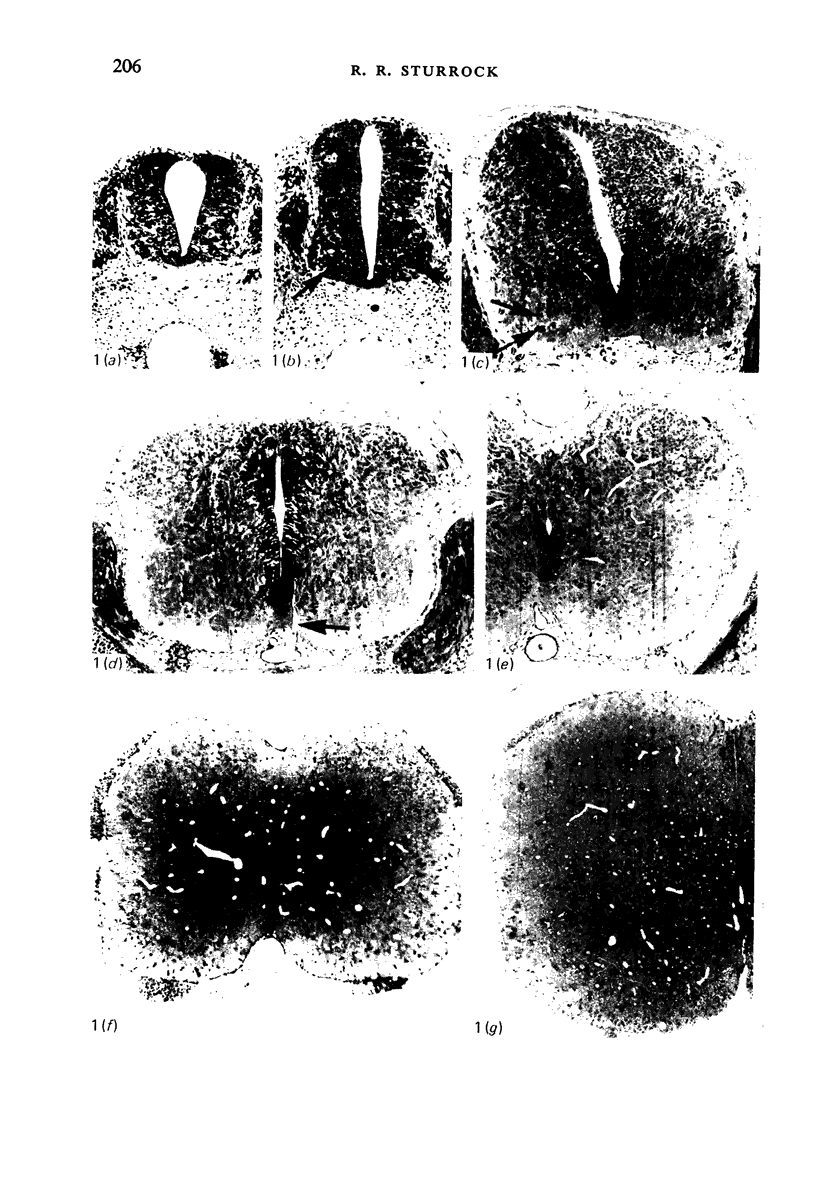
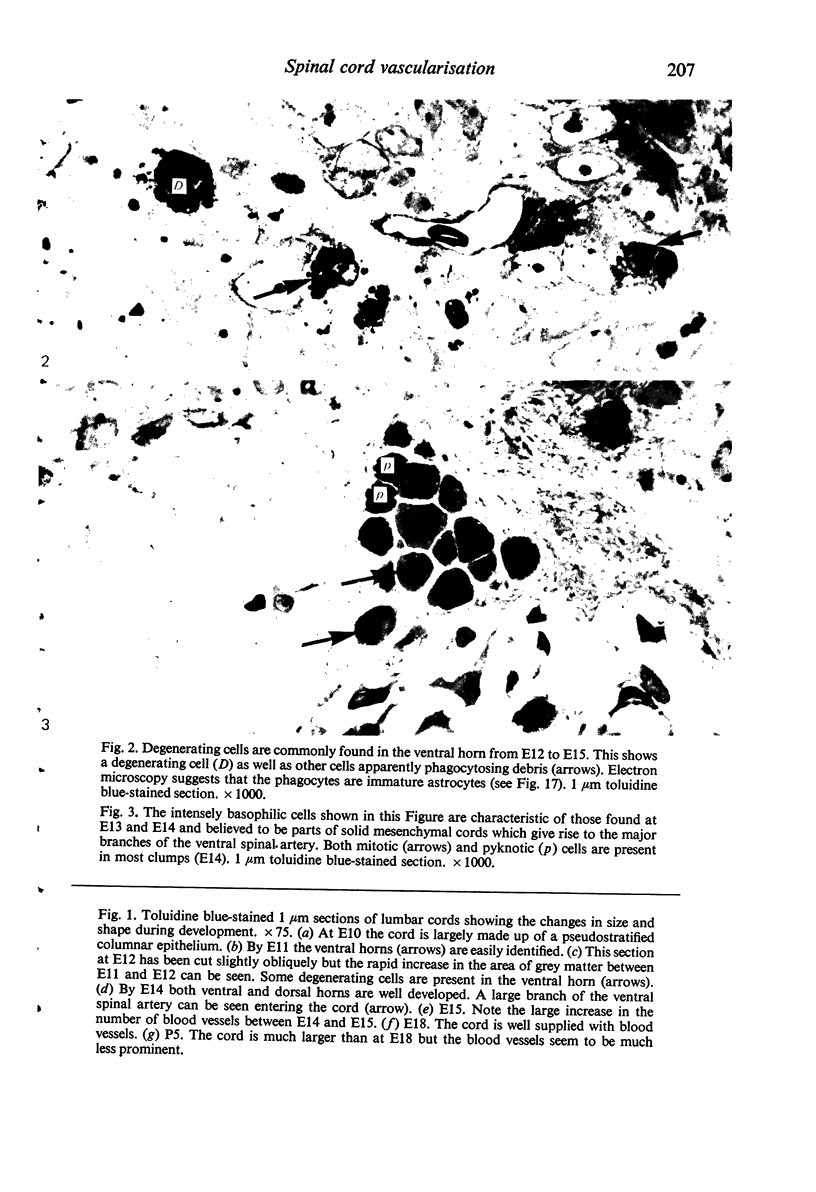
Abstract
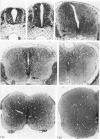
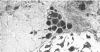
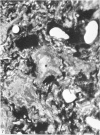
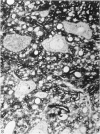
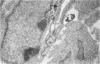
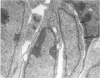
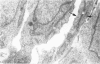
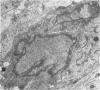
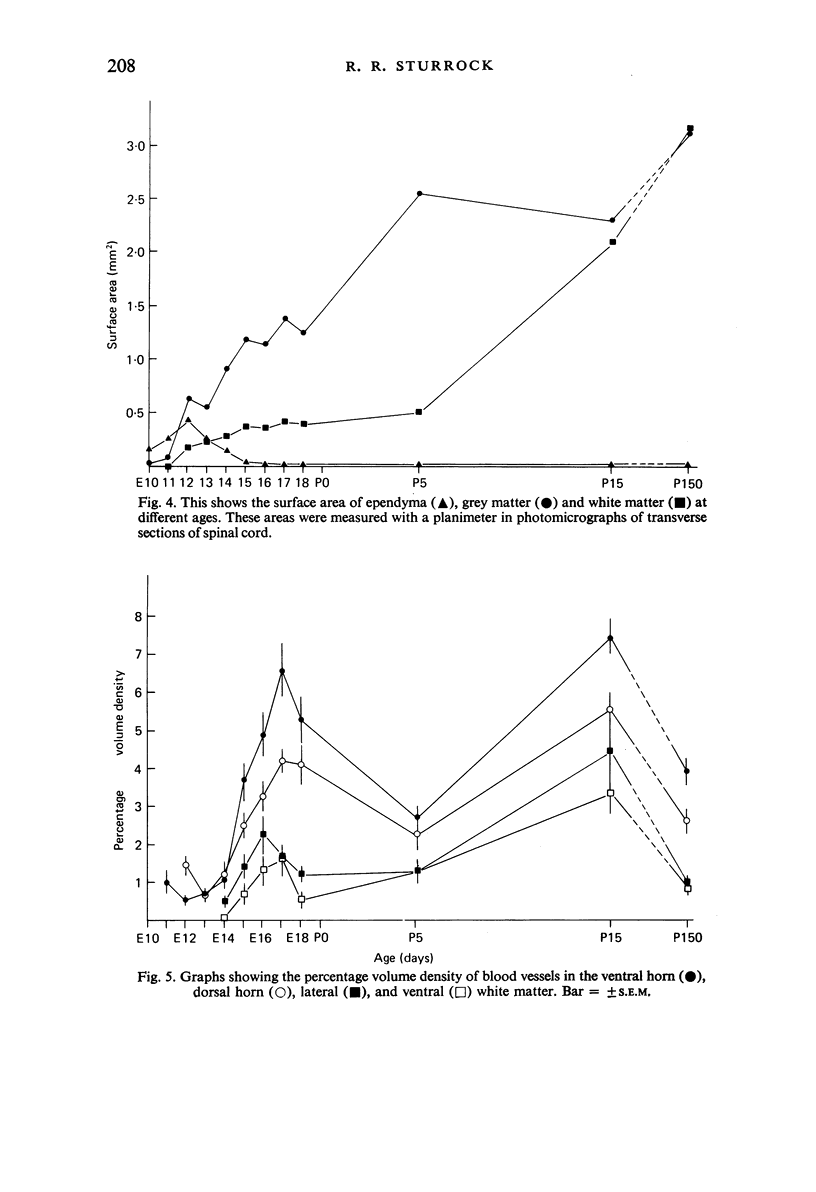
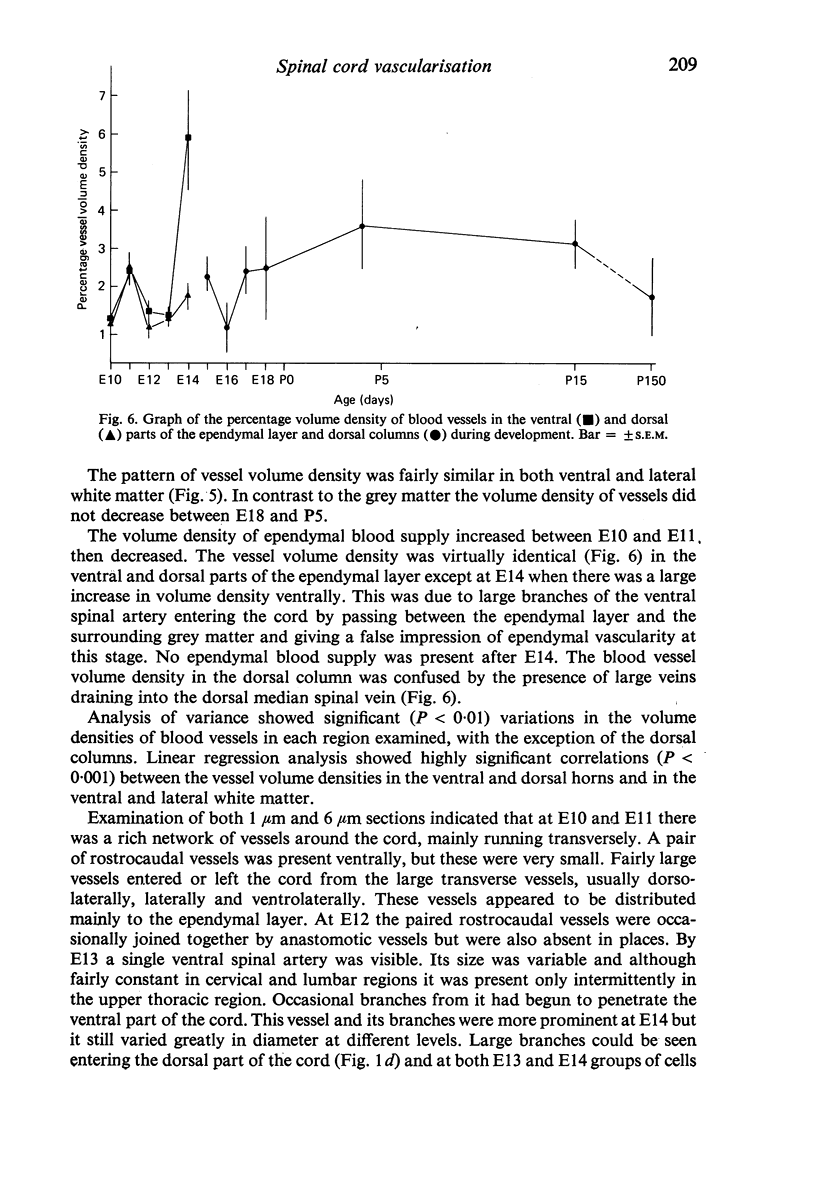
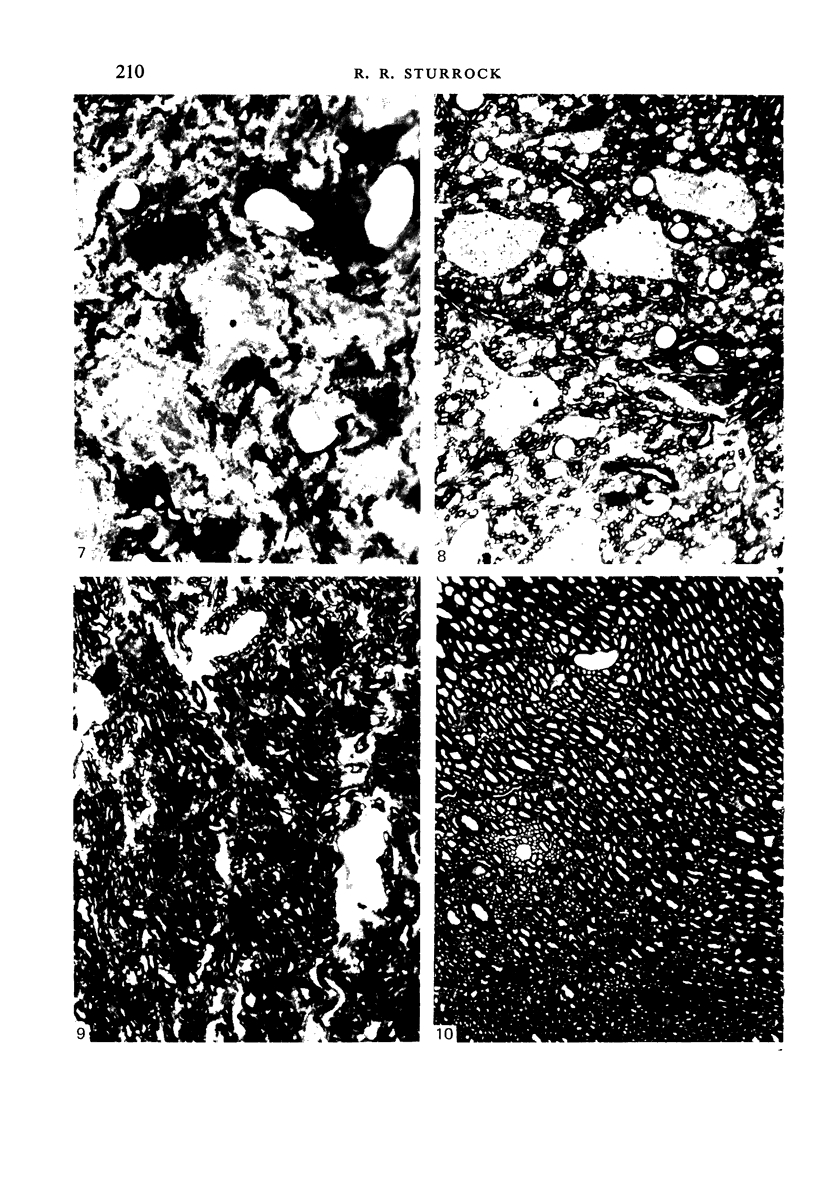
Vascularisation of the mouse spinal cord was examined by both quantitative histology and electron microscopy from E10 up to adult life, with particular emphasis on embryonic development. Blood vessels were present at all stages examined. From E10 to E14 blood vessels were most numerous in the ependymal layer. Ependymal layer vascularity declined as the layer itself decreased in size. Two rapid phases of vascularity were found. The first, from E14 to E18, coincided with the cessation of neuron production and early differentiation of grey matter. From E18 to P5 the volume density of blood vessels declined due to rapid growth of grey matter, presumably due to differentiation of the neuropil. A second rapid phase of vascularisation, associated with myelination, occurred between P5 and P15. The volume density of blood vessels declined between P15 and P150, probably due to a combination of cord growth and an actual decrease in size and/or number of blood vessels. The volume density of vessels in white matter increased from E14 to E18 and remained constant until P5. Since white matter volume increased rapidly between E18 and P5 due to the onset of myelination, the constant volume density indicated a substantial increase in the number of vessels during this time. The rapid increase in vascularisation noted in the grey matter between P5 and P15 also occurred in white matter, as did the later decline. It is suggested that there is a transient increase in vascularity associated with myelin production. At all stages spinal cord endothelial cells are joined by tight junctions. Primitive dark endothelial cells are present at E11 but many pale, well differentiated endothelial cells are also present. A basement lamina appears to be present on parts of vessels in contact with glioblasts. By E15 endothelial cells are well differentiated.
Full text
PDF




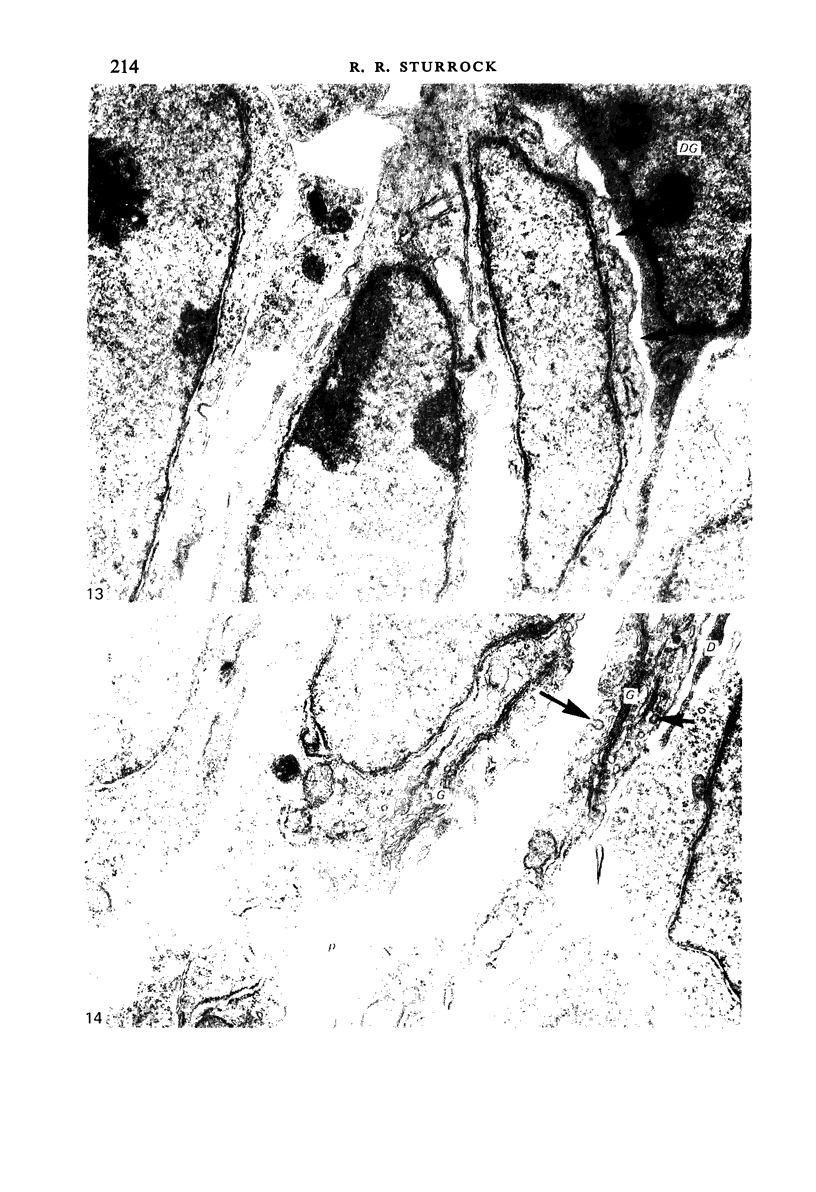

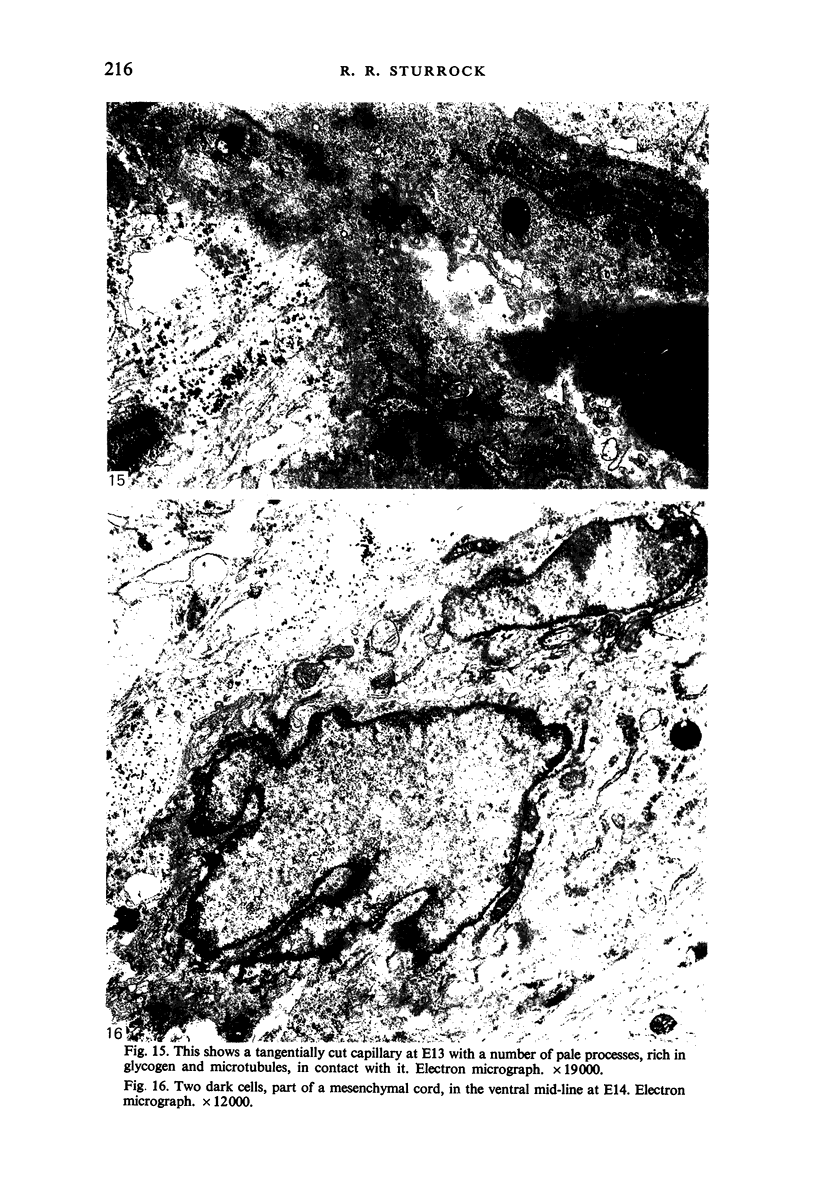
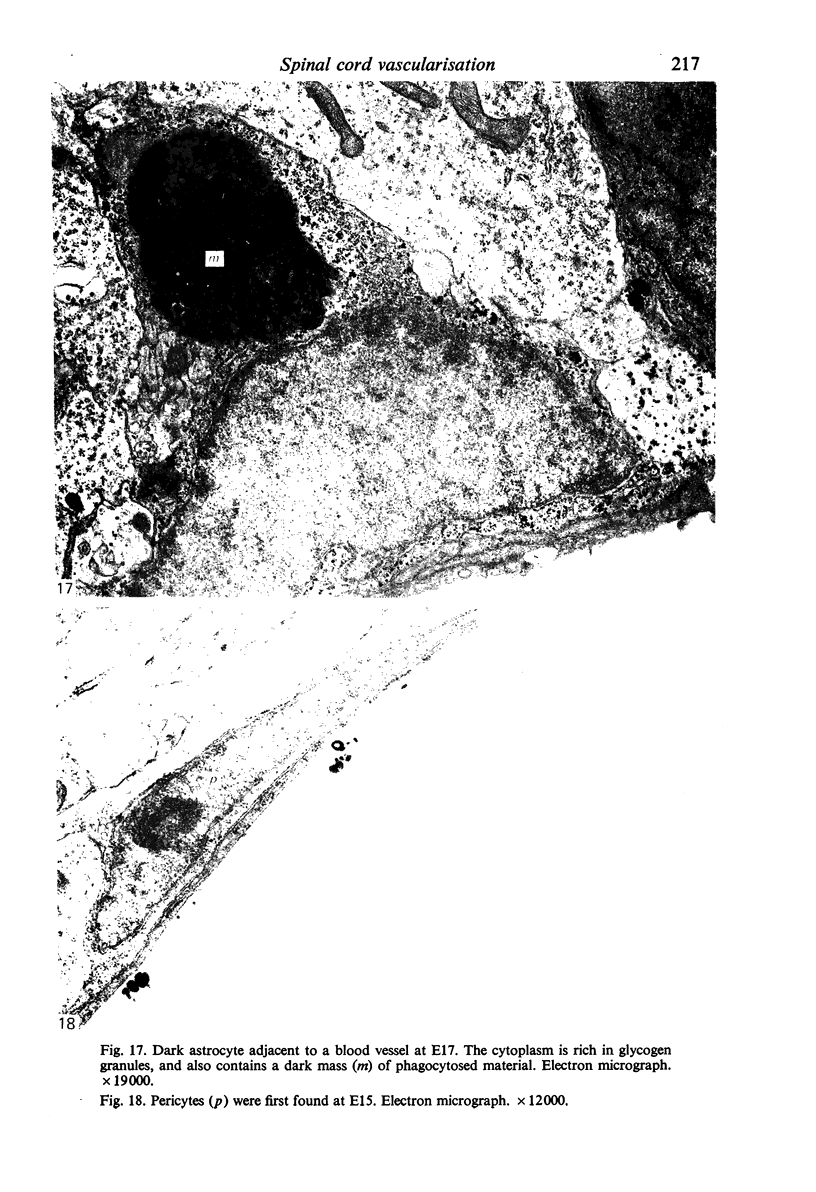
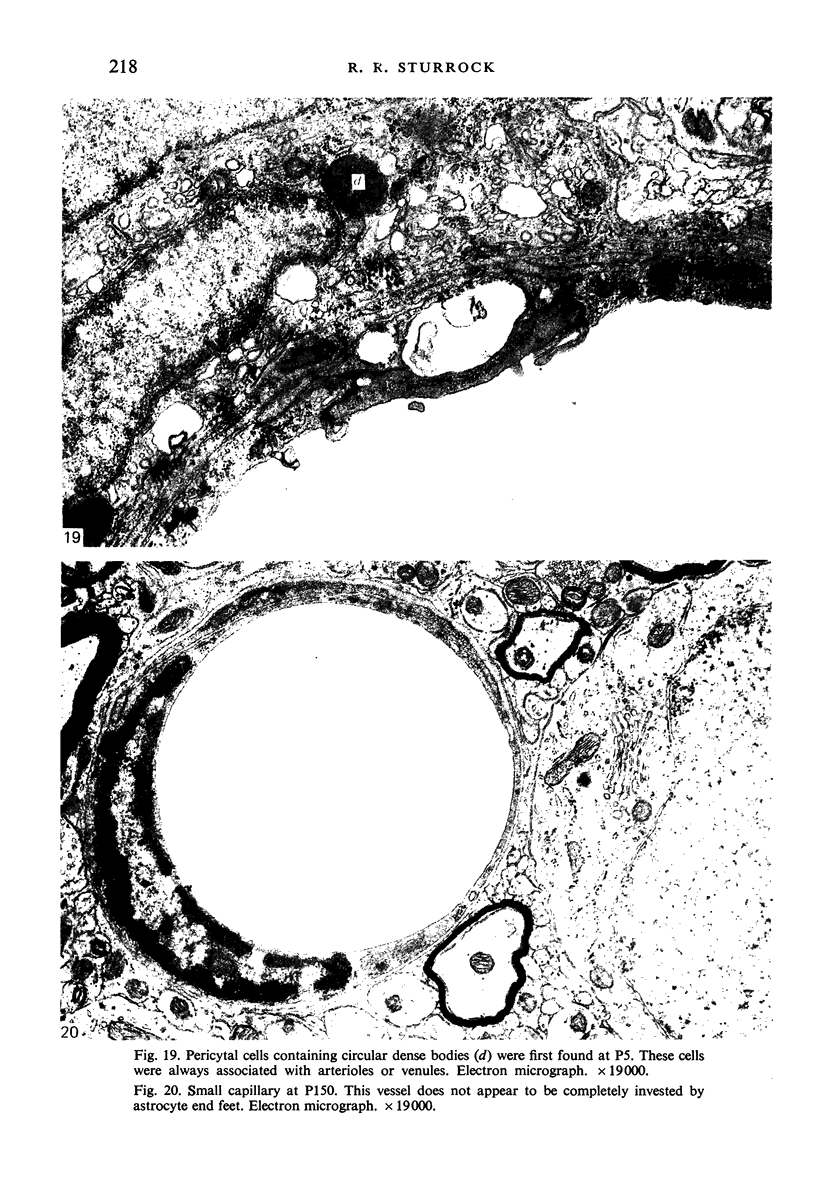



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bär T., Wolff J. R. The formation of capillary basement membranes during internal vascularization of the rat's cerebral cortex. Z Zellforsch Mikrosk Anat. 1972;133(2):231–248. doi: 10.1007/BF00307145. [DOI] [PubMed] [Google Scholar]
- Caley D. W., Maxwell D. S. Development of the blood vessels and extracellular spaces during postnatal maturation of rat cerebral cortex. J Comp Neurol. 1970 Jan;138(1):31–47. doi: 10.1002/cne.901380104. [DOI] [PubMed] [Google Scholar]
- Camosso M. E., Roncali L., Ambrosi G. Vascular patterns in the chick embryo spinal cord in normal and experimentally modified development. Acta Anat (Basel) 1976;95(3):349–367. doi: 10.1159/000144625. [DOI] [PubMed] [Google Scholar]
- Hannah R. S., Nathaniel E. J. The postnatal development of blood vessels in the substantia gelatinosa of rat cervical cord--an ultrastructural study. Anat Rec. 1974 Apr;178(4):691–709. doi: 10.1002/ar.1091780404. [DOI] [PubMed] [Google Scholar]
- Henrikson C. K., Vaughn J. E. Fine structural relationships between neurites and radial glial processes in developing mouse spinal cord. J Neurocytol. 1974 Dec;3(6):659–675. doi: 10.1007/BF01097190. [DOI] [PubMed] [Google Scholar]
- Ibrahim M. Z., Al-Wirr M. E., Bahuth N. The mast cells of the mammalian central nervous system. III. Ultrastructural characteristics in the adult rat brain. Acta Anat (Basel) 1979;104(2):134–154. doi: 10.1159/000145062. [DOI] [PubMed] [Google Scholar]
- Kalimo H. The role of the blood-brain barrier in perfusion fixation of the brain for electron microscopy. Histochem J. 1976 Jan;8(1):1–12. doi: 10.1007/BF01004000. [DOI] [PubMed] [Google Scholar]
- Phelps C. H. The development of glio-vascular relationships in the rat spinal cord. An electron microscopic study. Z Zellforsch Mikrosk Anat. 1972;128(4):555–563. doi: 10.1007/BF00306988. [DOI] [PubMed] [Google Scholar]
- Schmid A. H., Rohr H. P. Stereology, a complement to experimental neuropathology. I. Introduction into stereology. II. Ultrastructural-morphometric investigations (baseline data, axotomy) on the superior cervical ganglion of the rat. Acta Neuropathol. 1976 Oct 15;36(2):161–175. doi: 10.1007/BF00685278. [DOI] [PubMed] [Google Scholar]
- Smart I. H. Proliferative characteristics of the ependymal layer during the early development of the spinal cord in the mouse. J Anat. 1972 Apr;111(Pt 3):365–380. [PMC free article] [PubMed] [Google Scholar]
- Sturrock R. R. A developmental study of the mouse neostriatum. J Anat. 1980 Mar;130(Pt 2):243–261. [PMC free article] [PubMed] [Google Scholar]
- Sturrock R. R. A light and electron microscopic study of proliferation and maturation of fibrous astrocytes in the optic nerve of the human embryo. J Anat. 1975 Apr;119(Pt 2):223–234. [PMC free article] [PubMed] [Google Scholar]
- Sturrock R. R. An electron microscopic study of the development of the ependyma of the central canal of the mouse spinal cord. J Anat. 1981 Jan;132(Pt 1):119–136. [PMC free article] [PubMed] [Google Scholar]
- Sumi S. M. The extracellular space in the developing rat brain: its variation with changes in osmolarity of the fixative, method of fixation and maturation. J Ultrastruct Res. 1969 Dec;29(5):398–425. doi: 10.1016/s0022-5320(69)90062-8. [DOI] [PubMed] [Google Scholar]