones linked with subjective cognitive decline?. Climacteric. 2021;24(4):321‐332. doi: 10.1080/13697137.2021.1892627 [DOI] [PubMed] [Google Scholar]
- 26. Stone CP, Wechsler D. Wechsler Memory Scale Form II. Psychological Corporation; 1945. [Google Scholar]
- 27. Lezak MurielDeutsch. Neuropsychological Assessment. Oxford University Press; 2004. [Google Scholar]
- 28. Duff SJ, Hampson E. A sex difference on a novel spatial working memory task in humans. Brain Cogn. 2001;47(3):470‐493. doi: 10.1006/brcg.2001.1326 [DOI] [PubMed] [Google Scholar]
- 29. Blair JR, Spreen O. Predicting premorbid IQ: a revision of the national adult reading test. Clin Neuropsychol. 1989;3(2):129‐136. doi: 10.1080/13854048908403285 [DOI] [Google Scholar]
- 30. RadloffLS. The CES‐D Scale: a self‐report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385‐401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 31. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385‐396. [PubMed] [Google Scholar]
- 32. Team RC . A language and environment for statistical computing. R foundation for statistical computing. 2022. R Foundation for Statistical Computing; 2021. http://www.R-project.org/ [Google Scholar]
- 33. Gravelsins L, Duncan K, Einstein G. Do oral contraceptives affect young women's memory? Dopamine‐dependent working memory is influenced by COMT genotype, but not time of pill ingestion. Plos ONE. 2021;16(6):e0252807. doi: 10.1371/journal.pone.0252807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mielke MM. Sex and gender differences in Alzheimer's disease and Alzheimer's disease dementia. Psychiatr Times. 2018;35(11):14‐17. [PMC free article] [PubMed] [Google Scholar]
- 35. Calvo N, Anderson JA, Berkes M, Freedman M, Craik FI, Bialystok E. Gray matter volume as evidence for cognitive reserve in bilinguals with mild cognitive impairment. Alzheimer Dis Assoc Disord. 2023;37(1):7‐12. doi: 10.1097/WAD.0000000000000549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McEwen BS, Woolley CS. Estradiol and progesterone regulate neuronal structure and synaptic connectivity in adult as well as developing brain. Exp Gerontol. 1994;29(3‐4):431‐436. doi: 10.1016/0531-5565(94)90022-1 [DOI] [PubMed] [Google Scholar]
- 37. Roe CM, Xiong C, Miller JP, Morris JC. Education and Alzheimer disease without dementia: support for the cognitive reserve hypothesis. Neurology. 2007;68(3):223‐228. doi: 10.1212/01.wnl.0000251303.50459.8a [DOI] [PubMed] [Google Scholar]
- 38. Brigola AG, Manzini CSS, Oliveira GBS, Ottaviani AC, Sako MP, Vale FAC. Subjective memory complaints associated with depression and cognitive impairment in the elderly: a systematic review. Dement Neuropsychol. 2015;9:51‐57. doi: 10.1590/S1980-57642015DN91000009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Podlesek A, Komidar L, Kavcic V. The relationship between perceived stress and subjective cognitive decline during the COVID‐19 epidemic. Front Psychol. 2021;12:647971. doi: 10.3389/fpsyg.2021.647971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gervais NJ, Lauzon C, Brown A, et al. Effects of early surgical menopause on sleep, memory, and medial temporal lobe structure at midlife: developing topics. Alzheimers Dement. 2020;16:e047548. doi: 10.1002/alz.047548 [DOI] [Google Scholar]
- 41. Tierney MC, Yao C, Kiss A, McDowell I. Neuropsychological tests accurately predict incident Alzheimer disease after 5 and 10 years. Neurology. 2005;64(11):1853‐1859. doi: 10.1212/01.WNL.0000163773.21794.0B [DOI] [PubMed] [Google Scholar]
- 42. Abdi H, Williams LJ, Valentin D. Multiple factor analysis: principal component analysis for multitable and multiblock data sets. WIREs Comp Stat. 2013; 5(2): 149‐179. doi: 10.1002/wics.1246 [DOI] [Google Scholar]
- 43. Lê S, Josse J, Husson F. FactoMineR: an R package for multivariate analysis. J Stat Softw. 2008;25:1‐18. 10.18637/jss.v025.i01 [DOI] [Google Scholar]
- 44. Van Buuren Stef, Groothuis‐Oudshoorn Karin. mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1‐67. 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 45. Wang J, Yang Y, Zhao X, Zuo Z, Tan LH. Evolutional and developmental anatomical architecture of the left inferior frontal gyrus. NeuroImage. 2020;222:117268. doi: 10.1016/j.neuroimage.2020.117268 [DOI] [PubMed] [Google Scholar]
- 46. Joseph JE, Swearingen JE, Corbly CR, Curry TE Jr, Kelly TH. Influence of estradiol on functional brain organization for working memory. Neuroimage. 2012;59(3):2923‐2931. doi: 10.1016/j.neuroimage.2011.09.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Winecoff A, Clithero JA, Carter RM, Bergman SR, Wang L, Huettel SA. Ventromedial prefrontal cortex encodes emotional value. J Neurosci. 2013;33(27):11032‐11039. doi: 10.1523/JNEUROSCI.4317-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cao L, Xu J, Yang X, Li X, Liu B. Abstract representations of emotions perceived from the face, body, and whole‐person expressions in the left postcentral gyrus. Front Hum Neurosci. 2018;12:419. doi: 10.3389/fnhum.2018.00419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Luine VN. Estradiol increases choline acetyltransferase activity in specific basal forebrain nuclei and projection areas of female rats. Exp Neurol. 1985;89(2):484‐490. doi:10.1016/0014‐4886(85)90108‐6 [DOI] [PubMed] [Google Scholar]
- 50. Yonelinas AP, Hopfinger JB, Buonocore MH, Kroll NEA, Baynes K. Hippocampal, parahippocampal and occipital‐temporal contributions to associative and item recognition memory: an fMRI study. Neuroreport. 2001;12(2):359‐363. [DOI] [PubMed] [Google Scholar]
- 51. Sanabria A, Alegret M, Rodriguez‐Gomez O, et al. The Spanish version of Face‐Name Associative Memory Exam (S‐FNAME) performance is related to amyloid burden in subjective cognitive decline. Sci Rep. 2018;8(1):3828. doi: 10.1038/s41598-018-21644-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Humphreys GF, Ralph MAL, Simons JS. A unifying account of angular gyrus contributions to episodic and semantic cognition. Trends Neurosci. 2021;44(6):452‐463. doi: 10.1016/j.tins.2021.01.006 [DOI] [PubMed] [Google Scholar]
- 53. Buchsbaum BR, Hickok G, Humphries C. Role of left posterior superior temporal gyrus in phonological processing for speech perception and production. Cogn Sci. 2001;25(5):663‐678. doi: 10.1207/s15516709cog2505_2 [DOI] [Google Scholar]
- 54. Ramachandra A, Thomas EHX, Vincent AJ, et al. Subjective cognitive changes following premenopausal risk‐reducing bilateral salpingo‐oophorectomy. Climacteric. 2023;26(6):625‐631. doi: 10.1080/13697137.2023.2256659 [DOI] [PubMed] [Google Scholar]
- 55. Pulvers JN, Huttner WB, Brca1 is required for embryonic development of the mouse cerebral cortex to normal size by preventing apoptosis of early neural progenitors. 2009;136(11)1859‐1868. doi: 10.1242/dev.033498 [DOI] [PubMed] [Google Scholar]
- 56. Leung E, Hazrati LN. Breast cancer type 1 and neurodegeneration: consequences of deficient DNA repair. Brain Commun. 2021;3(2):fcab117. doi: 10.1093/braincomms/fcab117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Suberbielle E, Djukic B, Evans M, et al. DNA repair factor BRCA1 depletion occurs in Alzheimer brains and impairs cognitive function in mice. Nat Commun. 2015;6(1):8897. doi: 10.1038/ncomms9897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nakamura M, Kaneko S, Dickson DW, Kusaka H. Aberrant accumulation of BRCA1 in Alzheimer disease and other tauopathies. J Neuropathol Exp Neurol. 2020;79(1):22‐33. doi: 10.1093/jnen/nlz107 [DOI] [PubMed] [Google Scholar]
- 59. Hickey M, Moss KM, Brand A, et al. What happens after menopause?(WHAM): a prospective controlled study of depression and anxiety up to 12 months after premenopausal risk‐reducing bilateral salpingo‐oophorectomy. Gynecol Oncol. 2021;161(2):527‐534. doi: 10.1016/j.ygyno.2021.02.001 [DOI] [PubMed] [Google Scholar]
- 60. Riedel BC, Thompson PM, Brinton RD. Age, APOE and sex: triad of risk of Alzheimer's disease. J Steroid Biochem Mol Biol. 2016;160:134‐147. 10.1016/j.jsbmb.2016.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gong J, Harris K, Peters SA, Woodward M. Reproductive factors and the risk of incident dementia: a cohort study of UK Biobank participants. PLoS Med. 2022;19(4):e1003955. doi: 10.1371/journal.pmed.1003955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Minkova L, Habich A, Peter J, Kaller CP, Eickhoff SB, Klöppel S. Gray matter asymmetries in aging and neurodegeneration: a review and meta‐analysis. Hum Brain Mapp. 2017;38(12):5890‐5904. doi: 10.1002/hbm.23772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Witt ST, Brown A, Gravelsins L, et al. Gray matter volume in women with the BRCA mutation with and without ovarian removal: evidence for increased risk of late‐life Alzheimer's disease or dementia. Menopause. 2024;31(7):10‐1097. doi: 10.1097/GME.0000000000002361 [DOI] [PubMed] [Google Scholar]
- 64. Peter‐Derex L, Yammine P, Bastuji H, Croisile B. Sleep and Alzheimer's disease. Sleep Med Rev. 2015;19:29‐38. 10.1016/j.smrv.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 65. Brown A, Gervais NJ, Gravelsins L, et al. Effects of early midlife ovarian removal on sleep: polysomnography‐measured cortical arousal, homeostatic drive, and spindle characteristics. Horm Behav. 2024;165:105619. doi: 10.1016/j.yhbeh.2024.105619 [DOI] [PubMed] [Google Scholar]
- 66. Galea LA, Lee BH, Rajah MN, Einstein G. Beyond sex and gender differences: the case for women's health research. Principles of Gender‐Specific Medicine. Academic Press; 2023:699‐711. doi: 10.1016/B978-0-323-88534-8.00045-6 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


