Abstract
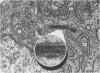
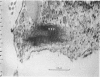
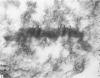
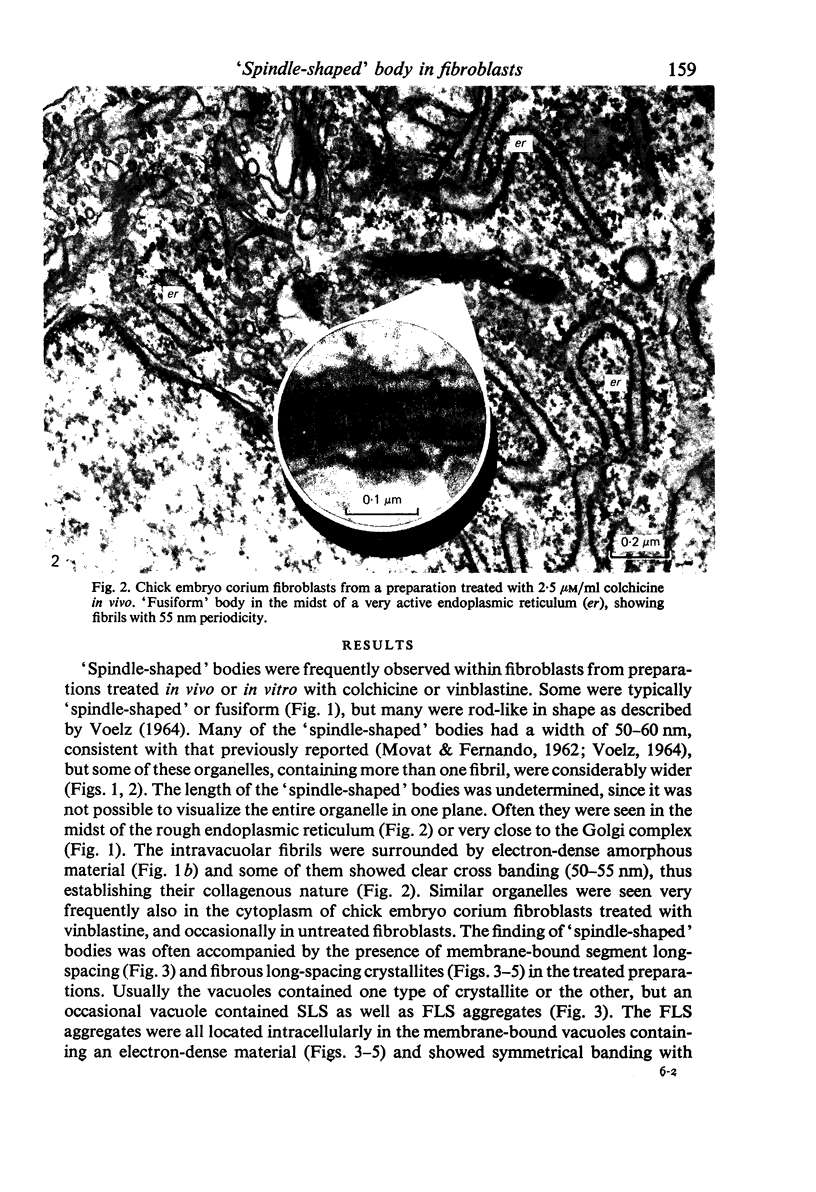
The "spindle-shaped" or "fusiform" bodies of fibroblasts are membrane-bound collagen fibrils. These cellular organelles, also described in the literature as rods or cigars, can be induced massively in chick embryo fibroblasts by treatment with colchicine in vinblastine. These alkaloids also induce massively the presence of non-striated fibrils, segment long-spacing (SLS) and fibrous long-spacing (FLS) crystallites in cytoplasmic vacuoles. The intracellular fibrillar structures described within the cytoplasmic vacuoles suggest the occurrence of tropocollagen interconversions, indication that under the experimental conditions used in this work some procollagen is processed intracellularly. Our findings may represent the first step of autophagocytosis of collagenous products when the secretory process is blocked. It is possible that these agents, by blocking secretion, magnify a normal process, and that under normal conditions a population of procollagen molecules may be processed intracellularly, thus contributing to the process of fibrillogenesis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bienkowski R. S., Baum B. J., Crystal R. G. Fibroblasts degrade newly synthesised collagen within the cell before secretion. Nature. 1978 Nov 23;276(5686):413–416. doi: 10.1038/276413a0. [DOI] [PubMed] [Google Scholar]
- Bienkowski R. S., Cowan M. J., McDonald J. A., Crystal R. G. Degradation of newly synthesized collagen. J Biol Chem. 1978 Jun 25;253(12):4356–4363. [PubMed] [Google Scholar]
- Blau S. A., Peacock E. E., Jr, Carlson E. C., Chvapil M. Effect of colchicine on cross-linking of collagen in rats. Surg Forum. 1975;26:65–67. [PubMed] [Google Scholar]
- Bruns R. R., Hulmes D. J., Therrien S. F., Gross J. Procollagen segment-long-spacing crystallites: their role in collagen fibrillogenesis. Proc Natl Acad Sci U S A. 1979 Jan;76(1):313–317. doi: 10.1073/pnas.76.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- Dehm P., Jimenez S. A., Olsen B. R., Prockop D. J. A transport form of collagen from embryonic tendon: electron microscopic demonstration of an NH 2 -terminal extension and evidence suggesting the presence of cystine in the molecule (chick embryo-tropocollagen-gel filtration). Proc Natl Acad Sci U S A. 1972 Jan;69(1):60–64. doi: 10.1073/pnas.69.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehm P., Prockop D. J. Time lag in the secretion of collagen by matrix-free tendon cells and inhibition of the secretory process by colchicine and vinblastine. Biochim Biophys Acta. 1972 Apr 21;264(2):375–382. doi: 10.1016/0304-4165(72)90302-9. [DOI] [PubMed] [Google Scholar]
- Diegelmann R. F., Peterkofsky B. Inhibition of collagen secretion from bone and cultured fibroblasts by microtubular disruptive drugs. Proc Natl Acad Sci U S A. 1972 Apr;69(4):892–896. doi: 10.1073/pnas.69.4.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich H. P., Bornstein P. Microtubules in transcellular movement of procollagen. Nat New Biol. 1972 Aug 30;238(87):257–260. doi: 10.1038/newbio238257a0. [DOI] [PubMed] [Google Scholar]
- Ehrlich H. P., Ross R., Bornstein P. Effects of antimicrotubular agents on the secretion of collagen. A biochemical and morphological study. J Cell Biol. 1974 Aug;62(2):390–405. doi: 10.1083/jcb.62.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREEMAN J. A., SPURLOCK B. O. A new epoxy embedment for electron microscopy. J Cell Biol. 1962 Jun;13:437–443. doi: 10.1083/jcb.13.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Madrid F., Noonan S., Riddle J., Karvonen R., Sasaki D. Intracellular processing of procollagen induced by the action of colchicine. J Anat. 1980 Mar;130(Pt 2):229–241. [PMC free article] [PubMed] [Google Scholar]
- Fernández-Madrid F. Biochemical and physicochemical characterization of collagen-synthesizing polyribosomes. J Cell Biol. 1967 Apr;33(1):27–42. doi: 10.1083/jcb.33.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler J. H., Fessler L. I. Biosynthesis of procollagen. Annu Rev Biochem. 1978;47:129–162. doi: 10.1146/annurev.bi.47.070178.001021. [DOI] [PubMed] [Google Scholar]
- Gokel J. M., Hübner G. Intracellular "fibrous long spacing" collagen in morbus dupuytren (Dupuytren's contracture). Beitr Pathol. 1977 Oct;161(2):176–186. doi: 10.1016/s0005-8165(77)80096-6. [DOI] [PubMed] [Google Scholar]
- HARKNESS R. D., MARKO A. M., MUIR H. M., NEUBERGER A. The metabolism of collagen and other proteins of the skin of rabbits. Biochem J. 1954 Apr;56(4):558–569. doi: 10.1042/bj0560558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGHBERGER J. H., GROSS J., SCHMITT F. O. The interaction of mucoprotein with soluble collagen; an electron microscope study. Proc Natl Acad Sci U S A. 1951 May;37(5):286–291. doi: 10.1073/pnas.37.5.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood R., Grant M. E., Jackson D. S. The route of secretion of procollagen. The influence of alphaalpha'-bipyridyl, colchicine and antimycin A on the secretory process in embryonic-chick tendon and cartilage cells. Biochem J. 1976 Apr 15;156(1):81–90. doi: 10.1042/bj1560081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON D. S. Connective tissue growth stimulated by carrageenin. I. The formation and removal of collagen. Biochem J. 1957 Feb;65(2):277–284. doi: 10.1042/bj0650277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAKUS M. A. Studies on the cornea. II. The fine structure of Descement's membrane. J Biophys Biochem Cytol. 1956 Jul 25;2(4 Suppl):243–252. doi: 10.1083/jcb.2.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krane S. M., Muñoz A. J., Harris E. D., Jr Collagen-like fragments: excretion in urine of patients with Paget's disease of bone. Science. 1967 Aug 11;157(3789):713–716. doi: 10.1126/science.157.3789.713. [DOI] [PubMed] [Google Scholar]
- Krane S. M., Muñoz A. J., Harris E. D., Jr Urinary polypeptides related to collagen synthesis. J Clin Invest. 1970 Apr;49(4):716–729. doi: 10.1172/JCI106284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDSTEDT S., PROCKOP D. J. Isotopic studies on urinary hydroxyproline as evidence for rapidly catabolized forms of collagen in the young rat. J Biol Chem. 1961 May;236:1399–1403. [PubMed] [Google Scholar]
- MOVAT H. Z., FERNANDO N. V. The fine structure of connective tissue. I. The fibroblast. Exp Mol Pathol. 1962 Dec;1:509–534. doi: 10.1016/0014-4800(62)90040-0. [DOI] [PubMed] [Google Scholar]
- NEUBERGER A., PERRONE J. C., SLACK H. G. B. The relative metabolic inertia of tendon collagen in the rat. Biochem J. 1951 Jul;49(2):199–204. [PMC free article] [PubMed] [Google Scholar]
- Nist C., Von Der Mark K., Hay E. D., Olsen B. R., Bornstein P., Ross R., Dehm P. Location of procollagen in chick corneal and tendon fibroblasts with ferritin-conjugated antibodies. J Cell Biol. 1975 Apr;65(1):75–87. doi: 10.1083/jcb.65.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen B. R., Prockop D. J. Ferritin-conjugated antibodies used for labeling of organelles involved in the cellular synthesis and transport of procollagen. Proc Natl Acad Sci U S A. 1974 May;71(5):2033–2037. doi: 10.1073/pnas.71.5.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price Z. H., Silk M. H. Observations on filamentous material in cultures of human skin fibroblasts. J R Microsc Soc. 1967 Apr;86(4):349–357. doi: 10.1111/j.1365-2818.1967.tb01029.x. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABATINI D. D., MILLER F., BARRNETT R. J. ALDEHYDE FIXATION FOR MORPHOLOGICAL AND ENZYME HISTOCHEMICAL STUDIES WITH THE ELECTRON MICROSCOPE. J Histochem Cytochem. 1964 Feb;12:57–71. doi: 10.1177/12.2.57. [DOI] [PubMed] [Google Scholar]
- SHELDON H., KIMBALL F. B. Studies on cartilage. III. The occurrence of collagen within vacuoles of the golgi apparatus. J Cell Biol. 1962 Mar;12:599–613. doi: 10.1083/jcb.12.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt F. O., Gross J., Highberger J. H. A New Particle Type in Certain Connective Tissue Extracts. Proc Natl Acad Sci U S A. 1953 Jun;39(6):459–470. doi: 10.1073/pnas.39.6.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelstad R. L. Vacuoles in the embryonic chick corneal epithelium, an epithelium which produces collagen. J Cell Biol. 1971 Mar;48(3):689–694. doi: 10.1083/jcb.48.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOELZ H. THE "SPINDLE-SHAPED BODY" IN FIBROBLASTS. J Cell Biol. 1964 Feb;20:333–337. doi: 10.1083/jcb.20.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock M. Collagen formation--observations on its intracellular packaging and transport. Z Zellforsch Mikrosk Anat. 1972;129(4):455–470. doi: 10.1007/BF00316743. [DOI] [PubMed] [Google Scholar]
- Weinstock M., Leblond C. P. Synthesis, migration, and release of precursor collagen by odontoblasts as visualized by radioautography after (3H)proline administration. J Cell Biol. 1974 Jan;60(1):92–127. doi: 10.1083/jcb.60.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]