Abstract
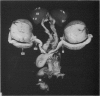
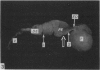
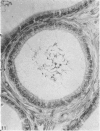
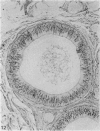
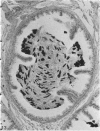
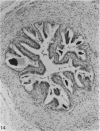
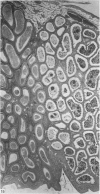
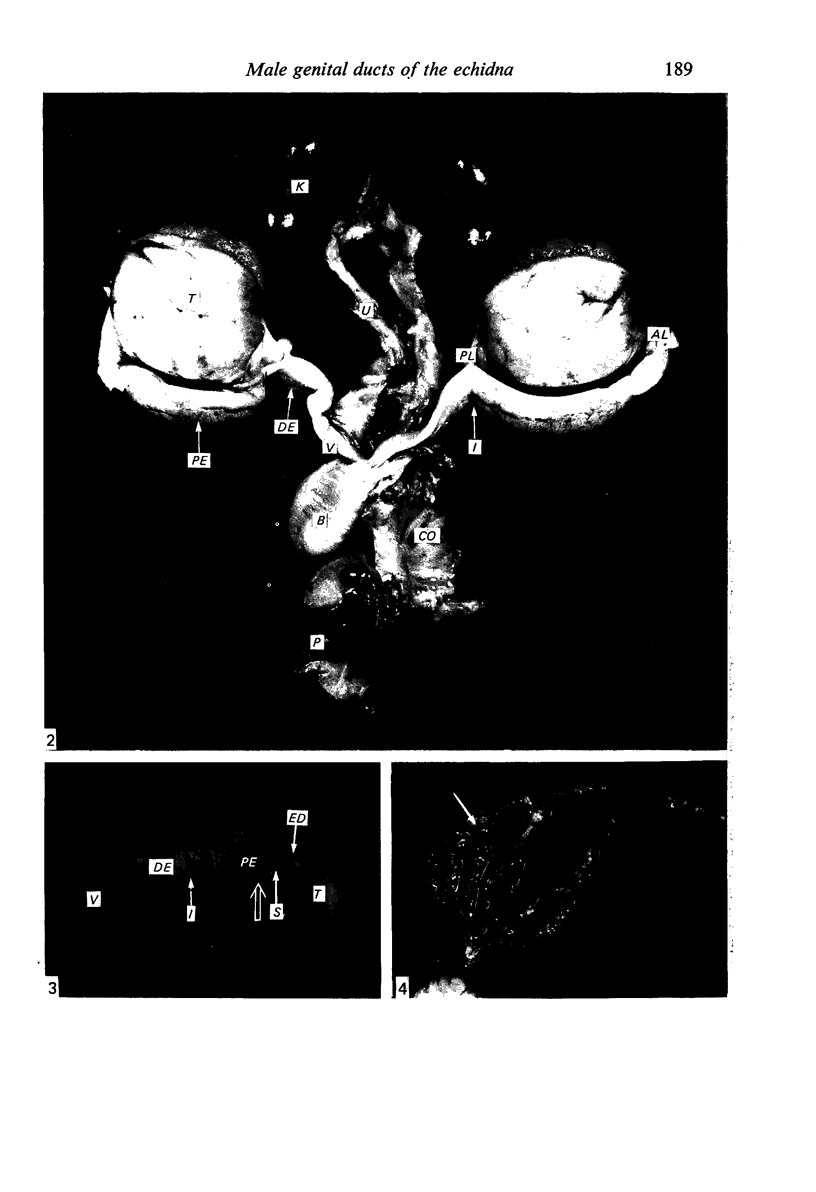
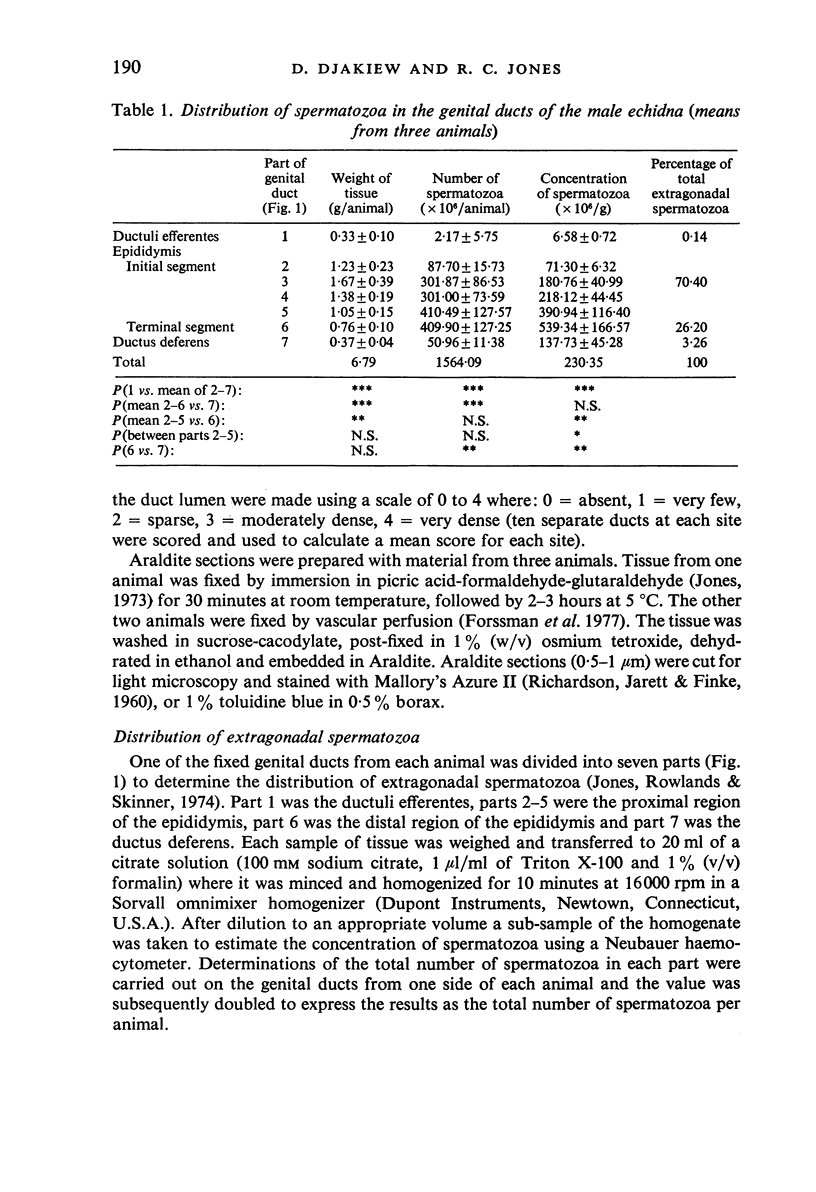
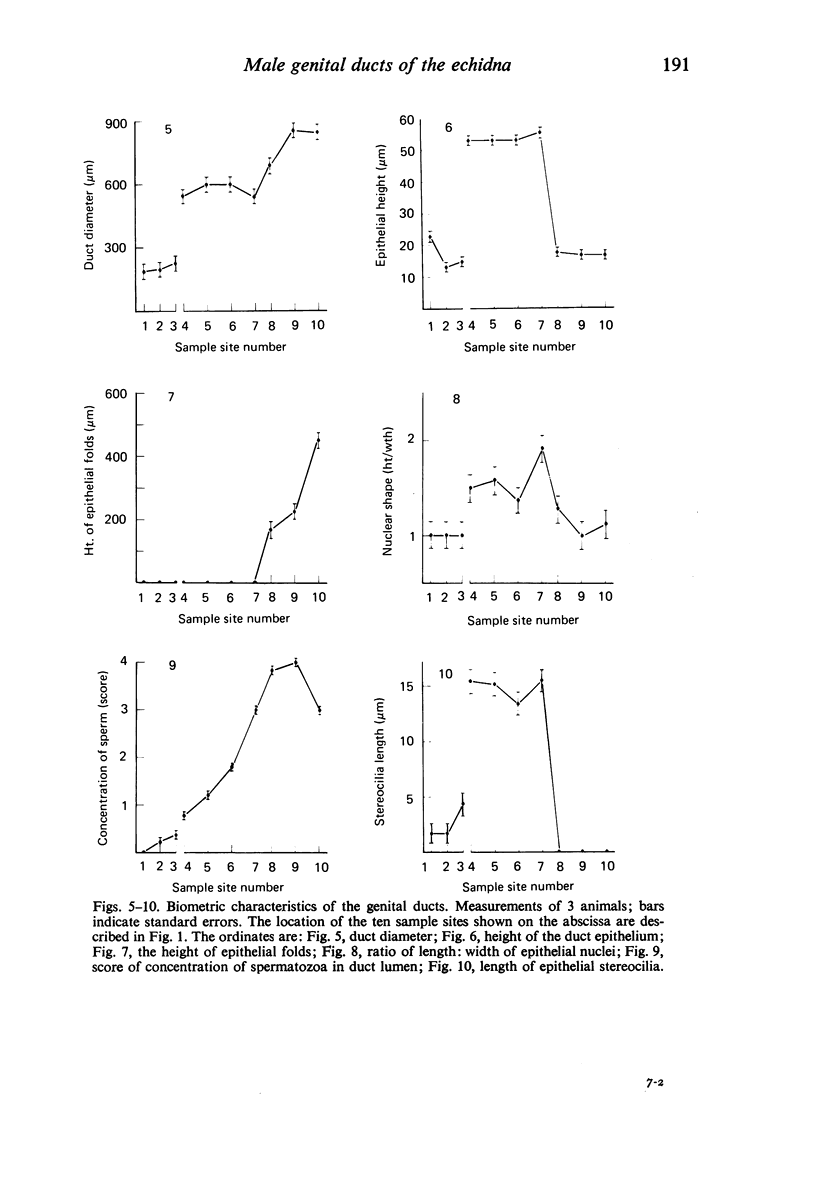
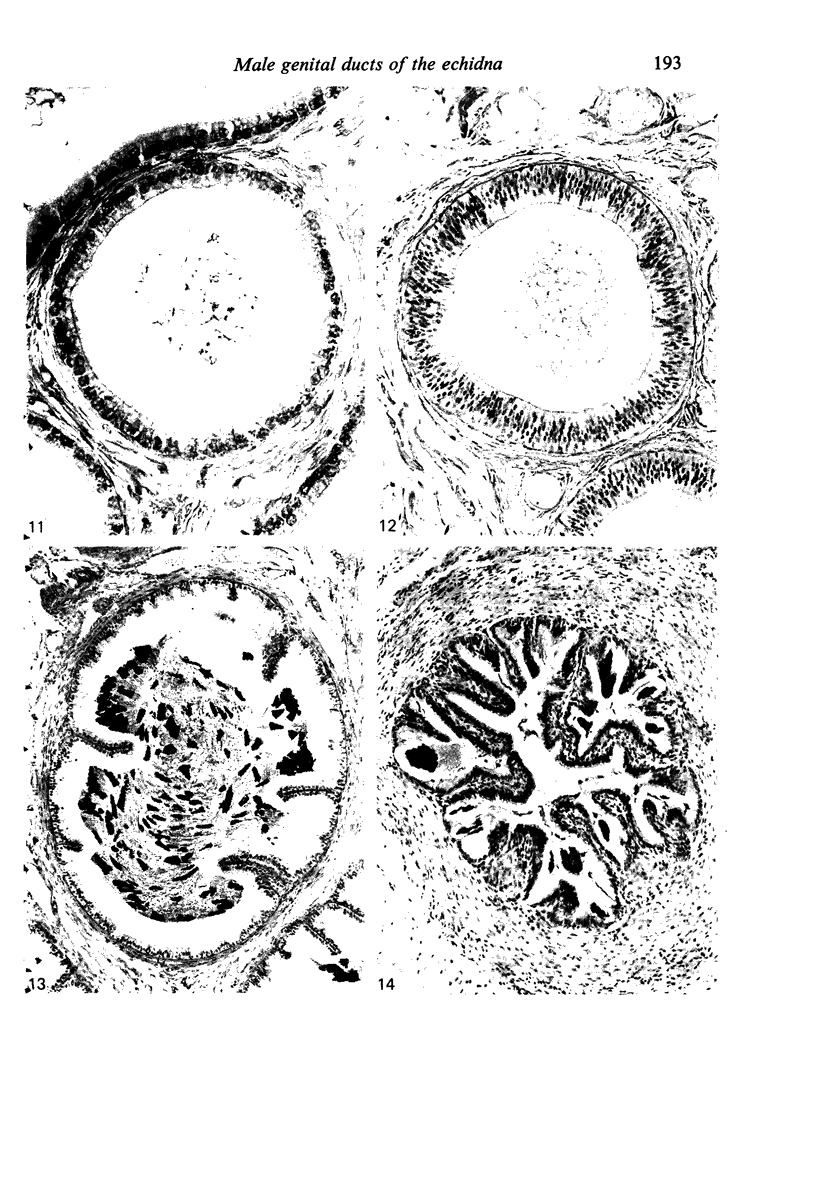
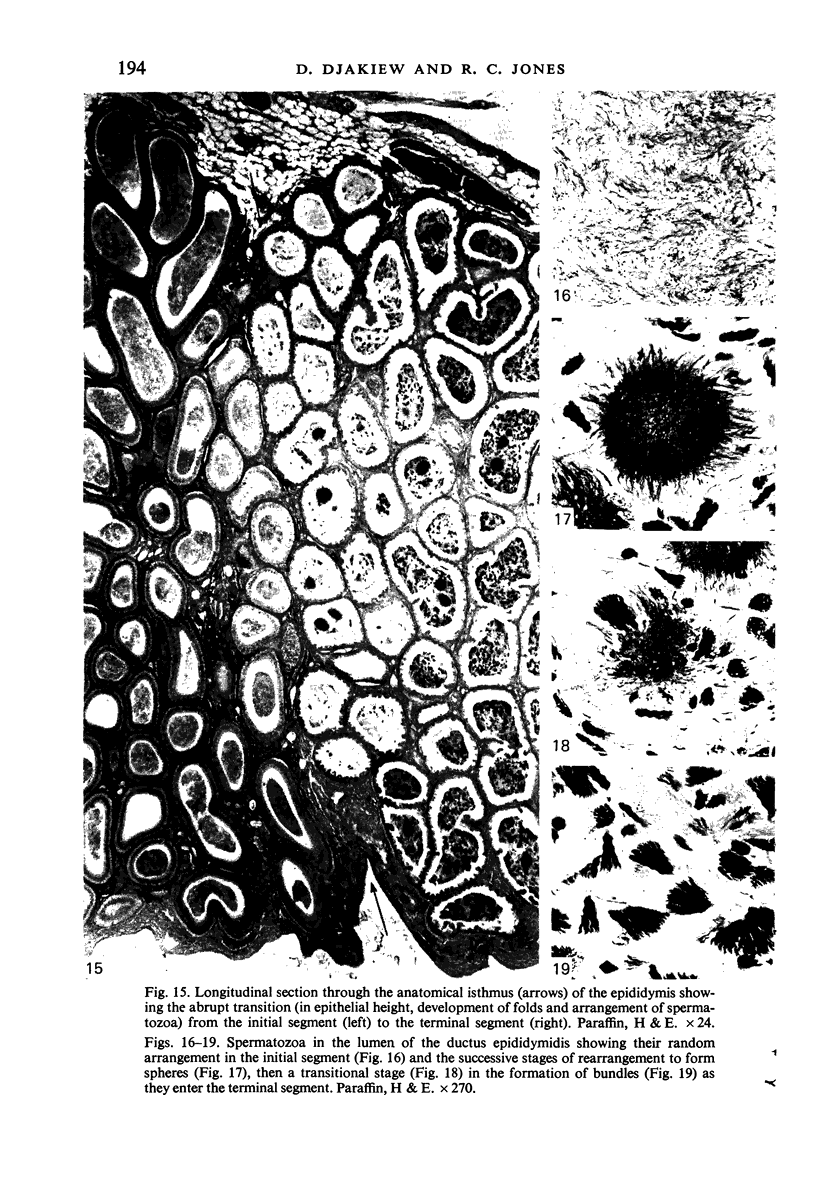
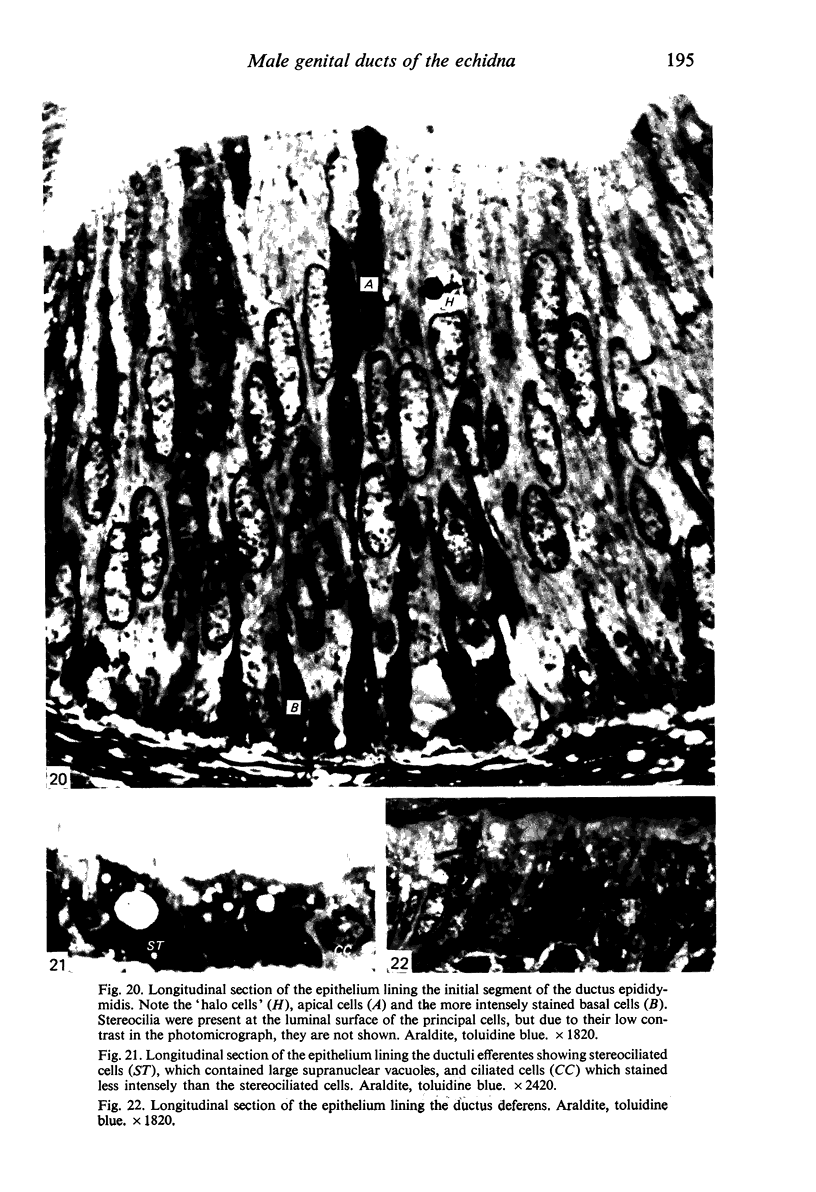
Seven ductuli efferentes radiate from the testis of the echidna and join the ductus epididymidis either directly or after joining one of their neighbours. They are pigmented brown and appear to be structurally and functionally similar to the ductuli efferentes of therian mammals. The epididymis is anatomically differentiated into a large head and small tail which appear to be, respectively, larger and smaller than the similar regions of the epididymis of scrotal mammals; they also contain, respectively, larger and smaller proportions of the animals' extragonadal spermatozoa. Only the head of the epididymis is adjacent to the testis: the tail and the ductus deferens are distal to the testis. The ductus epididymis is also histologically differentiated into two segments (initial and terminal segments) which correspond precisely with the anatomical differentiation. The initial segment is structurally similar to the initial segment of the epididymis of scrotal mammals (i.e. tall epithelium with long stereocilia, a farily homogeneous supranuclear cytoplasma containing Golgi apparatus and a low concentration of spermatozoa in the lumen). The terminal segment has adaptations of the duct (as in scrotal mammals) for the storage of spermatozoa such as a wide lumen containing a high concentration of spermatozoa, low epithelium and thick layers of periductal muscle. However, it is peculiar in that the duct epithelium is thrown into folds and it is involved in considerable apocrine secretion.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann R. P., Johnson L., Thompson D. L., Jr, Pickett B. W. Daily spermatozoal production, epididymal spermatozoal reserves and transit time of spermatozoa through the epididymis of the rhesus monkey. Biol Reprod. 1976 Dec;15(5):586–592. doi: 10.1095/biolreprod15.5.586. [DOI] [PubMed] [Google Scholar]
- Bedford J. M., Rifkin J. M. An evolutionary view of the male reproductive tract and sperm maturation in a monotreme mammal--the echidna, Tachyglossus aculeatus. Am J Anat. 1979 Oct;156(2):207–230. doi: 10.1002/aja.1001560204. [DOI] [PubMed] [Google Scholar]
- Brown P. D., Waites G. M. Regional blood flow in the epididymis of the rat and rabbit: effect of efferent duct ligation and orchidectomy. J Reprod Fertil. 1972 Feb;28(2):221–233. doi: 10.1530/jrf.0.0280221. [DOI] [PubMed] [Google Scholar]
- FAWCETT D. W., HOLLENBERG R. D. Changes in the acrosome of the guinea pig spermatozoa during passage through the epididymis. Z Zellforsch Mikrosk Anat. 1963;60:276–292. doi: 10.1007/BF00350481. [DOI] [PubMed] [Google Scholar]
- Forssmann W. G., Ito S., Weihe E., Aoki A., Dym M., Fawcett D. W. An improved perfusion fixation method for the testis. Anat Rec. 1977 Jul;188(3):307–314. doi: 10.1002/ar.1091880304. [DOI] [PubMed] [Google Scholar]
- Glover T. D. Aspects of sperm production in some East African mammals. J Reprod Fertil. 1973 Oct;35(1):45–53. doi: 10.1530/jrf.0.0350045. [DOI] [PubMed] [Google Scholar]
- Glover T. D., Nicander L. Some aspects of structure and function in the mammalian epididymis. J Reprod Fertil Suppl. 1971 May;13(Suppl):39–50. [PubMed] [Google Scholar]
- Hoffer A. P., Greenberg J. The structure of the epididymis, efferent ductules and ductus deferens of the guinea pig: a light microscope study. Anat Rec. 1978 Mar;190(3):659–677. doi: 10.1002/ar.1091900304. [DOI] [PubMed] [Google Scholar]
- Jones R. C. Preparation of spermatozoa for electron and light microscopy. J Reprod Fertil. 1973 Apr;33(1):145–149. doi: 10.1530/jrf.0.0330145. [DOI] [PubMed] [Google Scholar]
- Jones R. C., Rowlands I. W., Skinner J. D. Spermatozoa in the genital ducts of the African elephant, Loxodonta africana. J Reprod Fertil. 1974 Nov;41(1):189–192. doi: 10.1530/jrf.0.0410189. [DOI] [PubMed] [Google Scholar]
- Kormano M., Reijonen K. Microvascular structure of the human epididymis. Am J Anat. 1976 Jan;145(1):23–27. doi: 10.1002/aja.1001450103. [DOI] [PubMed] [Google Scholar]
- Kormano M., Suoranta H., Reijonen K. Blood supply to testes and excurrent ducts. Adv Biosci. 1973;10:73–82. [PubMed] [Google Scholar]
- LADMAN A. J., YOUNG W. C. An electron microscopic study of the ductuli efferentes and rete testis of the guinea pig. J Biophys Biochem Cytol. 1958 Mar 25;4(2):219–226. doi: 10.1083/jcb.4.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladman A. J. The fine structure of the ductuli efferentes of the opossum. Anat Rec. 1967 Apr;157(4):559–575. doi: 10.1002/ar.1091570403. [DOI] [PubMed] [Google Scholar]
- Levine N., Marsh D. J. Micropuncture studies of the electrochemical aspects of fluid and electrolyte transport in individual seminiferous tubules, the epididymis and the vas deferens in rats. J Physiol. 1971 Mar;213(3):557–570. doi: 10.1113/jphysiol.1971.sp009400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martan J., Hruban Z., Slesers A. Cytological studies of the ductuli efferentes of the opossum. J Morphol. 1967 Feb;121(2):81–102. doi: 10.1002/jmor.1051210202. [DOI] [PubMed] [Google Scholar]
- Morita I. [Some observations on the fine structure of the human ductuli efferentes testis]. Arch Histol Jpn. 1966 May;26(4):341–365. doi: 10.1679/aohc1950.26.341. [DOI] [PubMed] [Google Scholar]
- NICANDER L. On the regional histology and cytochemistry of the ductus epididymidis in rabbits. Acta Morphol Neerl Scand. 1957;1(2):99–118. [PubMed] [Google Scholar]
- NICANDER L. Studies on the regional histology and cytochemistry of the ductus epididymidis in stallions, rams and bulls. Acta Morphol Neerl Scand. 1958;1(4):337–362. [PubMed] [Google Scholar]
- Nicander L., Glover T. D. Regional histology and fine structue of the epididymal duct in the golden hamster (Mesocricetus auratus). J Anat. 1973 Apr;114(Pt 3):347–364. [PMC free article] [PubMed] [Google Scholar]
- Nicander L., Malmqvist M. Ultrastructural observations suggesting merocrine secretion in the initial segment of the mammalian epididymis. Cell Tissue Res. 1977 Nov 23;184(4):487–490. doi: 10.1007/BF00220971. [DOI] [PubMed] [Google Scholar]
- Orgebin-Crist M. C. Gonadal and epididymal sperm reserves in the rabbit: estimation of the daily sperm production. J Reprod Fertil. 1968 Feb;15(1):15–25. doi: 10.1530/jrf.0.0150015. [DOI] [PubMed] [Google Scholar]
- RICHARDSON K. C., JARETT L., FINKE E. H. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol. 1960 Nov;35:313–323. doi: 10.3109/10520296009114754. [DOI] [PubMed] [Google Scholar]
- SETCHELL B. P., WAITES G. M., TILL A. R. VARIATIONS IN FLOW OF BLOOD WITHIN THE EPIDIDYMIS AND TESTIS OF THE SHEEP AND RAT. Nature. 1964 Jul 18;203:317–318. doi: 10.1038/203317b0. [DOI] [PubMed] [Google Scholar]
- Suzuki F., Racey P. A. Fine structural changes in the epididymal epithelium of moles (Talpa europaea) throughout the year. J Reprod Fertil. 1976 May;47(1):47–54. doi: 10.1530/jrf.0.0470047. [DOI] [PubMed] [Google Scholar]
- Tuck R. R., Setchell B. P., Waites G. M., Young J. A. The composition of fluid collected by micropuncture and catheterization from the seminiferous tubules and rete testis of rats. Pflugers Arch. 1970;318(3):225–243. doi: 10.1007/BF00593663. [DOI] [PubMed] [Google Scholar]