Abstract
Membrane potential dynamics are crucial to the physiology of electrically excitable cells, such as neurons, cardiomyocytes, and pancreatic islet cells. Fluorescence voltage indicators have enabled the visualization of electrical activities at the single-cell level across large cell populations. However, most existing indicators have a negative fluorescence-voltage response, resulting in a high background signal when cells are at their resting state. To reduce background interference during voltage imaging, we developed a positive-going hybrid voltage indicator (HVI+), which has lower intensity at the resting state and becomes brighter upon membrane depolarization. HVI+-Cy3b exhibits a remarkable voltage sensitivity of 55% ΔF/F0 per 100 mV, enabling accurate reporting of action potential waveforms in beating cardiomyocytes and neurons. In addition, by combining HVI+-Cy3b with HVI-Cy3b, we demonstrate the capability to simultaneously assess the effects of glucose on the spike activities of two cell types in pancreatic islets.
A chemigenetic hybrid voltage indicator is developed for optical recording of membrane voltage in tissues with high sensitivity.
INTRODUCTION
Membrane potential is a fundamental biophysical property of cells and is dynamically regulated by the actions of ion channels and pumps. In electrically excitable cells, including neurons, cardiomyocytes, and pancreatic islet cells, their membrane potentials are intimately linked to cellular physiology, ranging from brain cognition and cardiac pacing to regulated hormone secretion (1–3). Our understanding of these excitable systems depends critically on our capability to monitor the spatiotemporal correlation of electrical signaling across a cell population with single-cell resolution. Compared with traditional microelectrode-based recording techniques, voltage imaging with genetically targetable indicators allows the spatiotemporally resolved measurement of membrane potential with higher throughput and lower invasiveness (4, 5).
Chemigenetic hybrid voltage sensors are constructed by site-specifically conjugating synthetic fluorescent reporters to voltage-sensing proteins, such as engineered rhodopsins and voltage-sensing domains (6). For instance, the Voltron series of indicators uses chemical dyes conjugated to the C terminus of Acetabularia acetabulum rhodopsin II (Ace2) mutants via HaloTag (7, 8). Meanwhile, hybrid voltage indicators (HVIs) attach fluorophores to the first extracellular loop of Ace2 through enzymatic and bioorthogonal chemical ligation (9–11). These hybrid indicators combine the superior photophysical properties of organic dyes with the genetic targetability of protein scaffolds (12). However, as cells depolarize, both HVIs and Voltron indicators exhibit a decrease in brightness (i.e., negative-going), which is an undesired feature for voltage imaging. A positive-going property is particularly attractive because sensors with this characteristic demonstrate lower intensity at the resting membrane potential. This results in reduced photobleaching and lower background fluorescence, which could be beneficial in densely labeled cell populations (Fig. 1A) (13–15). Besides, the signals of a positive-going indicator can be distinguished from a negative-going indicator, even when both indicators are spectrally indistinguishable, which enables encoding two cell types and recording their voltage signals in one channel (16).
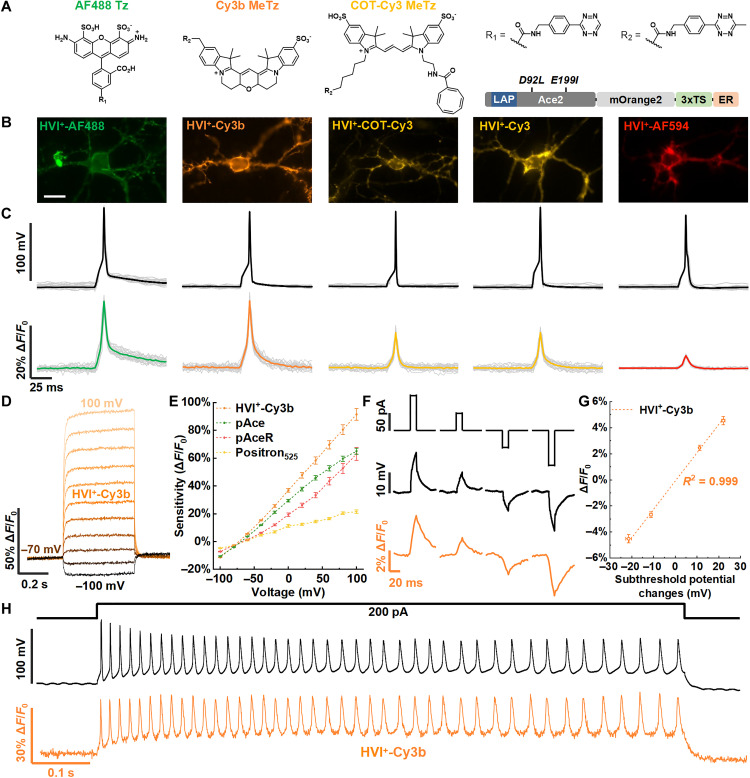
Fig. 1. Engineering of the HVI+.
(A) Schematic comparison of negative- and positive-going voltage indicators. (B) Scheme of the in situ HVI+ assembly via enzyme-mediated bioorthogonal chemical ligation (10) and its voltage sensing mechanism. (C) Voltage sensitivity of HVI mutants toward neuronal APs. All mutants were expressed in cultured rat hippocampal neurons and labeled with Cy3 (n = 2 to 6 cells). The mutant with the highest sensitivity, HVI (C81D D92L E199I), was named the HVI+. Error bars represent SEM.
In this study, we report the development and applications of a positive-going HVI (HVI+) with enhanced voltage sensitivity. Previously, we introduced a single mutation at the proton donor site of Ace2 (D92Q), resulting in an indicator with modest positive-going voltage sensitivity [2% per action potential (AP)] and fast kinetics (17). Coincidentally, Voltron was also engineered at the D92 site along with the proton release site E199, producing an Ace2(D92N E199V) mutant termed Positron, which demonstrated increased positive-going voltage sensitivity (14). Building upon these efforts, we screened a panel of HVI variants and identified a triple mutation, HVI(C81D D92L E199I), which produced an HVI+ with an impressive sensitivity of ΔF/F0 = 22.3 ± 0.8% per AP when used with the high-brightness orange dye Cy3b. This sensitivity is notably higher than that of the spectrally similar Positron525 (ΔF/F0 = 5.9 ± 0.8% per AP). The HVI+ enables accurate electrophysiological recording of beating cardiomyocytes through ratiometric voltage imaging. Moreover, it can be combined with the HVI to assess the impact of glucose on the spike activities of α and β cells in pancreatic islets.
RESULT
Engineering of the HVI+
The HVI+ uses the same electrochromic Förster resonance energy transfer (eFRET) mechanism as the HVI to detect alterations in membrane potential (Fig. 1B). In the HVI, membrane depolarization enhances the protonation of the retinal Schiff base in Ace2 rhodopsin, leading to increased absorbance in the visible range. This process boosts the FRET efficiency between the donor fluorophore and the retinal quencher, resulting in a decrease in the fluorescence emission from the dye upon membrane depolarization (9, 18).
The HVI contains a D81C mutation at the proton acceptor site of Ace2, which is responsible for its enhanced negative-going voltage sensitivity (9, 19). To develop an HVI+, we reverted this amino acid to the wild-type Ace2 sequence (C81D) and introduced mutations at the proton donor site D92 and the proton release site E199. In our initial screening, we expressed double mutants HVI(C81D D92Q) and HVI(C81D D92N) in cultured rat hippocampal neurons and triggered AP firing by injecting 200- to 400-pA current pulses for 10 ms through a whole-cell patch pipette. When labeled with Cy3, both mutants exhibited weakly positive responses to stimulated APs (<5% ΔF/F0 per AP; table S1). Consistent with the observation in Positron (14), introducing the E199V mutation further enhanced the sensitivities of these mutants (table S1). We then focused on the proton donor site and evaluated the sensitivities of 14 HVI(C81D D92X E199V) mutants. Among these, D92L demonstrated the highest sensitivity (10.6 ± 1.0% ΔF/F0 per AP) (Fig. 1C, fig. S1, and table S1). In the subsequent round of screening, we focused on the proton release site and characterized the sensitivities of 15 HVI(C81D D92L E199X) mutants. Among them, 14 mutants exhibited detectable responses, with E199I displaying the highest sensitivity in the Cy3 channel (12.5 ± 0.9% ΔF/F0 per AP; Fig. 1C, fig. S1, and table S1), approximately threefold higher than the initial HVI(C81D D92N) variant. Thus, we designated the triple mutant HVI(C81D D92L E199I) as the HVI+ and used it for all subsequent experiments.
Our next objective was to create a palette of HVI+’s. The modular design of hybrid indicators allows for conveniently changing fluorophore reporters without affecting the indicator expression and membrane trafficking. We screened a panel of fluorophores with emission spectra ranging from green to far-red. After optimizing the dye concentration (~1 μM) and chemical labeling time (~10 min) (fig. S2), we identified HVI+-AF488 as the brightest green indicator and HVI+-Cy3/Cy3b as the brightest orange indicators (fig. S3). For comparison, we also included Cy3 conjugated with cyclooctatetraene (HVI+-COT-Cy3) as the fluorescent reporter, which has been shown to exhibit improved photostability (20). At longer wavelengths, HVI+-AF594 and HVI+-Cy5/Cy5-COT were used as red and far-red indicators, respectively.
To evaluate the voltage sensitivities of the HVI+ conjugated with these fluorophores, we expressed the HVI+ in human embryonic kidney (HEK) 293T cells and varied the membrane potential from −70 to 30 mV using a whole-cell voltage clamp. The highest voltage sensitivity was observed in HVI+-Cy3b, with whole-cell fluorescence increasing by 55.0 ± 2.1% ΔF/F0 per 100 mV (fast component of rise time constant: 2.24 ± 0.38 ms; fast component of decay time constant: 1.33 ± 0.18 ms), followed by HVI+-AF488 (47.6 ± 1.6%), HVI+-COT-Cy3 (40.8 ± 1.8%), and HVI+-Cy3 (31.4 ± 1.4%). Voltage sensitivity decreased substantially at longer wavelengths, with HVI+-AF594 showing a sensitivity of 9.7 ± 0.4% and HVI+-Cy5 showing only 1.1 ± 0.1% ΔF/F0 per 100 mV (table S2). This spectral dependence of voltage sensitivity is consistent with the eFRET voltage sensing mechanism (18, 21). Notably, the three spectrally similar orange indicators (HVI+-Cy3/COT-Cy3/Cy3b) exhibited substantial variations in sensitivities. These differences might be attributed to subtle variations in fluorophore orientation and distinct excited state lifetime that influenced baseline FRET efficiency. A similar trend was observed in negative-going indicators HVI-Cy3/COT-Cy3/Cy3b, with HVI-Cy3b showing the highest voltage sensitivity (−61.3 ± 1.5% ΔF/F0 per 100 mV) (table S2). In summary, the above characterization identified HVI+-AF488 and HVI+-Cy3b as the most sensitive positive-going green and orange indicators, respectively.
We characterized the HVI+ for its ability to report neuronal APs and its sensitivity to subthreshold activities. When expressed in cultured rat hippocampal neurons, the HVI+ exhibited negligible steady-state photocurrent (fig. S4). HVI+-AF488 exhibited a sensitivity of 19.0 ± 1.4% ΔF/F0 per AP, which was higher than that of the negative-going sensor HVI-AF488. HVI+-Cy3b achieved a remarkable sensitivity of 22.3 ± 0.8% ΔF/F0 per AP, which is higher than the positive-going green voltage indicator pAce (19.9 ± 1.8% ΔF/F0 per AP) and outperforms the spectrally similar indicators (e.g., Positron525 for 5.9 ± 0.8% ΔF/F0 per AP; pAceR for 6.6 ± 0.5% ΔF/F0 per AP) (Fig. 2, A to E, and table S2). HVI+-Cy3b could respond linearly to subthreshold potentials with a sensitivity of 2.2% ΔF/F0 per 10 mV and distinguish bursts of APs at 42 Hz (Fig. 2, F to H, and table S3). These observations are consistent with the nearly linear fluorescence-voltage response curve of HVI+-Cy3b measured in cultured HEK293T cells (Fig. 2, D and E, and fig. S5). In addition, the HVI+ allowed for accurate recording of AP waveforms, especially at higher imaging sampling rates (fig. S6 and table S4). This improved resolution helped in observing the effect of AP broadening caused by treatment with 2 or 5 mM potassium channel blocker tetraethylammonium (TEA; Fig. 3, A and B) (22–24).
Fig. 2. Voltage response evaluation of a panel of HVI+’s in cultured cells.
(A) Chemical structures of Tz-conjugated fluorophores (Tz dyes) used in this study and domain structure of HVI+. (B) Representative epifluorescence images of rat hippocampal neurons expressing HVI+-AF488, HVI+-Cy3b, HVI+-COT-Cy3, HVI+-Cy3, and HVI+-AF594. Scale bar, 20 μm. (C) Electrical (top) and optical (bottom) recordings of single APs in neurons expressing HVI+. Averaged traces over 20 trials are shown in bold. The camera frame rate was 484 Hz. (D) Representative fluorescence responses of HVI+-Cy3b to voltage steps from −100 to 100 mV, recorded at 1058 Hz. (E) Fluorescence-voltage response curves of HVI+-Cy3b, pAce, pAceR, and Positron525 (n = 6 cells for each) measured in HEK293T cells. Fluorescence responses were normalized to baseline fluorescence at −70 mV. Error bars represent SEM. (F) Electrical (middle) and optical (bottom) recordings of subthreshold potentials in neurons expressing HVI+-Cy3b stimulated by 10-ms current injection (top). Traces are recorded at 484 Hz and averaged over 20 trials. (G) Linear regression of the fluorescence response of HVI+-Cy3b versus subthreshold potential changes (n = 6 cells). Error bars represent SEM. (H) Electrical recording (top) and HVI+-Cy3b fluorescence trace (bottom) of a 42-Hz AP burst triggered by injecting 200 pA into a neuron for 1 s. The camera frame rate was 2343 Hz.
Fig. 3. High-fidelity HVI+ voltage imaging of pharmacological effects in the neuronal AP waveform.
(A) Voltage imaging of neurons expressing HVI+-Cy3b at 1000 Hz in the absence (blank) versus the presence of 2 and 5 mM TEA. Zoom-in views of three shaded regions are shown on the right. (B) Statistical analysis of AP frequency (n = 11 cells), the FWHM of fluorescent signals (FWHMfluo., n = 6 cells), and their fold changes compared to the blank control recorded in the same neurons treated with various concentrations of TEA. Error bars represent SD. Statistical significance is determined by a paired two-sided t test (*P < 0.05 and **P < 0.01; n.s., not significant). Illumination intensities: 4 W cm−2 (488 nm), 5 W cm−2 (532 nm), 5 W cm−2 (561 nm), and 2 W cm−2 (594 nm).
At resting membrane potential, the fluorescence intensity of HVI+-Cy3b was approximately half of HVI-Cy3b. HVI+-Cy3b demonstrated greater photostability (half-life, 736 ± 113 s) than HVI-Cy3b (432 ± 111 s), Positron525 (207 ± 47 s), and Voltron2525 (180 ± 32 s) under identical illumination conditions (5 W cm−2) (figs. S5 and S7 and table S5). The above characterizations highlight the advantages of positive-going voltage indicators in reducing background fluorescence and improving photostability.
Ratiometric voltage imaging of cardiac APs with the HVI+
During voltage imaging, factors such as cell deformation and migration can interfere with the optical recording of voltage signals. This is particularly evident in the case of beating cardiomyocytes (25). Motion artifact can substantially interfere with voltage signal measurements. A common strategy to mitigate this is to coexpress a reference membrane-targeted fluorescent protein. However, differences in membrane localization between the voltage indicator and the reference can complicate image data analysis. An alternative strategy is to create a ratiometric indicator by fusing a large Stokes shift fluorescent protein, such as mCyRFP3, to the voltage indicator (26). In theory, pairing a green fluorophore (e.g., Alexa Fluor 488) with mCyRFP3 would allow excitation with a single 488-nm laser while simultaneously monitoring both emission channels using a dual-view setup. Unfortunately, fusing mCyRFP3 to the HVI+ severely impaired its membrane trafficking, resulting in a very low signal-to-noise ratio (fig. S8).
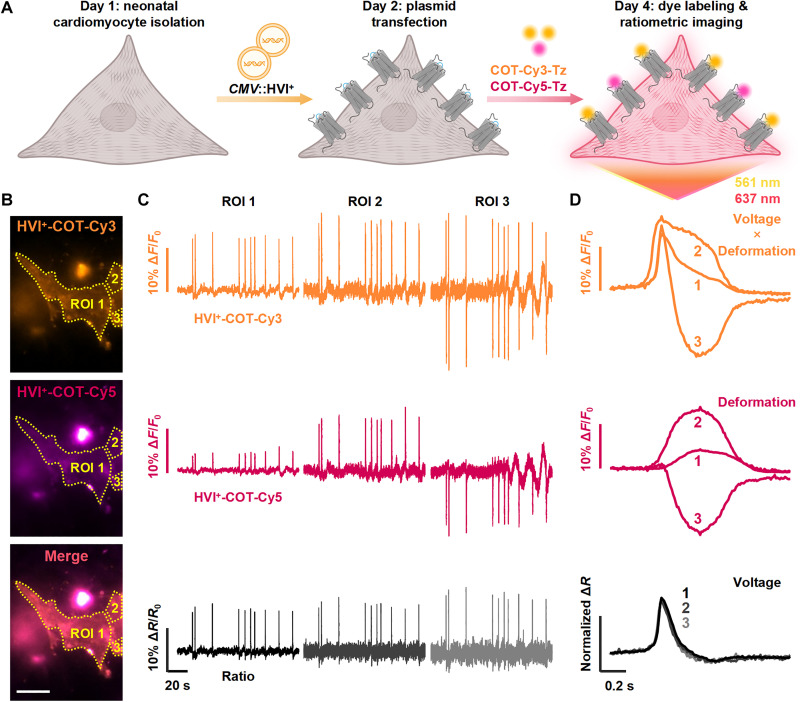
To overcome these limitations, we exploited the distinct voltage sensitivities of HVI+-COT-Cy3 and HVI+-COT-Cy5 to develop a ratiometric imaging method for motion-corrected optical recording of membrane voltage. This approach ensures that both dyes share identical spatial distributions, thereby simplifying data analysis. Cardiomyocytes expressing the HVI+ were labeled with a mixture of COT-Cy3 and COT-Cy5. This dual-color labeling strategy enables the inclusion of a reference imaging channel but results in a partial reduction of the HVI+-COT-Cy3 signal intensity (Fig. 4, A and B). Both fluorophores were COT-modified cyanine dyes with reduced phototoxicity and improved photostability (20). HVI+-COT-Cy5, which had minimal voltage sensitivity, was used as a reference to track cell morphology, while HVI+-COT-Cy3 reported the combined effects of cell deformation and membrane voltage changes.
Fig. 4. Ratiometric HVI+ voltage imaging of beating cardiomyocytes.
(A) Workflow of neonatal cardiomyocyte preparation for ratiometric voltage imaging with HVI+-COT-Cy3 and HVI+-COT-Cy5 [created with BioRender.com; Liu, S. (2025) https://BioRender.com/h1tpym1]. (B) Representative epifluorescence images of an HVI+-expressing rat cardiomyocyte competitively labeled with 1 μM COT-Cy3-Tz and 0.5 μM COT-Cy5-Tz for 15 min. Scale bar, 20 μm. (C) Fluorescence traces of Cy3 (top) and Cy5 (middle) and their ratiometric traces (bottom) were recorded from three ROIs marked in (A). (D) Overlays of the 2-min averaged fluorescence traces of Cy3 (top) and Cy5 (middle) and their ratio (bottom) recorded in (B). The camera frame rate was 100 Hz, and the illumination intensities of 561- and 637-nm lasers were 5.0 and 1.5 W cm−2, respectively.
As illustrated in multiple regions of interest (ROIs) in Fig. 4, substantial changes were recorded in both Cy3 and Cy5 channels during cardiomyocyte beating. It was challenging to extract membrane voltage information from the Cy3 channel alone, as the fluorescence traces in the ROIs even showed opposite signs. However, after calibrating the Cy3 signal using the Cy5 reference channel, the ratiometric optical signals from the three ROIs became highly similar (Fig. 4, C and D). Thus, by using ratiometric imaging with the HVI+, we achieved highly accurate recordings of the AP waveform and observed that the deformation occurred slightly later than the cardiac AP (Fig. 4D) (27).
Moreover, ratiometric imaging of the HVI+ was used to evaluate the effects of drugs on cardiomyocytes. 4-Aminopyridine (4-AP), a nonselective potassium channel inhibitor, binds to the cytoplasmic side of the channel on the cell membrane (Fig. 5A). Following treatment with 5 mM 4-AP, we observed prolonged AP waveforms, with the AP duration at 50% repolarization (APD50) nearly doubled (from 127 ± 28 to 242 ± 83 ms), indicating a partial inhibition of the hyperpolarization step (Fig. 5, B to D). In addition, the spike frequency and APD90 of cardiomyocytes were also significantly increased (Fig. 5D) (28, 29). To assess the variability of ratiometric labeling, we performed dual-color labeling with COT-Cy3 and COT-Cy5 in fixed HEK cells expressing the HVI+. The Cy3 and Cy5 intensities exhibited a strong linear correlation across cells, with minimal variability (Cy5/Cy3 ratio of 2.20 ± 0.11; fig. S8), likely due to minor nonspecific dye adsorption. This small degree of bias was sufficiently low to permit reliable motion-corrected voltage imaging. Overall, the HVI+ enables the accurate measurement of pharmacological effects on AP waveforms with high fidelity in both neurons and cardiomyocytes.
Fig. 5. Ratiometric HVI+ voltage imaging of drug-induced change in the cardiac AP waveform.
(A) Experimental scheme of pharmacologically perturbing voltage-gated potassium channels with 4-AP [created with BioRender.com; Li, C. (2025) https://BioRender.com/mrhvv65]. (B) Ratiometric voltage imaging of cardiomyocytes labeled with HVI+-COT-Cy3/Cy5 before and after treatment with 5 mM 4-AP. (C) Overlays of normalized ΔR traces averaged from (B). (D) Statistical analysis comparing the AP frequency (n = 10 cells), APD50 (n = 6 cells), and APD90 (n = 6 cells) recorded from the same cardiomyocytes treated with 4-AP versus the blank. Error bars represent SD. Statistical significance is determined by a two-sided paired t test (*P < 0.05, **P < 0.01, and ***P < 0.001). The illumination intensities of 532- and 637-nm lasers were 3.0 and 1.0 W cm−2, respectively.
Simultaneous voltage imaging with the HVI and HVI+ in pancreatic islets
We further applied the HVI and HVI+ to monitor cellular electrical activities in the pancreatic islets of mice. The islets of Langerhans are heterogeneous mini-organs composed mainly of β cells (60 to 80% in mice), with a smaller number of α cells (~20%) and a few δ and polypeptide cells (<10%) (30). In response to glucose changes, these islets precisely regulate the secretion of insulin from β cells and glucagon from α cells, which is crucial for maintaining glucose homeostasis (31, 32). It has been a long-standing goal to understand how pancreatic cells respond to glucose stimulation. Patch-clamp techniques have revealed the mechanism by which glucose triggers insulin release from β cells. It is well accepted that increased glucose levels depolarize β cells by closing the adenosine triphosphate (ATP)–dependent channel (KATP), which then opens the voltage-dependent L-type channel, allowing Ca2+ influx and triggering insulin secretion (3, 33, 34). However, the mechanism by which glucose inhibits glucagon secretion from α cells remains controversial. Both depolarization and hyperpolarization effects of high glucose on α cells have been reported (32, 35–37). In addition, autocrine and paracrine factors, such as somatostatin released from δ cells, are believed to play a role in regulating α cell secretion (38, 39). Therefore, it is crucial to understand how glucose inhibits α cells within the context of whole islets.
In this study, we used the preproglucagon promoter (ppg) to guide HVI expression in α cells and the rat insulin 2 promoter (rip2) to guide HVI+ expression in β cells, achieved through adenovirus infection (30). Immunofluorescence microscopy confirmed the high accuracy of the ppg promoter (28 of 29 cells) and the rip2 promoter (62 of 64 cells) (fig. S9). During wide-field imaging with HVI-Cy3b at 13 mM glucose, α cells displayed substantial diversity in both the frequency and shapes of electrical spike activities (fig. S10). In contrast, β cells exhibited more homogeneous electrical activities. Consistent with previous reports, the initial depolarization profiles from each β cell were synchronized, indicating the presence of electrical coupling among β cells (fig. S11), likely mediated by gap junctions (3, 40). However, the individual spikes were not synchronized.
To investigate the effect of glucose on α and β cells in intact pancreas islets, we recorded their spike activities at 3 and 13 mM glucose (Fig. 6). No significant difference in AP frequency was observed for α cells between low and high glucose levels (Fig. 6, A and B, and fig. S12). In contrast, most β cells were silent at 3 mM glucose, and their AP frequency significantly increased at 13 mM glucose (Fig. 6, C and D, and fig. S12), which is consistent with the established model (33, 34). In addition, the AP duration of both cells increased in 13 mM glucose treatment (Fig. 6E). Statistical analysis revealed strong heterogeneity in the electrical activities among α cells: The AP frequencies of some α cells decreased, while others increased or remained unchanged (Fig. 6F). This result is consistent with the reported U-shaped glucose response curve of glucagon secretion, which has the lowest secretion level at 7 mM glucose and comparable secretion levels between 4 and 16 mM glucose (32).
Fig. 6. Voltage imaging of glucose-induced AP frequency changes in α and β cells in intact pancreas islets.
(A) Representative wide-field image of α cells expressing HVI-Cy3b from a mouse pancreas islet. Scale bars, 20 μm. (B) Voltage imaging traces recorded from eight cells shown in (A) during 15-min treatment with 3 mM (left) and 13 mM (right) glucose. The camera frame rate was 200 Hz. (C) Representative wide-field image of β cells expressing HVI+-Cy3b from an intact islet. (D) Voltage imaging traces recorded from 12 cells shown in (C) during 30-min treatment with 3 mM (left) and 13 mM (right) glucose. (E) Overlays of averaged AP traces of α cell no. 5 (top) and β cell no. 8 (bottom) with the treatment of 3 or 13 mM glucose. (F) Statistical analysis of AP frequencies (n = 32 α cells and 26 β cells) and the full-width at half maxima of fluorescent recording (n = 18 α cells and 7 β cells) of the same islet cells treated with 3 or 13 mM glucose. Error bars represent SD. Statistical significance is determined by a two-sided paired t test (*P < 0.05, **P < 0.01, and ***P < 0.001). The illumination intensity of the 532-nm laser was 5.0 W cm−2.
Last, to further elucidate how glucose levels affect the electrical activities of α and β cells in intact islets, we simultaneously expressed the HVI and HVI+ in α and β cells of the same islet using a mixture of two adenoviruses. This allows us to simultaneously record the voltage signals of both cell types in a single imaging channel and distinguish their cell types by the response polarity from the indicators (Fig. 7A). Using this dual-polarity multiplexed voltage imaging (DUPLEX) scheme (16), we simultaneously recorded voltage activities from three β cells and two α cells in the same field of view within an islet, observing increased spike rates in response to 13 mM glucose (Fig. 7B and fig. S12).
Fig. 7. Single-channel simultaneous HVI/HVI+ voltage imaging of glucose-regulated AP firing in α and β cells in intact pancreatic islets.
(A) Workflow of islet preparation for simultaneous voltage imaging with HVI-Cy3b and HVI+-Cy3b (created with BioRender.com; agreement number: AG26ZLBTPN). (B and C) Representative wide-field voltage imaging of intact islets containing α cells expressing HVI-Cy3b and β cells expressing HVI+-Cy3b. Left: epifluorescence images of Cy3b, with cells of interest marked in dashed circles. Scale bar, 20 μm. Right: whole-cell fluorescence traces recorded from marked cells during treatment with 3 mM glucose (B, left), 13 mM glucose (B, right), or alternate treatment with 3 and 13 mM glucose over 40 min (C). (D) Overlays of averaged AP traces from the α cell (t = 15 min and 25 min) and β cell (t = 0 min and 15 min) in (C). (E) AP frequencies of the two islet cells in (C). (F) Statistical analysis of AP frequencies of the same islet cells (n = 8 α cells and 10 β cells) during the DUPLEX imaging. Error bars represent SD. Statistical significance is determined by a two-sided paired t test (*P < 0.05). The illumination intensity of the 532-nm laser was 5.0 W cm−2.
To demonstrate the capability of the HVI and HVI+ for imaging at a longer timescale, we recorded optical traces from a pair of cells within an islet over six rounds of alternating treatment with 13 and 3 mM glucose within 40 min (cells were imaged discontinuously, with each imaging session lasting for 30 s). The opposite spike polarities indicated their identities as α and β cells. Both cells displayed glucose concentration–dependent and reversible voltage responses. The activation effect of glucose on α and β cells occurred within 5 min, faster than the inhibition of their activities (~15 min for β cells; Fig. 7, C to E, and fig. S12) (3). We note, however, that the cell types of the silent cells could not be confirmed by DUPLEX alone without additional information. For example, two cells remained silent in both 3 and 13 mM glucose, potentially representing unexcited α cells (Fig. 7B). In addition, the statistical results of AP frequency from DUPLEX imaging are consistent with the findings recorded with a single indicator (Figs. 6F and 7F). Together, voltage imaging with the HVI and HVI+ revealed that in intact islets with a paracrine environment, switching from 3 to 13 mM glucose reversibly activated β cells. At the same time, both inhibitory and excitatory effects of glucose on α cells in the same islets were observed. The results also reflected the different timescales for the excitatory and inhibitory effects of glucose on islet cells.
DISCUSSION
In summary, we engineered a highly sensitive HVI+ and applied it to excitable cells and tissues. HVI+-Cy3b surpasses Positron525, exhibiting the highest sensitivity among positive-going orange voltage indicators. The HVI+ effectively calibrates the deformation of the beating cardiomyocytes and accurately identifies drug-induced changes in cardiac and neuronal AP waveforms. In addition, the HVI+ can be paired with the HVI, enabling the simultaneous recording of two cell types in the same pancreas islet through duplex imaging.
According to the eFRET mechanism, the sensitivity of HVI series indicators depends critically on the energy transfer efficiency between the fluorophore donor and the rhodopsin acceptor. Unlike HVI-Cy5, HVI+-Cy5 exhibits negligible voltage sensitivity (table S2). This difference may be due to the HVI and HVI+ using different Ace2 mutants, which likely have distinct absorption spectra. These variations can lead to different spectral overlaps between the FRET donor and acceptor, resulting in distinct sensitivity profiles (18). HVI+-Cy3b shows a sensitivity of 55.0 ± 2.1% ΔF/F0 per 100 mV, ~70% higher than the spectrally similar HVI+-Cy3 (table S2). This increased sensitivity could be attributed to the higher fluorescent quantum yield of Cy3b (41).
Because of challenges in delivering labeling reagents, HVI and HVI+ techniques are currently limited to imaging cultured cells and tissue sections. Ongoing efforts aim to overcome this limitation by expressing an evolved lipoate-protein ligase A (LplA) directly in neurons, thereby eliminating the need to deliver exogenous protein and ATP. Many electrophysiological questions in intact tissue remain unresolved. For example, advances in optical imaging and single-cell sequencing over the past decade have greatly enhanced our understanding of the heterogeneity among pancreatic islet cells, challenging and reshaping several classical theories in the field. Unfortunately, technical challenges make it nearly impossible to perform patch-clamp recordings on multiple cells within an intact islet. Consequently, our understanding of the electrophysiology and interplay between cells in pancreatic islets remains limited. Using the HVI and HVI+, our voltage imaging data show that glucose had both inhibitory and excitatory effects on α cells within the same islets, providing previously unknown insights into pancreatic physiology.
MATERIALS AND METHODS
Materials and reagents
The reagents used in this study are summarized in table S6. Primary cell isolation procedures were approved by the Peking University Animal Use and Care Committee and complied with the standards of the Association for Assessment and Accreditation of Laboratory Animal Care (code number IMM-ChenZX-1).
Chemical synthesis
For the synthesis of compound Cy3b-Tz (fig. S13), Cy3b-COOH (0.50 mg, 0.89 μmol, 1.0 equiv) was dissolved in 1.0 ml of anhydrous N,N-dimethyl-formamide (DMF). Then, O-(7-azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HATU; 0.68 mg, 1.8 μmol, 2.0 equiv) and N,N-diisopropylethylamine (DIPEA; 1.7 μl, 8.9 μmol, 10.0 equiv) were added to this solution followed by the addition of Tz-NH2·HCl (0.43 mg, 1.8 μmol, 2.0 equiv) in 1.0 ml of anhydrous DMF. The mixture was vortexed and stirred for 2 hours in the dark at room temperature. The reaction was monitored by liquid chromatography–mass spectrometry (LC-MS). Once completed, the reaction mixture was concentrated and dried in a vacuum oven. Then, the mixture was redissolved in 1.0 ml of DMF and then diluted to 4.0 ml with double-distilled H2O. Purification of the residue by high-performance liquid chromatography provided Cy3b-Tz (0.070 mg, 94 nmol, 11%) as a red solid. The purity of Cy3b-Tz was more than 95%, as determined by LC-MS. ESI-MS (electrospray ionization–mass spectrometry): m/z (mass/charge ratio) calculated for C41H42N7O5S [M + H]+, 744.3; found, 744.3.
General procedures on synthetic chemistry
Unless otherwise stated, all commercially available materials were purchased at the highest commercial quality and used without further purification. DMF was purchased from MREDA (China) or Innochem (China). Unless otherwise mentioned, all reactions were carried out under a nitrogen atmosphere with dry solvents.
Reactions were monitored by LC-MS [2.1-mm by 100-mm, 3-μm AQ C18 column; 2.0-μl injection; 5 to 100% CH3CN/H2O, linear gradient, with constant 0.1% (v/v) formic acid additive; 6- to 10-min run; 0.6 ml/min flow; ESI; positive or negative ion mode; UV (ultraviolet) detection with ACQUITY PDA]. Preparative high-performance liquid chromatography separations were performed using a Teledyne Isco EZ Prep UV-Vis and a RediSep Prep C18 column (100 Å, 5 μm, 20 by 150 mm).
Molecular cloning
For site-directed mutagenesis, the plasmids were constructed by using the QuickChange method. A pair of primers containing the desired mutation was used to introduce the mutation by polymerase chain reaction amplification.
The replacements of the vectors or promoters were achieved by Gibson assembly. Briefly, the vectors and inserts were polymerase chain reaction amplified into linear double-stranded DNA with overlapping sequences at the ends. Following the manufacturer’s instructions, these purified DNA fragments were mixed with Gibson assembly enzymes (Lightening Cloning Kit). Successful clones were verified by sequencing.
HEK293T cell culture and transfection
HEK293T cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco) supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco) at 37°C with 5% CO2. For imaging experiments, HEK293T cells were seeded in a 24-well plate. The cells were transfected with 500 ng of plasmid and 1 μl of Lipofectamine 2000 reagent mixed in Opti-MEM medium at 70 to 90% confluency. After the 4-hour incubation, the cells were digested with trypsin-EDTA (0.25%, Gibco) for 1 min, reseeded onto glass coverslips coated with Matrigel matrix, and cultured in cell medium for 24 hours before subsequent fluorophore labeling.
Neonatal rat neuron culture and transfection
For primary rat hippocampal neuron culture, glass coverslips were coated with a poly-d-lysine solution (Sigma-Aldrich) and Laminin Mouse Protein solution (Gibco). Neonatal Sprague-Dawley rats were used for neuron isolation. The heads of the rats were cut off with scissors, and their brains were isolated from the skull and placed in an ice-chilled dissection solution (DMEM with high glucose and penicillin-streptomycin antibiotics). The hippocampi were separated from the brains under the stereoscopic microscope, cut into small pieces, and incubated with trypsin-EDTA (0.25%) at 37°C for 15 min. Next, the trypsin solution was carefully replaced with DMEM containing 10% FBS. Then, the tissue fragments in a centrifuge tube were repeatedly pipetted for 1 min and incubated on ice for 5 min. The supernatant was collected and diluted with a neural culture medium (Neurobasal medium supplemented with B-27 supplement, GlutaMAX supplement, and penicillin-streptomycin) at the cell density of 7 × 104 cells/ml. Last, 1 ml of cell suspension was added to each well of a 24-well plate with a precoated coverslip. Every 4 days, half of the neural culture medium was replaced with fresh medium to ensure the neurons’ optimal growth and health.
For neuron transfection, cells were transfected at DIV8 (8 days in vitro). Neurons in each well of a 24-well plate were treated with a mixture of 0.5 to 1 μg of plasmids and 1 μl of Lipofectamine LTX for 40 to 45 min. The neurons expressing the voltage indicator for 4 to 6 days were labeled and further underwent imaging experiments.
Neonatal rat cardiomyocyte culture and transfection
Neonatal rat cardiomyocytes were isolated from Sprague-Dawley rats born within 24 hours. The whole hearts were isolated, minced, and rinsed in Tyrode’s buffer. Then, six cycles of digestion using collagenase type II (0.08%, w/v) and pancreatin (0.1%, w/v) were performed for each cycle for about 6 min at 37°C. At the end of each cycle, the suspension was centrifuged for 5 min at 100g, and the supernatant was collected, pooled, and resuspended in cardiomyocyte culture medium [DMEM containing 10% (v/v) FBS, 100 μM 5-bromo-2′-deoxyuridine, and penicillin-streptomycin]. Isolated cells were preplated for 2 hours on culture flasks in a humidified incubator at 37°C with 5% CO2 to separate cardiomyocytes and fibroblasts. Then, isolated cardiomyocytes were resuspended with cardiomyocyte culture medium. The density of cardiomyocytes was adjusted to 1.5 × 105 cells/ml. After neonatal rat cardiomyocytes had been cultured for 36 to 48 hours, the medium was rinsed for further experiments.
For cardiomyocyte transfection, the cells were transfected at DIV1 to DIV2. First, the cardiomyocytes were switched into Opti-MEM for more than 30 min in the incubator. Second, cells were transfected with 1.5 μg of plasmids, 1.5 μl of PLUS reagent, and 1.5 μl of Lipofectamine LTX reagent mixed in Opti-MEM medium. After 12 hours, the transfection mixture was replaced with the culture medium, and the transfected cardiomyocytes were further incubated for 2 to 4 days before the imaging experiments.
Pancreatic islet culture and virus infection
For pancreatic islet isolation and culture, 8-week-old C57BL/6N male mice (Beijing Vital River Laboratory Animal Technology Co., Ltd.) were used. Briefly, collagenase P Hanks’ balanced salt solution (0.5 mg/ml) was retrogradely injected into the pancreas via the common bile duct. The whole pancreas was carefully dissected and digested at 37°C for 16 min. The islets were handpicked under the stereoscopic microscope. The selected islets were cultured in complete RPMI 1640 medium, supplemented with 8 mM glucose, 10% FBS, and penicillin-streptomycin at 37°C in a 5% CO2 culture incubator for about 8 hours. For virus infection, after recovering from the isolation procedure, the islets were transferred into the complete RPMI 1640 culture medium containing diluted adenovirus and cultured overnight, following a 48-hour culture in fresh complete culture medium.
PRIME labeling
For PRIME labeling of transfected cells (10), the cells were rinsed with Tyrode’s Salts Solution (M&C Gene Technology) and then incubated with 5 μM W37VLplA (purified from cultured bacteria as previously described) (42), 100 μM 4-TCO [rel-(1R-4E-pR)-cyclooct-4-ene-1-yl-N-pentanoic acid carbamate] (9), 1 mM ATP, and 1 mM magnesium acetate in Tyrode’s buffer at 37°C for 30 min. Next, cells were gently rinsed three times with Tyrode’s buffer and subsequently labeled with 0.5 to 1.5 μM tetrazine (Tz) dyes in Tyrode’s buffer for 7.5 to 15 min (for instance, 0.5 μM Cy3b-Tz for 7.5 min or a mixture of 1 μM COT-Cy3-Tz and 0.5 μM COT-Cy5-Tz for 15 min for ratiometric imaging). Excess Tz dyes were removed, and cells were rinsed another three times before voltage imaging. For PRIME labeling of infected islets, all reagents were the same as above, except that Tyrode’s buffer was replaced by a Krebs-Ringer bicarbonate buffer (KRBB) solution containing 3 mM glucose.
Imaging apparatus and wide-field microscopy
All the imaging experiments were performed with an inverted fluorescence microscope (Nikon-TiE), equipped with a 40× 1.3–numerical aperture oil immersion objective lens, five laser lines (Coherent OBIS 488, 532, 561, 594, and 637 nm), and a scientific complementary metal-oxide semiconductor camera (Hamamatsu ORCA-Flash 4.0 version 2) and controlled by LabVIEW (National Instruments, version 15.0) software. Representative images were captured at 2 by 2–pixel binning with a 100-ms exposure time and were analyzed by ImageJ/Fiji (version 1.52d). For ratiometric imaging, a dual-view device (Photometrics DV2, dichroic mirror: T660lpxrxt) was used to split the emission into orange/red (filter: 600/50) and far-red (filter: 700/75) fluorescence channels.
Electrophysiology
For single-cell electrophysiological recording, borosilicate glass electrodes (Sutter) were pulled to a tip resistance of 2.5 to 5 megohms and were injected with the internal solution (125 mM potassium gluconate, 8 mM NaCl, 0.6 mM MgCl2, 0.1 mM CaCl2, 1 mM EGTA, 10 mM Hepes, 4 mM Mg-ATP, and 0.4 mM GTP·Na2, pH 7.3, 295 mosmol/kg). The position of a glass electrode was adjusted by a Sutter MP285 micromanipulator. The cultured neurons at DIV12 to DIV14 were incubated in Tyrode’s buffer with 20 μM gabazine, 10 μM 2,3-dioxo-6-nitro-7-sulfamoylbenzo[f]quinoxaline, and 25 μM d,l-2-amino-5-phosphonovaleric acid (and 0.5 μM tetrodotoxin for photocurrent measurement), and they were clamped by an Axopatch 200B (Axon Instruments) amplifier at room temperature. Electrophysiological data recorded from the patch amplifier were filtered by an internal 5-kHz Bessel filter and digitalized at 9681.48 Hz with a National Instruments PCIe-6353 data acquisition board.
The membrane potential of cultured cells was controlled via a whole-cell patch clamp. To evoke neuronal individual AP, a 200- to 400-pA current was injected into cultured neurons for 10 ms. Alternatively, neurons were stimulated with a 200-pA current for 1 s to fire a burst of APs. In addition, the neurons were stimulated by the injection of −100- to 100-pA current for 10 ms to induce subthreshold potentials. For photocurrent measurement, the neurons expressing the HVI or HVI+ were clamped at −70 mV. The photocurrent was measured with data acquisition and digitalized at 20 kHz when the cells were illuminated with 300-ms laser pulses.
Voltage imaging of pancreatic islets
The labeled islets were attached to the poly-l-lysine–coated coverslips and mounted in a self-made chamber. The chamber was placed on the stage of the fluorescence microscope, kept at 37°C, and superfused with Krebs-Ringer bicarbonate buffer at a rate of 0.3 to 0.5 ml/min. The imaging was acquired at 4 by 4–pixel binning at 200 Hz. All of the ROIs were selected manually.
Immunofluorescence staining
Forty-eight hours after adenovirus infection, the islets were fixed with phosphate-buffered saline (PBS) buffer containing 4% (w/v) paraformaldehyde at room temperature for 1 hour and subsequently were transferred into a 0.3% (v/v) Triton PBS solution overnight at 4°C. The islets were blocked in PBS with 0.3% Triton and 5% (w/v) bovine serum albumin (BSA) at room temperature for 1 hour, followed by washing in PBS with 0.3% Triton and 1% BSA. The islets were incubated with the 1:500 to 1000 diluted primary antibody (containing 0.3% Triton and 1% BSA) overnight. After rinsing three times, the islets were immersed into the 1:1000 diluted secondary antibody solution (with 0.3% Triton and 1% BSA) for 2 hours at room temperature and then kept in PBS at 4°C. Before imaging, the nucleus of islet cells was stained with Hoechst 33342 (1:1000) in PBS for 10 min at room temperature.
Data analysis
Both electrical data and fluorescence images were analyzed using custom MATLAB software (MathWorks, version R2019b). For the fluorescence intensity of a single cell, the mean pixel value of a manually created ROI around the soma was obtained and subtracted from the camera bias (100 and 400 for 1 by 1 and 2 by 2 binning, respectively). For the fluorescent signals of spontaneous electrical activities, the raw traces from individual ROIs were subtracted from the background trace. In ratiometric imaging, photobleaching in the traces was removed by normalizing them to the corresponding trend lines, generated using a smoothing function with a 2.5-s (999 consecutive data points) moving average applied to the background-subtracted data.
In pancreatic islet imaging, for the background calibration, the trace of a blank area was subtracted from the signals of islet cells. To prepare the signals for profiling AP frequency, the fluorescence traces of positive- and negative-going indicators from each ROI were normalized to a trace with a baseline of 1 and positive-going peaks. Then, the APs were identified by a threshold and double checked manually. For profiling the full width at half maximum (FWHM) of APs, baseline ranges of each AP were manually defined within 500 ms before and after the peak. Specifically, the rising (depolarization) and falling (repolarization) edges of each AP were normalized using the baseline defined before the peak for the rising edge and the baseline defined after the peak for the falling edge. The rising and falling edges of each AP were then separately scaled to 0 to 1, and the FWHM was calculated as the time interval between the 50% maximum points on rising and falling edges. Statistical analyses were conducted using Excel (Microsoft Excel 2019) and OriginPro (2022). The schematic graphs of Figs. 4A, 5A, and 7A were created with BioRender.com (agreement numbers: BQ28CF1GXP and AG26ZLBTPN).
Acknowledgments
We thank A. Tengholm from Uppsala University for providing pancreatic islet cell–specific promoters. P.Z. is sponsored by Bayer Investigator Award.
Funding: P.Z. was supported by the National Natural Science Foundation of China (32088101), the Ministry of Science and Technology of China (2022YFA1304700), and Beijing National Laboratory for Molecular Sciences (BNLMS-CXXM-202403). Z.C. was supported by the Ministry of Science and Technology of China (2021YFF0502904) and Beijing Municipal Science & Technology Commission (Z221100003422013). C.T. was supported by the National Natural Science Foundation of China (32088101 and 12090053) and the Ministry of Science and Technology of China (2021YFF1200500).
Author contributions: Writing—original draft: S.L., J. Ling, B.X., Y.Z., L.P., L.Y., Y.Y., C.T., Z.C., and P.Z. Conceptualization: S.L., J. Ling, Y.Z., L.Y., C.T., Z.C., and P.Z. Investigation: S.L., J. Ling, B.X., Y.Z., L.Y., J. Lin, and P.Z. Writing—review and editing: S.L., J. Ling, B.X., L.P., L.Y., Y.Y., C.T., Z.C., and P.Z. Methodology: S.L., J. Ling, B.X., L.Y., Z.C., and P.Z. Resources: S.L., J. Ling, L.Y., Z.C., and P.Z. Funding acquisition: C.T., Z.C., and P.Z. Data curation: J. Ling, L.P., L.Y., Y.Y., and P.Z. Validation: S.L., J. Ling, B.X., L.Y., J. Lin, and P.Z. Supervision: C.T., Z.C., and P.Z. Formal analysis: S.L., B.X., L.Y., Y.Y., and P.Z. Software: L.P., Y.Y., and P.Z. Project administration: S.L., J. Ling, C.T., Z.C., and P.Z. Visualization: S.L., J. Ling, B.X., L.Y., and P.Z.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The amino acid sequence of HVI+ is shown in table S7. The MATLAB code can be downloaded from Dryad at https://datadryad.org/dataset/doi:10.5061/dryad.m63xsj4dd.
Supplementary Materials
The PDF file includes:
Figs. S1 to S13
Tables S1, S3 to S7
Legend for table S2
Other Supplementary Material for this manuscript includes the following:
Table S2
REFERENCES AND NOTES
- 1.Levin M., Molecular bioelectricity: How endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo. Mol. Biol. Cell 25, 3835–3850 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C. L.-H., Lei M., Cardiomyocyte electrophysiology and its modulation: Current views and future prospects. Philos. Trans. R. Soc. Lond. B Biol. Sci. 378, 20220160 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rorsman P., Ashcroft F. M., Pancreatic β-cell electrical activity and insulin secretion: Of mice and men. Physiol. Rev. 98, 117–214 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Y., Zou P., Cohen A. E., Voltage imaging with genetically encoded indicators. Curr. Opin. Chem. Biol. 39, 1–10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bando Y., Grimm C., Cornejo V. H., Yuste R., Genetic voltage indicators. BMC Biol. 17, 71 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang A., Feng J., Li Y., Zou P., Beyond fluorescent proteins: Hybrid and bioluminescent indicators for imaging neural activities. ACS Chem. Nerosci. 9, 639–650 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Abdelfattah A. S., Kawashima T., Singh A., Novak O., Liu H., Shuai Y., Huang Y.-C., Campagnola L., Seeman S. C., Yu J., Zheng J., Grimm J. B., Patel R., Friedrich J., Mensh B. D., Paninski L., Macklin J. J., Murphy G. J., Podgorski K., Lin B.-J., Chen T.-W., Turner G. C., Liu Z., Koyama M., Svoboda K., Ahrens M. B., Lavis L. D., Schreiter E. R., Bright and photostable chemigenetic indicators for extended in vivo voltage imaging. Science 365, 699–704 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Abdelfattah A. S., Zheng J., Singh A., Huang Y.-C., Reep D., Tsegaye G., Tsang A., Arthur B. J., Rehorova M., Olson C. V. L., Shuai Y., Zhang L., Fu T.-M., Milkie D. E., Moya M. V., Weber T. D., Lemire A. L., Baker C. A., Falco N., Zheng Q., Grimm J. B., Yip M. C., Walpita D., Chase M., Campagnola L., Murphy G. J., Wong A. M., Forest C. R., Mertz J., Economo M. N., Turner G. C., Koyama M., Lin B.-J., Betzig E., Novak O., Lavis L. D., Svoboda K., Korff W., Chen T.-W., Schreiter E. R., Hasseman J. P., Kolb I., Sensitivity optimization of a rhodopsin-based fluorescent voltage indicator. Neuron 111, 1547–1563.e9 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu S., Lin C., Xu Y., Luo H., Peng L., Zeng X., Zheng H., Chen P. R., Zou P., A far-red hybrid voltage indicator enabled by bioorthogonal engineering of rhodopsin on live neurons. Nat. Chem. 13, 472–479 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Liu D. S., Tangpeerachaikul A., Selvaraj R., Taylor M. T., Fox J. M., Ting A. Y., Diels-Alder cycloaddition for fluorophore targeting to specific proteins inside living cells. J. Am. Chem. Soc. 134, 792–795 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Y., Peng L., Wang S., Wang A., Ma R., Zhou Y., Yang J., Sun D.-E., Lin W., Chen X., Zou P., Hybrid indicators for fast and sensitive voltage imaging. Angew. Chem. Int. Ed. Engl. 57, 3949–3953 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Liu S., Yang J., Zou P., Bringing together the best of chemistry and biology: Hybrid indicators for imaging neuronal membrane potential. J. Neurosci. Methods 363, 109348 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Platisa J., Vasan G., Yang A., Pieribone V. A., Directed evolution of key residues in fluorescent protein inverses the polarity of voltage sensitivity in the genetically encoded indicator ArcLight. ACS Chem. Nerosci. 8, 513–523 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdelfattah A. S., Valenti R., Zheng J. H., Wong A., GENIE Project Team, Podgorski K., Koyama M., Kim D. S., Schreiter E. R., A general approach to engineer positive-going eFRET voltage indicators. Nat. Commun. 11, 3444 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans S. W., Shi D.-Q., Chavarha M., Plitt M. H., Taxidis J., Madruga B., Fan J. L., Hwang F.-J., van Keulen S. C., Suomivuori C.-M., Pang M. M., Su S., Lee S., Hao Y. A., Zhang G., Jiang D., Pradhan L., Roth R. H., Liu Y., Dorian C. C., Reese A. L., Negrean A., Losonczy A., Makinson C. D., Wang S., Clandinin T. R., Dror R. O., Ding J. B., Ji N., Golshani P., Giocomo L. M., Bi G.-Q., Lin M. Z., A positively tuned voltage indicator for extended electrical recordings in the brain. Nat. Methods 20, 1104–1113 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kannan M., Vasan G., Haziza S., Huang C., Chrapkiewcz R., Luo J., Cardin J. A., Schnitzer M. J., Pieribone V. A., Dual-polarity voltage imaging of the concurrent dynamics of multiple neuron types. Science 378, eabm8797 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Y., Deng M., Zhang S., Yang J., Peng L., Chu J., Zou P., Imaging neuronal activity with fast and sensitive red-shifted electrochromic FRET indicators. ACS Chem. Nerosci. 10, 4768–4775 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Zou P., Zhao Y., Douglass A. D., Hochbaum D. R., Brinks D., Werley C. A., Harrison D. J., Campbell R. E., Cohen A. E., Bright and fast multicoloured voltage reporters via electrochromic FRET. Nat. Commun. 5, 4625 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han Y., Yang J., Li Y., Chen Y., Ren H., Ding R., Qian W., Ren K., Xie B., Deng M., Xiao Y., Chu J., Zou P., Bright and sensitive red voltage indicators for imaging action potentials in brain slices and pancreatic islets. Sci. Adv. 9, eadi4208 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu S., Ling J., Chen P., Cao C., Peng L., Zhang Y., Ji G., Guo Y., Chen P. R., Zou P., Chen Z., Orange/far-red hybrid voltage indicators with reduced phototoxicity enable reliable long-term imaging in neurons and cardiomyocytes. Proc. Natl. Acad. Sci. U.S.A. 120, e2306950120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jares-Erijman E. A., Jovin T. M., FRET imaging. Nat. Biotechnol. 21, 1387–1395 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Bean B. P., The action potential in mammalian central neurons. Nat. Rev. Neurosci. 8, 451–465 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Kita H., Kita T., Kitai S. T., Regenerative potentials in rat neostriatal neurons in an in vitro slice preparation. Exp. Brain Res. 60, 63–70 (1985). [DOI] [PubMed] [Google Scholar]
- 24.Hlubek M. D., Cobbett P., Differential effects of K+ channel blockers on frequency-dependent action potential broadening in supraoptic neurons. Brain Res. Bull. 53, 203–209 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Herron T. J., Lee P., Jalife J., Optical imaging of voltage and calcium in cardiac cells & tissues. Circ. Res. 110, 609–623 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim B. B., Wu H., Hao Y. A., Pan M., Chavarha M., Zhao Y., Westberg M., St-Pierre F., Wu J. C., Lin M. Z., A red fluorescent protein with improved monomericity enables ratiometric voltage imaging with ASAP3. Sci. Rep. 12, 3678 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wood E. H., Heppner R. L., Weidmann S., Inotropic effects of electric currents. I. Positive and negative effects of constant electric currents or current pulses applied during cardiac action potentials. II. Hypotheses: Calcium movements, excitation-contraction coupling and inotropic effects. Circ. Res. 24, 409–445 (1969). [DOI] [PubMed] [Google Scholar]
- 28.Tahara S., Fukuda K., Kodama H., Kato T., Miyoshi S., Ogawa S., Potassium channel blocker activates extracellular signal-regulated kinases through Pyk2 and epidermal growth factor receptor in rat cardiomyocytes. J. Am. Coll. Cardiol. 38, 1554–1563 (2001). [DOI] [PubMed] [Google Scholar]
- 29.Reppel M., Igelmund P., Egert U., Juchelka F., Hescheler J., Drobinskaya I., Effect of cardioactive drugs on action potential generation and propagation in embryonic stem cell-derived cardiomyocytes. Cell. Physiol. Biochem. 19, 213–224 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Shuai H., Xu Y., Yu Q., Gylfe E., Tengholm A., Fluorescent protein vectors for pancreatic islet cell identification in live-cell imaging. Pflugers Arch. 468, 1765–1777 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell J. E., Newgard C. B., Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat. Rev. Mol. Cell Biol. 22, 142–158 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gylfe E., Gilon P., Glucose regulation of glucagon secretion. Diabetes Res. Clin. Pract. 103, 1–10 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Ashcroft F. M., Harrison D. E., Ashcroft S. J., Glucose induces closure of single potassium channels in isolated rat pancreatic β-cells. Nature 312, 446–448 (1984). [DOI] [PubMed] [Google Scholar]
- 34.Cook D. L., Hales C. N., Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature 311, 271–273 (1984). [DOI] [PubMed] [Google Scholar]
- 35.Walker J. N., Ramracheya R., Zhang Q., Johnson P. R., Braun M., Rorsman P., Regulation of glucagon secretion by glucose: Paracrine, intrinsic or both? Diabetes Obes. Metab. 13, 95–105 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Gromada J., Ma X., Hoy M., Bokvist K., Salehi A., Berggren P. O., Rorsman P., ATP-sensitive K+ channel-dependent regulation of glucagon release and electrical activity by glucose in wild-type and SUR1−/− mouse α-cells. Diabetes 53, S181–S189 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Barg S., Galvanovskis J., Göpel S. O., Rorsman P., Eliasson L., Tight coupling between electrical activity and exocytosis in mouse glucagon-secreting α-cells. Diabetes 49, 1500–1510 (2000). [DOI] [PubMed] [Google Scholar]
- 38.Olsen H. L., Theander S., Bokvist K., Buschard K., Wollheim C. B., Gromada J., Glucose stimulates glucagon release in single rat α-cells by mechanisms that mirror the stimulus-secretion coupling in β-cells. Endocrinology 146, 4861–4870 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Johansson H., Gylfe E., Hellman B., Cyclic AMP raises cytoplasmic calcium in pancreatic α2-cells by mobilizing calcium incorporated in response to glucose. Cell Calcium 10, 205–211 (1989). [DOI] [PubMed] [Google Scholar]
- 40.Meissner H. P., Electrophysiological evidence for coupling between β cells of pancreatic islets. Nature 262, 502–504 (1976). [DOI] [PubMed] [Google Scholar]
- 41.Cooper M., Ebner A., Briggs M., Burrows M., Gardner N., Richardson R., West R., Cy3b™: Improving the performance of cyanine dyes. J. Fluoresc. 14, 145–150 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Uttamapinant C., Sanchez M. I., Liu D. S., Yao J. Z., White K. A., Grecian S., Clark S., Gee K. R., Ting A. Y., Site-specific protein labeling using PRIME and chelation-assisted click chemistry. Nat. Protoc. 8, 1620–1634 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S13
Tables S1, S3 to S7
Legend for table S2
Table S2