Abstract
Active restoration strategies targeting corals with elevated heat tolerance have the potential to enhance reef resistance under a warming climate. While stress-tolerant corals have been documented in extreme systems such as mangrove lagoons, it is critical to assess the ability of these corals to maintain tolerance when moved to a more benign habitat. Here, we translocated corals from a mangrove lagoon to an adjacent reef and evaluated the thermal thresholds of corals from both locations before translocation and after 1 year. We demonstrate that mangrove colonies have higher thermal tolerance than reef corals, and, critically, mangrove colonies exhibited no loss in thermal tolerance following 1-year translocation to a less extreme reef habitat. Up-regulation of genes associated with DNA repair, metabolism, and homeostasis indicates the importance of these pathways in helping mangrove corals mitigate thermal stress. Our findings suggest the use of heat tolerant corals from extreme systems holds promise as part of intervention strategies aiming to increase reef resistance.
Corals from extreme environments hold promise for active intervention strategies in reef conservation.
INTRODUCTION
Coral reefs worldwide are facing an uncertain future, where ocean warming (1), ocean acidification (2), deoxygenation (3), and storm activity (4) are eroding coral reef persistence. Globally, live coral cover has declined by 50% since the 1950s (5), resulting in substantial shifts in community assemblage and population dynamics across many reef systems (6, 7). The natural adaptive capacity of corals is unlikely to keep pace with the rapidly changing climate (8), and traditional approaches of reef management are no longer adequate (9), catalyzing the urgent need for novel tools and techniques for sustaining future reef health (10) and buying time as we continue to address climate change (11). Although a suite of active interventions aimed at protecting coral reefs have been suggested [e.g., selective breeding and microbiome manipulation; (12)], one approach gaining attention is the integration of resilient coral material, particularly to elevated temperatures, into active management plans (13). Selection and propagation of coral colonies that have a naturally elevated heat tolerance could bolster population resilience to stressful environmental conditions through increased abundance of tolerant individuals [e.g., (14, 15)]. If such individuals become reproductively viable, then they also have the potential to facilitate the exchange of adaptive traits between populations through gene flow (16).
Corals living in extreme shallow water systems and marginal environments [sensu (17)] could provide one strategy for bolstering population resilience through targeted propagation and subsequent translocation of tolerant individuals into new populations (13). For example, corals growing in back-reef pools with naturally high-temperature variability in American Samoa have been used to construct heat-tolerant coral nurseries by integrating colonies with greater heat tolerance than conspecifics growing in pools with moderate temperature variability (18). Another example of extreme coral systems includes mangrove lagoons, where environmental conditions are highly variable for multiple parameters compared to adjacent reefs [e.g., New Caledonia (19), the Caribbean (20), and the Great Barrier Reef (GBR) (21)]. Mangrove lagoons on the GBR are characterized by reduced oxygen and pH alongside elevated maximum temperatures and temperature variability (22). Yet, these systems house taxonomically diverse coral communities (34 species to date), highlighting their potential use in targeted adaptive management strategies (21). Where such habitats host naturally stress-resilient coral populations (23) in proximity to adjacent reefs, they could aid conservation strategies by reducing the effort and costs involved with translocation. Such benefits require that resilient colonies originating from extreme systems will maintain their capacity for tolerance following translocation to a novel environment [see (24)]. Although relocation of corals between reef environments has been shown to lead to complex trait interactions to varying environmental conditions during bleaching (25), the retention of resilience following transplantation has yet to be examined over broad spatial and temporal scales (26).
Extreme systems that host coral populations have emerged as key natural laboratories to enhance knowledge on mechanisms driving coral resilience. Factors including phenotypic plasticity (27), regulation of gene expression (28), epigenetics [e.g., heritable modification to DNA without changing the DNA sequence; (29)], or association with heat-tolerant Symbiodiniaceae (30) can contribute to the resilience of coral individuals and do not necessarily occur in isolation. For example, studies from American Samoa have revealed that coral tolerance to highly variable back-reef pools is driven by a combination of up-regulated gene expression and phenotypic acclimatization (31). Corals which host naturally resilient Symbiodiniaceae species, for example, some species from the genus Durusdinium, have been shown to mitigate bleaching susceptibility (30, 32), with coral-Durusdinium associations often observed in suboptimal or disturbed environments, including mangrove lagoons (22). Acclimatization to more extreme environmental conditions can also occur through epigenetic mechanisms such as histone modification or DNA methylation via changes to gene expression (33). DNA methylation [i.e., the addition of a methyl group generally to the C-5 position of a cytosine; (34)] can be triggered by the environment and occur rapidly [i.e., within hours to days; (35, 36)], leading to real-time changes in phenotypic responses (33). For example, Hackerott et al. (37) showed that variations in DNA methylation among Acropora cervicornis genetic clones across environmental gradients were related to seasonal conditions, with observable differences in colony physiology aligned with epigenetic variation. Regulation of gene expression such as alterations of key genes or proteins associated with metabolic pathways, oxidative stress responses, DNA repair, and apoptosis have been documented in corals in response to increased environmental stress (28, 38) and, in some cases, can reflect local acclimation or adaptation to elevated abiotic conditions (31). Notably, corals from the naturally extreme Persian-Arabian Gulf have exhibited marked up-regulation of heat shock proteins as a defensive mechanism during peak periods of heat stress (39), while mangrove corals on the GBR have an enrichment of genes associated with cellular maintenance and respiration compared to their reef conspecifics, likely necessary for their survival in an extreme habitat (40). Collectively, these studies on “natural laboratories” and the molecular mechanisms involved in bolstering stress tolerance provide evidence of the ability of corals to withstand environmental extremes and could help resolve pathways involved in promoting coral fitness. A greater understanding of these mechanisms could allow for more optimized active management initiatives targeting the use of corals from extreme habitats.
Recent use of rapid phenotyping devices has begun to reveal how coral species and genotypes vary widely in their capacity to withstand environmental stress (41, 42) and the putative underlying mechanisms (43). One approach, the Coral Bleaching Automated Stress System (CBASS) has been developed to better resolve differences in coral thermal stress responses via acute heat stress assays (43). Although phenotyping has been used increasingly as a powerful means to identify coral individuals with enhanced thermotolerance, determining whether corals selected from naturally resilient populations can retain enhanced tolerance when translocated to a new, more benign, environment remains uncertain. Therefore, we examined differences in thermal tolerance (via photophysiological responses of Fv/Fm, maximum photosynthetic efficiency) of Pocillopora acuta from a mangrove system compared to the adjacent reef via CBASS acute heat stress assays before and after 1-year translocation of colonies from mangrove to reef, as well as differences in thermal tolerance compared to wild counterparts after 1 year. Our goal was to determine whether observed thermal tolerance would be retained following long-term exposure to the less variable or extreme reef habitat, with the study designed specifically to assess coral tolerance to heat stress in isolation from other stressors that could be present. Additionally, temperature profiles 12 months before each stress assay were investigated to understand whether differences in annual temperature regimes influenced thermal thresholds. We specifically examined gene expression profiles and total quantification of methylated DNA to investigate underlying mechanisms contributing to potential retention or erosion of thermal tolerance. Using our combined phenotypic and molecular approaches, we show that mangrove corals have greater thermotolerance to short-term heat stress and retain their superior tolerance following 1 year transplantation to a more benign reef system. By comparison, back-transplanted corals (i.e., reef corals translocated to a reef environment) retained the same thermal tolerance as before transplantation, excluding the transplantation process as the driver behind the observed pattern. We discuss the potential value of using corals from extreme systems as part of active intervention strategies with such enhanced retained thermal tolerance. Although promising, these strategies represent one approach within the larger framework of global reef conservation but do not replace continued action to mitigate climate change (11).
RESULTS
Fv/Fm ED50-based thermal tolerance response
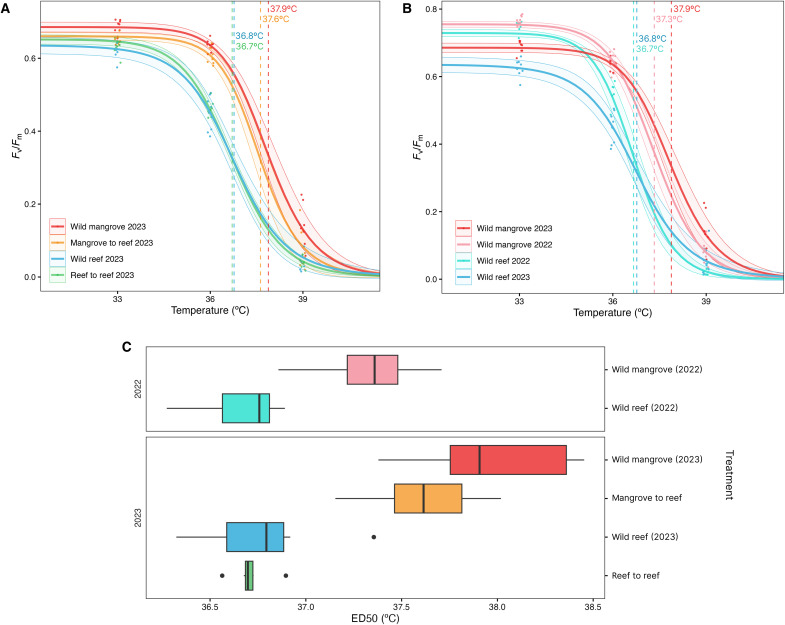
Photophysiological responses of Fv/Fm were examined to investigate the thermal tolerance of mangrove and reef corals before and following translocation. Across years (2022 versus 2023) and treatments (wild versus transplant), corals originating from the mangroves had higher values of mean effective dose (ED50) for Fv/Fm than the reef native corals. In February 2022, the ED50 was +0.6°C higher for the wild mangrove than the wild reef colonies [t test: t13 = 5.313, P < 0.001; 37.3°C (±0.3°C) (means ± SD) versus 36.7°C (±0.2°C), respectively; fig. S1]. In February 2023, the ED50 again differed between groups [Kruskal-Wallis test χ2(3) = 23.804, P < 0.001], with wild mangrove corals exhibiting the highest ED50 at 37.9°C (±0.4°C) and reef-to-reef transplanted corals the lowest at 36.7°C (±0.1°C) (Fig. 1A). Pairwise comparisons revealed that both wild mangrove and mangrove-to-reef transplanted colonies had significantly higher ED50 values than both reef groups (wild reef and reef to reef; table S1). No differences were found in ED50 between wild mangrove and mangrove-to-reef corals (Dunn’s test: adjusted P value = 1.00), with a mean of 37.9°C (±0.4°C) and 37.6°C (±0.3°C), respectively (Fig. 1A). Similarly, wild reef and reef-to-reef corals exhibited no difference (0.1°C) in mean ED50 (Dunn’s test: adjusted P value = 1.00; Fig. 1A). In summary, corals originating from the mangroves consistently had a higher mean ED50 than reef native corals across both years (2022 and 2023) and treatments (wild and transplant), while no differences were found between groups originating from the same habitat (i.e., reef or mangrove) following translocation (Fig. 1C).
Fig. 1. Thermal tolerance responses of mangrove and reef corals to short term heat stress.
P. acuta Fv/Fm (maximum photosynthetic efficiency of photosystem II) thermal performance curves modeled using a three parameter log logistic regression model, with dashed vertical lines indicating the mean ED50 values for (A) February 2023 wild groups (wild mangrove and wild reef) versus 1-year transplanted groups (mangrove to reef and reef to reef; Kruskal-Wallis test, P < 0.001) and (B) wild mangrove and reef corals between years (February 2022 versus 2023). Shaded areas around the curves represent the 95% confidence interval and data points indicate the mean Fv/Fm for each colony within the group. (C) Box plots of group ED50 values by treatment (wild versus translocated) and year (2022 versus 2023) showing median value with upper and lower quartiles, n = 8 per group except wild reef 2022 (n = 7).
To investigate potential differences in photophysiological values influenced by annual variations in temperature regimes, wild mangrove and reef corals were compared across years (February 2022 versus February 2023). Reef corals exhibited no differences in ED50 responses across years (t test: t13 = 0.718, P = 0.486), with a difference of 0.1°C between 2022 and 2023 (36.7° and 36.8°C, respectively). In contrast, mangrove corals differed between years (t test: t14 = −3.931, P = 0.002), where the mean ED50 of wild mangrove corals was +0.6°C higher in February 2023 (37.9°C) compared to that in February 2022 (37.3°C; Fig. 1B). Notably, this higher ED50 value of mangrove corals in February 2023 is consistent with patterns in temperature profiles between years, whereby Low Isles reef flat had a higher mean temperature (+0.1°C) and a greater temperature range (±4.6°C) in February 2022–2023 (year 2) compared to those in 2021–2022 (year 1; fig. S2).
Differential gene expression
To explore molecular drivers underlying the photophysiological response differences, we compared the gene expression of wild (wild mangrove and wild reef) and 1-year transplanted (mangrove to reef and reef to reef) corals subject to acute heat assays via CBASS in February 2023. A principal components analysis (PCA) of transcript abundance revealed separation of samples by both temperature and habitat, with principal component 1 (PC1) and PC2 explaining 54% of the variation in response patterns (Fig. 2A). Discrete clusters were evident for mangrove-to-reef and wild mangrove groups, particularly under exposure to 33° and 36°C (Fig. 2A). Wild reef colonies at 36°C also showed notable separation to all other groups. Conversely, the lowest temperature (30°C) had a lesser effect on gene expression, as wild reef, reef-to-reef, and wild mangrove corals exhibited overlap.
Fig. 2. Gene expression patterns across habitat groups and temperature.
(A) Principal components analysis (PCA) of transcript abundance counts of P. acuta habitat groups (mangrove to reef, reef to reef, wild mangrove, and wild reef) across temperatures (30°, 33°, and 36°C), with ellipses representing 80% confidence intervals. (B) Count of differentially expressed genes (DEGs; via differential gene expression analysis) between temperatures, groups and their combined interaction. (C) Count of DEGs which were up- and down-regulated (dark colors and faded colors, respectively) compared between groups for each temperatures (Wald test). See table S2 for all sample sizes.
Differential gene expression analysis of 32,733 genes showed variation in differentially expressed genes (DEGs) across temperatures, habitat groups, and their combined interaction, with temperature exerting the overall greatest effect on gene expression (Fig. 2B). Across temperature treatments (30°, 33°, and 36°C), 16,445 genes were differentially expressed, while 13,640 genes were differentially expressed between habitat groups. When considered by the combined interaction of habitat group and temperature, a smaller number of DEGs were found, with a total of 4745 (Fig. 2B). When further investigated by pairwise comparisons (Wald test; table S2), the largest difference in expression was observed between mangrove-to-reef and wild reef corals under the greatest temperature stress (5954 up-regulated and 5460 down-regulated genes at 36°C). Of all comparisons, mangrove-to-reef and wild mangrove colonies had the smallest number of DEGs across temperature treatments (Fig. 2C and table S2).
Patterns in gene enrichment across habitat groups and temperatures
Counts of DEGs revealed that temperature followed by habitat group (mangrove versus reef) effects were dominant over interactive effects (Fig. 2B). To investigate differences in gene expression patterns across temperature (irrespective of habitat group) and habitat group (irrespective of temperature), we examined the higher order functions of up- and down-regulated genes. Gene ontology (GO) enrichment analysis found that, irrespective of temperature exposure, mangrove corals were enriched in genes associated with protein binding and up-regulated in metabolic processes including macromolecule biosynthesis and lipoprotein lipase activity compared to reef corals. Similarly, irrespective of habitat group, temperature had a strong consistent effect, with P. acuta colonies within the 36°C treatment enriched in DEGs related to metabolism and energy [adenosine 5′-triphosphate (ATP) metabolic process, l-aspartate transport, and oxidative phosphorylation] as well as protein regulation (protein folding chaperone and unfolded protein binding) compared to 30° and 33°C treatments (supplementary data accessible online at https://zenodo.org/records/15208057).
Differences in gene enrichment between habitat groups under heat stress
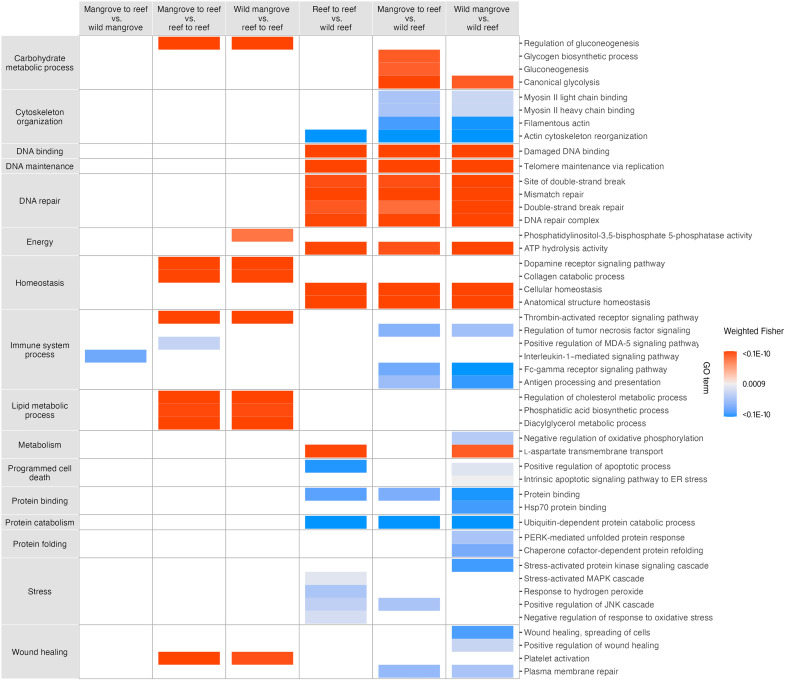
To assess whether groups from different habitats (mangrove or reef habitats) varied in their gene expression response under acute heat stress, we further examined up- and down-regulated genes in mangrove versus reef corals occurring at 36°C. GO enrichment analysis found that wild mangrove versus wild reef had the greatest number of differentially enriched genes (n = 328 differentially enriched GO terms) of all comparisons under heat stress, while only six genes were found to differ between mangrove groups (wild mangrove versus mangrove to reef) at 36°C (table S3). Categorical expression patterns of genes contributing to enriched GO terms summarized via GO Slims revealed that mangrove-to-reef and wild mangrove colonies exhibited similar enriched categories, with differences in expression primarily associated with signaling and DNA binding (supplementary data accessible online at https://zenodo.org/records/15208057).
In contrast to similarities between mangrove corals, reef groups (wild reef versus reef-to-reef corals) exhibited a number of functional annotation differences under heat stress (supplementary data accessible online at https://zenodo.org/records/15208057). DEGs associated with DNA damage and repair (double-strand break repair, mismatch repair, and telomere maintenance) and homeostasis (cellular homeostasis and anatomical structure homeostasis) were enriched in reef-to-reef corals. Conversely, wild reef groups were enriched in several stress responses, including up-regulation of stress-activated mitogen-activated protein kinase (MAPK) cascade, response to hydrogen peroxide, as well as negative regulation of cellular response to oxidative stress (Fig. 3).
Fig. 3. Relative expression of enriched GO terms under heat stress (36°C).
Gene ontology (GO) slim categories (left panel) of significantly enriched GO terms (right side) between groups (comparisons in the top panel) at 36°C (assessed via GO enrichment analysis). The color gradient indicates GO terms that were significantly (weighted Fisher, P < 0.001) enriched in the first group (red) or in the second group (blue) shown in the top panel. GO terms without functional categorization into GO Slims by the generic obo database were manually categorized based on literature searches of each term. For visualization purposes, a subset of GO terms were presented here; see supplementary data accessible online at https://zenodo.org/records/15208057 for entire list of enriched GO terms (n = 5 per sample group).
Comparisons between wild reef corals and mangrove groups (both mangrove to reef and wild mangrove) demonstrated differences in expression patterns between environments. Mangrove groups were up-regulated in genes important in metabolic and energy processes (glycolysis process, ATP hydrolysis activity, and l-aspartate transport), cellular homeostasis, and DNA repair (double-strand break repair, mismatch repair, and DNA repair complex). In contrast, wild reef corals were enriched in certain pathways related to immune response regulation (Fc-gamma receptor signaling and tumor necrosis factor–mediated signaling pathway), cytoskeleton organization, as well as processes related to protein regulation and binding when compared to mangrove groups (Fig. 3; supplementary data accessible online at https://zenodo.org/records/15208057). Of all group comparisons, wild mangrove and wild reef colonies exhibited the greatest number of differences in expression patterns of genes contributing to GO enrichment differences. Notably, wild reef corals were up-regulated in apoptotic signaling pathways, cellular stress cascades (stress-activated protein kinase signaling), wound healing, and suppression of energy regulating processes (negative regulation of oxidative phosphorylation) compared to wild mangrove corals (Fig. 3).
Last, examination of DEGs revealed that gene expression patterns of mangrove groups (mangrove to reef and wild mangrove) were more closely aligned with reef-to-reef corals than wild reef corals, although differences were still exhibited (Fig. 3). Mangrove-to-reef and wild mangrove corals were enriched in a number of processes related to carbohydrate and lipid metabolism (gluconeogenesis, diacylglycerol metabolic process, and cholesterol metabolic process), homeostasis (dopamine receptor signaling and collagen catabolic process), and signaling processes, including those related to activation of inflammatory responses (thrombin-activated receptor signaling) compared to reef-to-reef corals.
Methylated DNA across habitat groups and temperatures
Percent DNA methylation varied only by the interaction of group and temperature [aligned rank transform (ART) analysis of variance (ANOVA), F4 = 3.102, P = 0.033; table S4], whereby reef-to-reef individuals had 1.8 times higher DNA methylation under 33° and 36°C treatments compared to that under ambient (30°C) conditions. Additionally, mangrove-to-reef colonies had 1.9 times lower DNA methylation compared to reef-to-reef colonies at 36°C (post hoc comparison, P = 0.015; Fig. 4). No differences were found in percent DNA methylation for mangrove corals between temperatures (table S5).
Fig. 4. Percent DNA methylation of habitat groups across temperatures.
Percent DNA methylation relative to total DNA (means ± SE) of P. acuta groups (mangrove to reef, reef to reef, and wild mangrove) under acute temperature stress (30°, 33°, and 36°C) during the February 2023 CBASS experiment (n = 4 per sample group; ART ANOVA, P = 0.033).
Population genetic structure
To ensure that phenotypic responses were not influenced by clonal individuals, the presence of clones within habitat groups was assessed via a genomic relationship matrix (GRM) that revealed no presence of clones among individuals (kinship coefficient of 0.45; fig. S3A). A PCA used to visualize genetic structure showed distinct clustering of habitat groups, particularly along PC1, of the mangrove versus reef P. acuta individuals (fig. S3B). Further investigation via STRUCTURE analysis indicated the presence of two population clusters (fig. S3C), which was additionally supported by a genetic differentiation analysis that demonstrated strong differentiation between habitat groups [FST = 0.811; (44)].
DISCUSSION
Active management strategies targeting the use of coral colonies with stress-tolerant phenotypes is a suggested approach for bolstering reef resilience under a changing climate (15, 45). However, assessing whether colony resilience can be retained following transplantation to a novel environment, as well as the mechanisms underpinning thermal tolerance, is a critical first step to understanding the suitability of this approach for reef conservation. Here, we provide evidence that corals growing within naturally extreme mangrove lagoons (with high-temperature maxima and variability) exhibited greater photophysiological performance under acute heat stress compared to reef conspecifics. Furthermore, this elevated performance under heat stress is maintained following long-term (1 year) translocation to a new, more benign reef environment, supporting the notion of relocating corals from more extreme environments to more benign environments to enhance long-term resilience and survival. Differential gene expression and up-regulation of processes involved in stress and homeostasis, DNA repair, and metabolism in mangrove corals compared to reef colonies suggest the importance of these pathways for mitigating heat stress in P. acuta colonies. Colony DNA methylation primarily did not vary across environments and temperatures. An exception was the highly methylated reef-to-reef corals compared to mangrove-to-reef corals under high heat stress (36°C), matching previous well-known patterns of the inverse relationship between DNA methylation and phenotypic plasticity (46). Our findings provide evidence that P. acuta colonies with elevated thermal resilience can maintain tolerance over time and space, demonstrating the viability of using stress-hardened corals in future stock selection strategies as a means of enhancing reef resilience through proactive management actions.
Extreme corals retain their heat tolerance following translocation to a new environment
From a restoration perspective, the ability for corals to retain tolerance across novel habitats is critical, as translocation of corals is an often-necessary step in coral restoration initiatives (12). Movement to a new environment introduces a suite of unique biotic and abiotic factors that may lead to trade-offs in coral fitness (24). Thus, understanding the ability of corals to retain beneficial traits outside their native environment is a fundamental first step for assessing the efficacy of propagating and transplanting resilient corals for active management strategies. In our study, corals native to the more extreme mangrove lagoon at Woody Isles retained the same thermal thresholds (Fv/Fm ED50 responses) after 1 year growing on the Low Isles reef as they did in their native habitat. When considered alongside the overall greater thermotolerance observed for mangrove groups (mangrove to reef and wild mangrove) compared to reef groups (reef to reef and wild reef), this strategy of leveraging extreme systems for stock selection of naturally tolerant individuals holds considerable promise for restoration initiatives. As we assessed thermal thresholds over time as well as in parallel with colonies from native habitats, we were able to ascertain that neither the physical translocation nor the potential consequences associated with acclimatizing to a new environment had any discernible effect on phenotypic responses. Prior work by Haydon et al. (22) at the same location revealed that P. acuta transplanted for 9 months between the Woody Isle mangroves and adjacent Low Isles reef retained their native symbionts but experienced a shift in their microbiome in response to changing environmental conditions. Thus, the highly stable Symbiodiniaceae associated communities may contribute to the retained thermal tolerance of P. acuta when moved to new environments as seen in this study. Additionally, Woody Isles mangrove corals are associated with Symbiodiniaceae communities dominated by species from the Durusdinium genus (22), which may not only contribute to their ability to better withstand bleaching events (18), but, if proliferated during stress, can also allow for increased acclimatization and resilience (32). Future work could consider assessing coral responses over a greater geographic distance, as the mangrove and reef habitats are relatively close in this study. However, previous work has also shown that mangrove corals have higher performance (in this case, greater calcification under low pH) in New Caledonia, suggesting that tolerance of mangrove corals is geographically widespread and extends beyond temperature (47). Despite the proximity between habitats in this study, we still observed differences in environmental conditions (fig. S4) and thermal performance of corals across habitats, indicating that variations in traits are likely related to differences in adaptation to distinct environments.
To date, few studies have assessed the persistence of coral thermal tolerance with exposure to new habitats (26) or propagation environments (18, 48), despite broad interest in leveraging stress-resilient corals for active reef management. In alignment with our findings, the bleaching response of two Hawaiian coral species was unaltered following 6-month exposure to a new reef characterized by a range of distinct physicochemical conditions (26), suggesting that heat tolerance could be a relatively fixed trait. While additional work is needed to understand the multiyear and transgenerational persistence of these beneficial traits, the fixed nature of these effects evidenced by this study and others (26) provides encouragement for at least conferred benefits of heat tolerance on a 1-year time frame. Additionally, given that environmental pressures rarely occur in isolation in real-world situations, future research using phenotyping tools that integrate multiple stressors (e.g., light intensity, nutrients, and turbidity) and can capture a suite of physiological parameters will be especially beneficial to help us better understand the interplay between multiple stressors and how they influence the fitness and response of corals (41).
Elevated tolerance to temperature stress even over the short term is likely to benefit recipient populations as heat events are predicted to accelerate in frequency over the coming years (49). Although the findings of this study hold promise for bolstering reef resilience from a restoration perspective, there are potential trade-offs in other fitness traits associated with the use of “extreme” corals that will require longer-term assessments to ascertain impacts beyond simply increasing the retention of resilient coral biomass. For example, Scucchia et al. (40) showed lower skeletal density and reduced genetic diversity in mangrove corals that could compromise their ability to cope with future stress, particularly if translocated to an area where reductions in skeletal density are especially risky such as reefs with high wave action. Further, Bartels et al. (50) have revealed the high risk of mangrove corals to freshwater bleaching, highlighting that tolerance of extreme corals to key stressors (e.g., temperature and pH) does not guarantee their tolerance to other stressors (e.g., reduced salinity) or multistressor events. If enhanced thermal tolerance comes at the expense of other key fitness traits, such as growth (51) or fecundity (52), then this could result in considerable costs at an ecological level, including diminished ecological function. Such consequences could negatively affect natural regeneration (via reduced reproductive output) or threaten biodiversity, which, in many cases, are key objectives of reef conservation. With this knowledge, the use of extreme corals could form one part of a restoration strategy designed to enhance benefits to recipient populations while minimizing risks associated with their utilization (13) and should be complimented with long-term monitoring to understand potential impacts to ecosystem services (e.g., such as reef biogeochemistry).
Heat loading associated with thermal regimes can vary seasonally and annually, with recent thermal history shown to influence coral thermal performance (53). Thus, by comparing thermal thresholds of wild colonies across years (February 2022 and 2023), we were able to assess potential differences in photophysiological phenotypic performance influenced by the recent thermal history. While no difference was found in ED50 of reef corals between years, February 2023 wild mangrove corals had a higher thermal threshold than wild mangrove corals in February 2022 (differences of +0.6°C). Evaluation of temperature profiles of the Low Isles reef flat from the year immediately before each sampling period (e.g., February 2021–2022 and February 2022–2023) showed that mean and maximum temperature, as well as temperature fluctuations, were higher in the second year compared to year 1 (see fig. S2) (54). Thus, mangrove corals may have acclimated to a slighter warmer environment in the 12 months, leading up to the experiment in the second year, which would explain their higher thermal performance. The mangrove lagoon at Woody Isles represents a shallow, semienclosed environment, known to experience greater temperature extremes likely due to reduced tidal flushing compared to the adjacent reef (21, 55). As such, we could postulate that the small differences in mean annual temperature observed between the 2 years on the reef flat could have been exacerbated in the mangrove lagoon and would additionally explain why differences in ED50 thermal performance were only observed in mangrove corals between years. It is also pertinent to note that, while environmental heterogeneity can lead to variation in phenotypic responses, differences in bleaching thresholds could also be related to the underlying genetic architecture (56). It is well-documented that host genotype can contribute to bleaching responses (57, 58) and thus could also be responsible for variations in thermal thresholds between years as unique genotypes were assessed in the wild groups (wild mangrove and wild reef colonies) in 2023. Further work tracking coral individuals across years and environments will help better resolve underlying mechanisms contributing to difference in heat tolerance.
Mechanisms of thermal tolerance underlying coral habitat group response differences
Expression patterns of habitat groups under heat stress of 36°C (i.e., interactive effect) revealed that genes of mangrove corals (wild mangrove and mangrove-to-reef corals) were up-regulated relative to reef corals (wild reef and reef to reef) in processes necessary for maintaining function under stress including DNA repair, cell homeostasis, and metabolism. At Woody Isle, the mangrove lagoons are known to experience highly variable temperatures and reduced pH and oxygen levels (21, 55). Consequently, management of energy budgets through up-regulation of key lipid and carbohydrate metabolic pathways (such as canonical glycolysis, gluconeogenesis, and diacylglycerol processes) is likely critical to satisfy cellular demands and maintain responses under suboptimal environmental conditions. Previous work in the same mangrove lagoon has found that P. acuta colonies are able to withstand high-temperature exposure through metabolic plasticity (55). This is also consistent with evidence from corals growing in thermally stressful inshore environments that are able to withstand high-temperature exposure through up-regulation of key metabolism pathways, thereby highlighting the role of energy budget management in the adaptation of coral thermotolerance (38). Such up-regulation of metabolic pathways may have contributed to the greater thermal thresholds of mangrove corals observed in this study and could additionally help explain the increased thermal sensitivity in wild reef corals given their observed suppression of oxidative phosphorylation, a key process important in regulating an organism’s energy needs (59), which can be suppressed due to the accumulation of reactive oxygen species (ROS) in the mitochondria during stress (60). Alternatively, up-regulation of metabolic pathways could be enabled by greater nutritional acquisition through enhanced heterotrophy in mangrove corals, as previously inferred by Polapa et al. (61) in corals from extreme systems. Alongside energy pathways, proteins responsible for DNA repair can be activated in response to heat stress-driven cellular damage (62), and up-regulation of DNA repair processes likely indicates that mangrove corals were able to sense and activate repair mechanisms better than their reef conspecifics that could have contributed to their observed elevated photophysiological performance.
Under the same interactive scenario (differences in habitat group gene expression at 36°C), reef corals exhibited up-regulation of DEGs associated with immune responses, intrinsic apoptotic signaling, and cytoskeleton organization compared to mangrove corals, which may have contributed to the reduced thermal thresholds observed under heat stress. Cytoskeletal organization has been implicated as having an active role in response to stress in corals, with regulation of myosin proteins and actin remodeling likely representing an attempt to protect cells against damage in response to heat stress (62). However, cytoskeletal components are highly redox sensitive (63), and these pathways have also been linked to declines in cell viability under persistent heat stress (64). Thus, enrichment in factors involved in cytoskeletal reorganization can indicate direct damage to the integrity of the cytoskeleton (65) and could have led to the intrinsic programmed cell death also exhibited by reef corals. Reef corals exhibited a stronger enrichment in pathogen-related immune responses, including pathways related to Fc gamma receptor signaling, melanoma differentiation-associated gene 5 (MDA-5) signaling, and wound healing. Down-regulation of immunity genes can result as a consequence of trade-offs in resources under thermal stress where immunity reactions or pathogen defense may not be immediately required (66) and, hence, could explain the general reduction of immunity genes in mangrove corals coupled with their enhanced thermal tolerance.
In alignment with the minimal variation in ED50 thermal thresholds exhibited by translocated and wild mangrove colonies, enriched DEGs differed very little between the mangrove groups at 36°C (six in total), suggesting that mechanisms of heat tolerance may be well conserved in mangrove P. acuta colonies. Comparatively, reef-to-reef and wild reef corals were found to vary more widely in their expression to thermal stress, with several processes likely important in mediating their responses. Following translocation to the reef frames for 1 year, reef-to-reef corals were enriched in processes important in DNA repair and cellular homeostasis in response to heat stress, while wild reef corals exhibited active regulation of several stress responses and regulated cell death. Given the proximity between the wild reef corals and the reef frames housing the translocated colonies, variations in gene expression may have been affected by potential differences in microhabitat such as light or pathogen exposure (67, 68) or could be a consequence of variations in symbiont composition (68). Homeostasis-regulating genes help to mitigate disruptions caused by stress by orchestrating various mechanisms necessary for the internal steady state of cells (69), while DNA repair was likely responsible for maintaining cellular integrity. Conversely, stress signaling elevated in wild reef colonies, such as those through MAPK and c-Jun N-terminal kinase (JNK) cascades, are known to play an important role in protection against oxidative stress (70), with JNK activity previously implicated in the early activation and protection of corals to ROS during thermal and ultraviolet stress (71). Additionally, wild reef corals were characterized by up-regulation in their response to the ROS hydrogen peroxide, a potential trigger for bleaching if not sufficiently processed by antioxidant systems (72). Enrichment of such a response pathway provides evidence of the high levels of ROS likely experienced under the acute heat stress of this experiment and, which alongside up-regulation of pro-apoptosis, may have contributed to greater sensitivity in wild reef individuals. Given the number of differences in DEGs between reef groups, yet the overall similarity in ED50 thermal thresholds (a difference of 0.1°C), these expression patterns suggest that wild reef and reef-to-reef groups may have differed in their strategies of homeostasis and coping with heat stress. This could have been the result of regulation of molecular functions that differed but ultimately resulted in the same overall response to stress, potentially driven by their inability to successfully respond under high heat loading.
One important consideration is the observable morphological differences between P. acuta colonies across environments (Fig. 5, C and E). Morphological plasticity (73) and cryptic and hybrid species are known to occur in the genus Pocillopora, all of which have the potential to influence thermotolerance and gene expression patterns. To assess such possibilities, population structure and genetic differentiation were examined, which revealed P. acuta individuals from the mangroves and reef likely belong to separate populations, with little evidence of hybridization or clonal individuals present. Notably, within each habitat, all individuals belonged to a single population, supporting the absence of hybridization and suggesting that within-population phenotypic variation in heat tolerance is not driven by the presence of cryptic species. As both heat tolerance and gene expression were conserved across individuals within habitat groups, it is highly likely these responses are driven by the genetic architecture. We acknowledge the possibility that underlying genetic differences such as speciation could contribute to the observed variation in phenotypic responses. However, given the relatively small sample size in this study for assessing population structure, targeted research with greater replication across environmental gradients is needed to better contextualize the Pocillopora population between Low Isles reef and Woody Isles mangrove lagoon. Critically, these considerations underscore the importance of resolving and preserving genetic diversity across unique habitats, especially in extreme environments, as a priority for conservation biology.
Fig. 5. Location of translocation experiment, collection sites and nursery frames.
(A) Location of Low Isles in the northern Great Barrier Reef as indicated by the white square; Great Barrier Reef area depicted as per light blue shading in inset map. (B) Low Isle and Woody Isle surrounded by Low Isles reef, with the two collection sites indicated by pins: Low Isles reef flat (blue pin) and Woody Isle mangrove lagoon (red pin). Note that the blue pin also indicates the location of the aluminum nursery frames upon which the mangrove and reef corals were reared for 1 year from February 2022 to February 2023. (C) Image of P. acuta colony growing on Low Isles reef flat [photo credit: E.F.C., University of Technology Sydney (UTS)]. (D) Image of Woody Isle mangrove lagoon (photo credit: C.D.R., UTS). (E) Image of P. acuta colony growing in Woody Isle mangrove lagoon (photo credit: E.F.C., UTS). (F) Image of collected mangrove and reef P. acuta colonies on nursery frames following transplantation to the reef (photo credit: E.F.C., UTS).
Patterns in DNA methylation
Epigenetic mechanisms are becoming increasingly recognized for their potential to help buffer the adverse impacts of rapidly changing environmental conditions by facilitating phenotypic plasticity. DNA methylation [most commonly occurring on CpG (cytosine-phosphate-guanine) dinucleotides] is one of the most well-studied epigenetic modifications across taxa (35) and can promote or suppress gene expression by regulating access of transcriptional proteins to the DNA, thereby altering the response of an organism in absence of changes to the underlying DNA sequence. In corals, DNA methylation can occur in response to ocean acidification (74), nutrient enrichment (36), and heat and symbiont presence (75), with correlations between DNA methylation patterns and phenotypic traits of polyp size and skeletal porosity found in Stylophora pistillata under chronic pH stress (33). In our study, patterns of whole-genome DNA methylation only varied by the interaction of group and temperature and primarily for the reef translocated group. While reef-to-reef colonies were found to have the highest percent DNA methylation under moderate- to high-temperature stress (33° and 36°C), they exhibited the poorest ED50 thermal performance. In alignment with our study, Dixon et al. (46) demonstrated using the well-established metric of normalized CpG (i.e., the observed CpG versus expected CpG: CpGO/E) that strong methylation is associated with weak plasticity, while weak methylation is associated with strong plasticity in the coral Acropora millepora. This could, in part, explain our findings of highest percent DNA methylation exhibited in the poorest performer (i.e., reef-to-reef corals) and is further supported by the significantly lower DNA methylation in high-performing mangrove-to-reef colonies versus reef-to-reef corals at 36°C. That said, such patterns were not consistent across temperatures and groups; for example, no difference was exhibited in DNA methylation between wild mangrove versus reef to reef despite significant differences in ED50 thermal thresholds. This emphasizes the dynamic role of epigenetic mechanisms in shaping phenotypic plasticity, as previously observed by differences in the sensitivity for inducible epigenetic modification between two coral species under the same treatment (74), and underscores the need to further our understanding of direct involvement of the epigenome in coral acclimatization. Overall, questions remain about the exact mechanisms through which epigenetics contribute to the overall plasticity in corals and warrant further investigation such as the concurrent analysis of DNA methylation alongside RNA sequencing (RNA-seq) to elucidate the relationship between epigenetic modifications and gene expression patterns and better understand the environmental responses of heat-stressed corals.
Future considerations
Although identification of symbiont communities was outside the scope of this study given our primary interest in host responses to heat stress, previous work (22, 76) has shown that P. acuta colonies from the same mangrove lagoon and reef flat examined here are predominantly associated with species from Durusdinium and Cladocopium genera, respectively. Given the well-established role of Durusdinium in mitigating bleaching (32) and the ability of symbionts to influence gene expression (77), it should be acknowledged that such differences in symbiont communities would likely have contributed, at least in part, to the distinct photophysiological phenotypic responses observed between habitat groups. However, it is becoming increasingly clear that coral thermal tolerance is shaped by a complex interplay of mechanisms involving multiple members of the coral holobiont, including the host, algal symbionts, and associated microbial communities (78). Notably, the stability of symbiont communities in mangrove-associated P. acuta colonies following transplantation (22), along with evidence that host-regulated functional genes can influence bleaching susceptibility independent of symbiont composition (79), supports both the observed duration of thermal tolerance and the role of host gene expression in this study. Given these findings, it is important to consider timescales for the loss or retention of thermal tolerance, as different mechanisms will confer tolerance over varying temporal periods. While symbiont shuffling or microbial shifts have been shown to occur relatively rapidly in corals (80), changes to host standing genetic variation are likely to occur on slower time frames (81). Future work should evaluate the combined contribution of host and symbiont responses to better understand mechanisms contributing to stress tolerance of mangrove corals and the timescales over which said mechanisms are likely to vary.
While light intensities have been demonstrated as similar in both mangrove and reef environments at Low Isles (21), future work should incorporate a more comprehensive characterization of light history and the influence of morphological variation when investigating thermal tolerance, as these factors can affect coral physiological responses. Notably, the slight morphological differences observed between mangrove and reef P. acuta colonies highlight the importance of accounting for within-species variations in traits such as internal light fields, which may contribute to differences in thermal sensitivity. While we do not believe that these factors alter the overall conclusions of this study, incorporating them more explicitly will further our understanding and enhance our confidence in interpreting coral responses to climate stressors.
In conclusion, our study supports the potential utility of stress-tolerant corals from extreme environments in restoration initiatives by selecting and propagating resilient individuals that have the capacity to retain their tolerance when moved to a different environment. Regulation of genes associated with DNA repair, metabolism, and homeostasis is likely important for P. acuta corals to cope with and maintain tolerance to acute heat stress. Comparisons between mangrove and reef corals revealed that the largest proportion of DEGs occurred under the greatest heat stress (36°C). Taken into consideration with the high thermal thresholds observed for mangrove corals, these results are highly encouraging, as they suggest that mangrove corals maintain the ability to respond to heat stress under the most extreme conditions assessed in this study (+6°C above baseline) via molecular mechanisms. Stock selection of heat resilient corals from extreme systems could be particularly useful for “high-value sites” that are near to extreme coral habitats, providing easily implementable strategies for reef managers. For example, Low Isles reef represents a culturally, economically and ecologically important reef (82), which supports local communities through high tourism value (83), and, thus, active management initiatives aiming to build reef resilience into this location (such as through propagation of heat tolerance corals) will provide a range of ecological and socioeconomic benefits. While our findings hold great promise in the utility of stress-resilient corals for supporting reef restoration strategies, we urge caution and consideration of risks associated with the use of extreme systems as well as acclimating corals to a novel environment. We highlight the need to carefully consider and assess these factors (e.g., such as through risk-benefit analyses) and suggest extreme corals could form one part of a restoration strategy aiming to increase benefits to recipient populations while minimizing risks (13). With these considerations, harnessing the natural resilience of corals in situ could improve the efficacy of adaptive management and help promote reef resilience in the face of a changing climate. Critically, we stress that, while novel active management techniques and strategies can buy time for coral reefs (11), they cannot replace systemic efforts to mitigate climate change which is of the highest priority to ensure the long-term survival and resilience of coral ecosystems.
MATERIALS AND METHODS
Experimental design and coral collection
Experimentation and sample collection were undertaken at Low Isles reef (16°23′06.1″S, 145°33′45.0″E), an inshore reef on the northern GBR (Fig. 5A). Low Isles reef surrounds the sand cay Low Isle and mangrove island Woody Isle (Fig. 5B), providing a unique opportunity to study corals growing in both a reef and mangrove environment. Woody Isle features a semienclosed lagoon surrounded by mangrove trees (Fig. 5D) where corals are exposed to more extreme environmental conditions including high-temperature variations (14.7° to 35.0°C), reduced pH (min of 5.8), and low oxygen (<1 mg liter−1) compared to corals growing on the Low Isles reef flat (fig. S4) (23, 56). In February 2022, eight P. acuta colonies ~15 cm in diameter were collected from both Low Isles reef flat (Fig. 5C) and Woody Isle mangrove lagoon (Fig. 5, D and E) for use in the subsequent acute heat stress CBASS assay (described below). Colonies were collected with bone cutters from a depth of ~1.5 m to ensure consistency across the two habitats and transferred in trays kept in native seawater to the research vessel. Onboard the vessel, colonies were divided into two pieces. One piece was used for CBASS analysis, while the other piece was tagged to allow for resampling and returned to the reef, secured to small aluminum frames (~19 cm by 39 cm) attached to concrete blocks on the reef flat (Fig. 5F). One year later (February 2023), a partial colony was again collected from all corals moved to the reef in 2022. In addition, eight wild mangrove and wild reef colonies were also sampled. This approach resulted in four experimental groups: (i) mangrove to reef (mangrove colonies translocated to the aluminum frames on the reef for 1 year), (ii) reef to reef (native reef colonies growing on the same frames for 1 year), (iii) wild mangrove, and (iv) wild reef (native mangrove and reef colonies collected the day of the CBASS experiment). Last, to characterize each environment (mangrove and reef), HOBO MX2501 loggers (calibration temperature 25°C) were used to measure temperature and pH every hour from February 2022 to February 2023. Calibration of pH probes was conducted using National Bureau of Standards (NBS) and tris buffers for total scale (calibration temperature, 25.0°C), and the accuracy of HOBO MX2501 loggers was tested in the lab before deployment by measuring the pH of artificial seawater under incremental increases in temperature. Measures of pH started to decline at ~34°C, and, thus, pH values corresponding to temperatures of ≥35°C were removed.
To understand how annual temperature may have contributed to variations in P. acuta thermal threshold responses for both years, the in situ temperature profiles of Low Isles reef flat were investigated. Temperature data from the Australian Institute of Marine Science (AIMS) Temperature Logger Program [https://apps.aims.gov.au/metadata/view/4a12a8c0-c573-11dc-b99b-00008a07204e; (54)] were used to examine the 12 months before each CBASS experiment (i.e., February 2021–2022 and February 2022–2023). Temperature data were measured every hour via VEMCO Minilog-II-T loggers, and temperature profiles were summarized and visualized using R (version 4.3.1).
CBASS experimental setup and photophysiological measurements
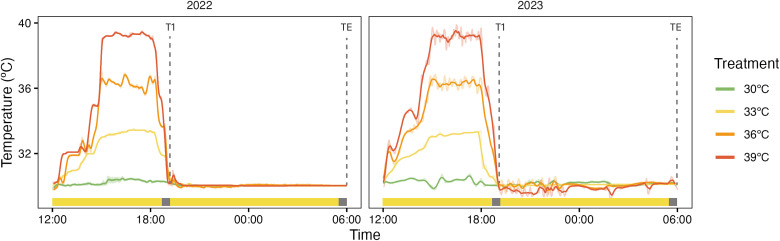
Corals were tested via CBASS acute heat stress assays to determine the standardized thermal tolerance of mangrove and reef P. acuta fragments in February 2022 and 2023. In 2022, wild reef and wild mangrove colonies were tested. These same colonies were reassessed in 2023 following 1-year transplantation (i.e., mangrove to reef and reef to reef), along with additional wild reef and wild mangrove samples collected on the day of the CBASS experiment. Note that n = 8 for all groups with the exception of the February 2022 wild reef group (n = 7), as, due to time constraints of conducting the CBASS experiment and establishing the translocated corals on the same day, only seven of the eight colonies were included in the CBASS measurements for this group in this year. As in previous studies (43), CBASS-derived thresholds in the present study should be considered in the context of acute heat stress and do not necessarily capture the effects of long-term cumulative heat stress. A portable, basic setup of CBASS (43) was used for our field-based assessment, previously described by Alderdice et al. (48). In brief, the system consisted of eight 21-liter flow-through tanks, each connected to an independent sump containing in situ seawater pumped at 5000 liters/hour (Eheim CompactON). Each tank was supplied with a submersible powerhead (Aqua One 320 lph) for water circulation and white Hydra light-emitting diode lights (Aqua Illumination, Ames, IA, USA) set to an irradiance of 350 μmol quanta m−2 s−1. The optimal light intensity was selected on the basis of an evaluation of the light saturation coefficient (Ek), which reflects the coral’s long-term light acclimation history (21). One fragment (~4 cm in diameter) from each of the collected mangrove lagoon and reef flat colonies was transferred into each of the experimental tanks consisting of four temperature treatments: ambient (30°C, as determined by the in situ HOBO temperature loggers), +3°, +6°, and + 9°C above ambient temperature. Each temperature treatment had two replicate tanks, for a total of eight tanks. The 18 hour temperature assay consisted of a replicated hold at 30°C and three replicated heat stress treatments consisting of a 3 hour temperature ramp to reach the respective target temperature (33°, 36°, or 39°C), a 3-hour hold, a 1-hour ramp down to ambient temperature (30°C; T1), and, lastly, a recovery phase held at ambient temperature for 11 hours (TE; Fig. 6). The temperature of each sump was controlled via a custom-made bar heater connected to a thermostat and checked manually each hour using a handheld temperature probe (Multimeter 340 WTW, Weilheim, Germany) to maintain target temperatures. Each temperature treatment also contained a HOBO Pendant Temperature Data Logger (OneTemp) logging temperature every minute for the duration of the experiment (Fig. 6).
Fig. 6. CBASS temperature profiles in year 1 and year 2.
Temperature profiles of 2022 and 2023 CBASS experiments measured via HOBO Pendant Temperature Data Loggers (OneTemp) recorded every 1 min within each of the temperature treatments (30°, 33°, 36°, and 39°C). Experiments ran for 18 hours starting at 13:00 local time on 9 February 2022 and at 12:00 local time on 2 February 2023. Sampling points occurred following temperature ramp-down (T1) and recovery (TE), indicated by the dashed vertical lines. The yellow bar at the bottom indicates when the lights were on during the experiment, with gray boxes indicating the lights turned off 30 min before Fv/Fm measurements being taken.
Pulse-amplitude-modulated (PAM) fluorometry (Diving-PAM underwater fluorometer; Heinz Walz GmbH, Effeltrich Germany) was used to assess the photophysiological response of each coral fragment during the acute heat stress assay. Dark-acclimated maximum photosynthetic efficiency of photosystem II (Fv/Fm) was measured for each coral at T1 and TE (Fig. 6) to provide a proxy of broader holobiont stress (43). Corals were dark acclimated for 30 min following temperature ramp down, and multiple measurements of Fv/Fm were taken by holding the probe 3-mm distance from the coral (standardized by use of a black rubber spacer to ensure a consistent distance from the fragment and angle of measure) and measuring along the length of the branch from base to tip to capture variations in acclimation (n = 3 per fragment). Only live fragments with areas of intact coral tissue were measured. As per Alderdice et al. (48), the following settings were used for optimized measurements: measuring light intensity of 8, saturation pulse intensity of 11, saturating width of 0.8, damp of 2, and gain of 4. Last, a sample of each colony was collected from one of each of the technical replicate tanks of the four temperature treatments (i.e., 32 fragments per tank) following temperature ramp-down (T1; Fig. 6) in February 2023 by cutting with bone cutters, immediately wrapping in aluminum foil and freezing in liquid nitrogen for subsequent gene expression and DNA methylation analysis.
RNA extraction and sequencing
Total RNA was extracted from frozen P. acuta samples collected during CBASS assays in February 2023 from the four sample groups (mangrove to reef, reef to reef, wild mangrove, and wild reef) and three temperatures (30°, 33°, and 36°C) during CBASS assays in February 2023 (n = 4 to 5 per treatment, see table S6 for sample sizes). As corals experienced tissue sloughing and mortality in the 39°C treatment, this treatment was excluded from RNA analysis as per other CBASS studies [e.g., (48)]. A QIAGEN RNeasy mini kit (QIAGEN, Germany) was used for RNA extractions following kit protocol. While being kept on dry ice, coral samples were stripped of their tissue using an airbrush and 600 μl of prechilled kit RLT buffer (RNA Lysis Buffer). Isolated tissue was homogenized using a bead beater for 2 min at 50 Hz, and sample supernatant was collected following centrifugation for 3 min at 15,000 rpm. The supernatant was mixed with a 1:1 volume of 70% ethanol and centrifuged for 15 s at 10,000 rpm in a spin column. The flow-through was discarded and three additional wash steps conducted using kit buffers added to the spin column [700 μl of RW1 buffer and duplicate washes with 500 μl of buffer RNA Purification Ethanol Buffer (RPE)]. Samples were centrifuged for 15 s at 10,000 rpm between each buffer wash step and the flow-through discarded. Last, RNA was eluted into 20 μl of RNA-free water after centrifuging for 1 min at 10,000 rpm. RNA quality and quantity were evaluated via spectrophotometry (NanoDrop 2000, Thermo Fisher Scientific, Massachusetts, USA), and only samples with a concentration of >90 ng/ μl and A260:280 value of >1.95 were retained for RNA sequencing. Samples that did not pass quality control and were identified as DNA contaminated (presence of nucleotides > 6000) underwent a repeat extraction, which included the use of DNase treatment (QIAGEN RNAse-Free DNase set) before the buffer wash steps. Total RNA for each sample underwent RNA sequencing conducted by the Australian Genome Research Facility (Melbourne, Australia) to generate 2 × 150–base pair (bp) paired-end mRNA libraries. Sequencing was performed on the Illumina NovaSeq X 10B flow cell (300 cycles) with five additional lanes to increase sequencing depth. RNA-seq data are available at http://www.ncbi.nlm.nih.gov/bioproject/1189141.
RNA-seq analysis
Demultiplexed reads were merged across five Illumina lanes and underwent quality check via FastQC (version 0.12.1). Sequences were trimmed using Trimmomatic (version 0.39) to filter and remove Illumina adaptors (Nextera 2:30:10), poor quality reads (SLIDINGWINDOW:4:20), and reads with less than 50 bp. Leading and trailing bases were removed if the quality score was less than 3 (LEADING:3 TRAILING:3). Following trimming, sample reads were mapped to the reference genomic gene set (n = 33,730 predicted protein-coding genes) of Hawaiian P. acuta (84) using STAR (version 2.7.11b), and read counts were quantified using the function “quantMode GeneCounts.” Any sample that did not pass FastQC quality checks following trimming (<94% paired-end read counts) and mapping [<5 million mapped reads; (85)] were removed and excluded from downstream analyses (table S7). HTSeq (version 0.12.3) was used to estimate abundances of transcripts of genes to generate count data (86). The R script is available at https://github.com/ChristineRoper/Mangrove-coral-thermotolerance-retained/tree/main/RNA%20Seq. To visualize differences in gene expression across temperatures (30°, 33°, and 36°C) and groups (mangrove to reef, reef to reef, wild mangrove, and wild reef), a PCA was plotted in R. Differential gene expression analysis was used to examine DEGs across treatments (temperatures and groups) on the basis of the raw count data. The R package “DESeq2” was used to perform a likelihood ratio test to explore differences in gene regulation by temperature, group, and the interaction of temperature and group. Pairwise analyses between temperatures and groups were performed via Wald testing with a negative binomial general linear model and Benjamini-Hochberg correction for multiple comparisons (cutoff of 0.05). DEGs were investigated through GO enrichment analysis via the “TopGO” package, using the “weight01” algorithm and “fisher” test statistic, and a P value cutoff of P < 0.001. The resulting enriched GO terms were then grouped into broader functional categories using the “goSlim” function in the GSEABase R package with GOslim generic obo as the reference database (40) to allow for a summary of key functions enriched across groups (with temperatures combined) and across temperatures (with groups combined). Additionally, to understand how mangrove and reef corals differ in their response to heat stress, enriched genes were investigated between groups under the greatest heat stress (36°C). Enriched GO terms and their corresponding GO Slim categories were compared between the four groups at 36°C and were visualized via a heatmap.
DNA extraction and methylated DNA quantification
Genomic DNA was extracted using the CTAB method (dx.doi.org/10.17504/protocols.io.dyq7vv) from the February 2023 P. acuta samples across three of the four temperature treatments (30°, 33°, and 36°C) with the exclusion of wild reef samples due to constraints in number of samples that could be processed in the methylated DNA quantification kit (n = 4 per treatment for mangrove-to-reef, reef-to-reef, and wild mangrove corals). Samples were submerged in a SDS and DNA buffer mix and stripped of their tissue using an air gun. The resulting tissue slurry was bead beaten, and the host tissue and Symbiodiniaceae cells were separated by centrifuging at 3000g for 2 min at 4°C. Samples in SDS were incubated for 60 min at 65°C, and 150 μl of each sample was combined with 15 μl of proteinase K. Following a second incubation (2 hours at 55°C), twice the sample volume of CTAB mix was added, and a third incubation was undertaken for 60 min at 65°C. Samples were allowed to cool to room temperature, and an equal volume of chloroform was added followed by several rounds of vortexing and rotating. Samples were centrifuged at 10,000g for 10 min and the separated, clear top layer, retained followed by the addition of twice the volume of prechilled (−20°C) ethanol to promote DNA precipitation. Samples were centrifuged at 10,000g for 10 min at 4°C, and the ethanol carefully decanted. This ethanol wash step was repeated a second time, and tubes were allowed to air dry for 30 min to ensure ethanol evaporation. Once fully dry, 50 μl of MilliQ water was added, and each sample was vortexed to resuspend the DNA pellet. DNA quality and quantity were assessed via NanoDrop 2000 (Thermo Fisher Scientific, Massachusetts, USA) before methylated DNA quantification.
Whole-genome DNA methylation of the coral host was quantified according to the assay protocol of the colorimetric methylated DNA quantification kit for absolute quantification of 5-mC (Abcam, Cambridge, UK, ab117128). Samples were prepared alongside duplicate positive and negative controls to generate a standard curve and measured via spectrophotometer (Spark Microplate Reader, Tecan, Mannedorf Switzerland) at 450 nm. Methylated DNA was reported as a percentage relative to the DNA quantity input for each sample. Percent methylation data were compared across treatments using an ART ANOVA followed by contrast tests [art.con function from the ARTools package; (87)] for post hoc pairwise comparisons as assumptions of normality were not met (Shapiro-Wilk test).
Population genetic structure and differentiation
Given morphological differences observed between P. acuta colonies from the mangrove lagoon versus the reef (Fig. 5, C and E), population structure and genetic differentiation was investigated using single-nucleotide polymorphism (SNP) data generated via genome complexity reduction-based sequencing by Diversity Arrays Technology (DarT) Pty Ltd. (University of Canberra, ACT). Sequencing of 18 individuals yielded 36,706 SNPs across the two habitat groups, which were filtered in R (version 4.3.1) for (i) duplicate loci (secondaries), (ii) reproducibility (0.99), (iii) call rate by loci (0.6), (iv) call rate by individuals (0.8), (v) low cover loci (≤5 and ≥85), (vi) monomorphic loci, and (vii) minor allele frequencies (<0.01). Following filtering, 2340 high-quality SNPs from 16 individuals were retained and were assessed for algal symbiont contamination via a BLAST alignment [function gl.blast; (88)] against 12 Symbiodiniaceae reference genomes (89). Next, the presence of clones was investigated using a GRM with a kinship coefficient of 0.45 on the basis of suggested clonal kinship values (44). To assess potential genetic divergence, a PCA (using the gl.dist.pop function) and STRUCTURE analysis (burn-in period and numreps set to 10,000, and five runs per iteration of K), along with a fixation index (FST; 999 permutations using the gl.FST.pop function), was calculated.
Statistical analysis
As a proxy for measures of thermal tolerance across individuals, the temperature at which Fv/Fm declined to 50% of its initial value was determined by fitting the data to a log logistic model using the “drm” function provided by the R package DRC. Defined as the median effective dose (herein known as ED50), values of ED50 have been used in a range of studies to characterize thermal tolerance in coral individuals and populations (43, 53). ED50 provides a standardized and comparable proxy based on the notion that corals with lower ED50 values are likely to have higher bleaching susceptibility at lower temperatures (42, 90); however, how this standardized thermal tolerance threshold ultimately relates to mortality remains unknown, as factors including rates of bleaching and recovery can have a major influence on mortality. As we were interested in heat tolerance in the present study, ED50 provided a useful metric for assessing differences in thermal thresholds across habitats. Comparisons of mean ED50 between sample groups and years were assessed via t tests where assumptions of normality (Shapiro-Wilk test) and equality of variance (Bartlett’s tests) were met; otherwise, a Kruskal-Wallis test was used as follows: (i) 2022 wild mangrove and wild reef coral ED50 values were compared via an unpaired two-sample t test; (ii) to examine whether 1-year transplantation influenced thermal performance to acute stress, 2023 groups (mangrove to reef, reef to reef, wild mangrove, and wild reef) were compared using a Kruskal-Wallis test, with pairwise comparisons calculated via Dunn’s test with P value adjustment for multiple testing (Bonferroni correction); and (iii) to assess how photophysiological parameters could have been driven by environmental differences, wild populations (mangrove and reef) were compared between years using independent t tests by habitat. R code for all analyses presented in this study can be accessed at https://github.com/ChristineRoper/Mangrove-coral-thermotolerance-retained, alongside data available at https://zenodo.org/records/15208057.
Acknowledgments
We wish to acknowledge and pay our respects to the Traditional Owners of the land and sea upon which this research took place, the Yirrganydji and Kuku Yalanji people. We thank the Great Barrier Reef Marine Park Authority, whose support established the permit for coral collection at Low Isles (G18/40023.1), as well as the owners and staff (notably John and Jenny Edmondson) from Wavelength Reef Cruises who provided access and support for data collection.
Funding: This work was supported by the partnership between the Australian Government’s Reef Trust and Great Barrier Reef Foundation, University of Technology Sydney Chancellor’s Postdoctoral Research Fellowship (to E.F.C.), ARC Discovery Early Career Researcher Award DE190100142 (to E.F.C.), Rolex Awards for Enterprise (to E.F.C.). David and Susan Rockefeller awards (to E.F.C.), and Australian Research Council Discovery Grant DP230100210 (to E.F.C.).
Author contributions: Conceptualization: E.F.C., D.J.S., and C.R.V. Methodology: E.F.C., J.E., C.D.R., D.J.S., S.G., R.A., C.R.V., and H.E. Investigation: E.F.C., J.E., C.D.R., S.G., T.D.H., H.E., and C.M.D. Data curation: C.D.R., H.E., C.R.V., and K.S. Data analysis: C.D.R., R.A., and C.R.V. Formal analysis: C.D.R., E.F.C., K.S., and R.A. Validation: C.D.R., E.F.C., C.R.V., and HE. Visualization: C.D.R. Software: C.D.R., K.S., and H.E. Supervision: E.F.C., D.J.S., C.R.V., and S.G. Writing—original draft: C.D.R. and E.F.C. Writing—review and editing: C.D.R., E.F.C., D.J.S., C.R.V., K.S., J.E., S.G., T.D.H., H.E., and R.A. Funding acquisition: E.F.C., D.J.S., and C.R.V. Resources: E.F.C., D.J.S., C.R.V., and K.S. Project administration: E.F.C.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials or online at https://zenodo.org/records/15208057. Raw sequencing data can be found at www.ncbi.nlm.nih.gov/bioproject/1189141. R code for all analyses presented in this study can be accessed at https://zenodo.org/records/15208057 and https://github.com/ChristineRoper/Mangrove-coral-thermotolerance-retained.
Supplementary Materials
This PDF file includes:
Figs. S1 to S4
Tables S1 to S7
REFERENCES AND NOTES
- 1.Hughes T. P., Kerry J. T., Baird A. H., Connolly S. R., Dietzel A., Eakin C. M., Heron S. F., Hoey A. S., Hoogenboom M. O., Liu G., McWilliam M. J., Pears R. J., Pratchett M. S., Skirving W. J., Stella J. S., Torda G., Global warming transforms coral reef assemblages. Nature 556, 492–496 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Hoegh-Guldberg O., Poloczanska E. S., Skirving W., Dove S., Coral reef ecosystems under climate change and ocean acidification. Front. Mar. Sci. 4, 158 (2017). [Google Scholar]
- 3.Hughes D. J., Alderdice R., Cooney C., Kühl M., Pernice M., Voolstra C. R., Suggett D. J., Coral reef survival under accelerating ocean deoxygenation. Nat. Clim. Change 10, 296–307 (2020). [Google Scholar]
- 4.Puotinen M., Drost E., Lowe R., Depczynski M., Radford B., Heyward A., Gilmour J., Towards modelling the future risk of cyclone wave damage to the world’s coral reefs. Glob. Chang. Biol. 26, 4302–4315 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Eddy T. D., Lam V. W. Y., Reygondeau G., Cisneros-Montemayor A. M., Greer K., Palomares M. L. D., Bruno J. F., Ota Y., Cheung W. W. L., Global decline in capacity of coral reefs to provide ecosystem services. One Earth 4, 1278–1285 (2021). [Google Scholar]
- 6.Cramer K. L., Donovan M. K., Jackson J. B. C., Greenstein B. J., Korpanty C. A., Cook G. M., Pandolfi J. M., The transformation of Caribbean coral communities since humans. Ecol. Evol. 11, 10098–10118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tebbett S. B., Connolly S. R., Bellwood D. R., Benthic composition changes on coral reefs at global scales. Nat. Ecol. Evol. 7, 71–81 (2023). [DOI] [PubMed] [Google Scholar]
- 8.van Oppen M. J. H., Gates R. D., Blackall L. L., Cantin N., Chakravarti L. J., Chan W. Y., Cormick C., Crean A., Damjanovic K., Epstein H., Harrison P. L., Jones T. A., Miller M., Pears R. J., Peplow L. M., Raftos D. A., Schaffelke B., Stewart K., Torda G., Wachenfeld D., Weeks A. R., Putnam H. M., Shifting paradigms in restoration of the world’s coral reefs. Glob. Chang. Biol. 23, 3437–3448 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Kleypas J., Allemand D., Anthony K., Baker A. C., Beck M. W., Hale L. Z., Hilmi N., Hoegh-Guldberg O., Hughes T., Kaufman L., Kayanne H., Magnan A. K., Mcleod E., Mumby P., Palumbi S., Richmond R. H., Rinkevich B., Steneck R. S., Voolstra C. R., Wachenfeld D., Gattuso J. P., Designing a blueprint for coral reef survival. Biol. Conserv. 257, 109107 (2021). [Google Scholar]
- 10.Anthony K., Bay L. K., Costanza R., Firn J., Gunn J., Harrison P., Heyward A., Lundgren P., Mead D., Moore T., Mumby P. J., van Oppen M., Robertson J., Runge M. C., Suggett D. J., Schaffelke B., Wachenfeld D., Walshe T., New interventions are needed to save coral reefs. Nat. Ecol. Evol. 1, 1420–1422 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Peixoto R. S., Voolstra C. R., Baums I. B., Camp E. F., Guest J., Harrison P. L., Montoya-Maya P. H., Pollock F. J., Smith D. J., Wangpraseurt D., Banaszak A. T., Chui A. P. Y., Shah N., Moore T., Fabricius K. E., Vardi T., Suggett D. J., The critical role of coral reef restoration in a changing world. Nat. Clim. Change 14, 1219–1222 (2024). [Google Scholar]
- 12.Suggett D. J., van Oppen M. J. H., Horizon scan of rapidly advancing coral restoration approaches for 21st century reef management. Emerg. Top. Life. Sci. 6, 125–136 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camp E. F., Contingency planning for coral reefs in the Anthropocene; the potential of reef safe havens. Emerg. Top. Life. Sci. 6, 107–124 (2022). [DOI] [PubMed] [Google Scholar]
- 14.Howells E. J., Abrego D., Liew Y. J., Burt J. A., Meyer E., Aranda M., Enhancing the heat tolerance of reef-building corals to future warming. Sci. Adv. 7, eabg6070 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Oppen M. J. H., Oliver J. K., Putnam H. M., Gates R. D., Building coral reef resilience through assisted evolution. Proc. Natl. Acad. Sci. U.S.A. 112, 2307–2313 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quigley K. M., Bay L. K., van Oppen M. J. H., The active spread of adaptive variation for reef resilience. Ecol. Evol. 9, 11122–11135 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoepf V., Baumann J. H., Barshis D. J., Browne N. K., Camp E. F., Comeau S., Cornwall C. E., Guzmán H. M., Riegl B., Rodolfo-Metalpa R., Sommer B., Corals at the edge of environmental limits: A new conceptual framework to re-define marginal and extreme coral communities. Sci. Total Environ. 884, 163688 (2023). [DOI] [PubMed] [Google Scholar]
- 18.Morikawa M. K., Palumbi S. R., Using naturally occurring climate resilient corals to construct bleaching-resistant nurseries. Proc. Natl. Acad. Sci. U.S.A. 116, 10586–10591 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maggioni F., Pujo-Pay M., Aucan J., Cerrano C., Calcinai B., Payri C., Benzoni F., Letourneur Y., Rodolfo-Metalpa R., The Bouraké semi-enclosed lagoon (New Caledonia)–a natural laboratory to study the lifelong adaptation of a coral reef ecosystem to extreme environmental conditions. Biogeosciences 18, 5117–5140 (2021). [Google Scholar]
- 20.Stewart H. A., Kline D. I., Chapman L. J., Altieri A. H., Caribbean mangrove forests act as coral refugia by reducing light stress and increasing coral richness. Ecosphere 12, e03413 (2021). [Google Scholar]
- 21.Camp E. F., Edmondson J., Doheny A., Rumney J., Grima A. J., Huete A., Suggett D. J., Mangrove lagoons of the Great Barrier Reef support coral populations persisting under extreme environmental conditions. Mar. Ecol. Prog. Ser. 625, 1–14 (2019). [Google Scholar]
- 22.Haydon T. D., Seymour J. R., Raina J. B., Edmondson J., Siboni N., Matthews J. L., Camp E. F., Suggett D. J., Rapid shifts in bacterial communities and homogeneity of Symbiodiniaceae in colonies of Pocillopora acuta transplanted between reef and mangrove environments. Front. Microbiol. 12, 756091 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.C. S. Rogers, J. J. Herlan, “Life on the edge: Corals in mangroves and climate change,” in Proceedings of the 12th International Coral Reef Symposium (National Coral Reef Institute, 2012), pp. 9–13.
- 24.Schoepf V., Carrion S. A., Pfeifer S. M., Naugle M., Dugal L., Bruyn J., McCulloch M. T., Stress-resistant corals may not acclimatize to ocean warming but maintain heat tolerance under cooler temperatures. Nat. Commun. 10, 4031 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drury C., Lirman D., Genotype by environment interactions in coral bleaching. Proc. Biol. Sci. B 288, 20210177 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barott K. L., Huffmyer A. S., Davidson J. M., Lenz E. A., Matsuda S. B., Hancock J. R., Innis T., Drury C., Putnam H. M., Gates R. D., Coral bleaching response is unaltered following acclimatization to reefs with distinct environmental conditions. Proc. Natl. Acad. Sci. U.S.A. 118, e2025435118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bay R. A., Palumbi S. R., Rapid acclimation ability mediated by transcriptome changes in reef-building corals. Genome Biol. Evol. 7, 1602–1612 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han T., Liao X., Guo Z., Chen J. Y., He C., Lu Z., Deciphering temporal gene expression dynamics in multiple coral species exposed to heat stress: Implications for predicting resilience. Sci. Total Environ. 912, 169021 (2024). [DOI] [PubMed] [Google Scholar]
- 29.Liew Y. J., Howells E. J., Wang X., Michell C. T., Burt J. A., Idaghdour Y., Aranda M., Intergenerational epigenetic inheritance in reef-building corals. Nat. Clim. Change 10, 254–259 (2020). [Google Scholar]
- 30.Berkelmans R., van Oppen M. J. H., The role of zooxanthellae in the thermal tolerance of corals: A ‘nugget of hope’for coral reefs in an era of climate change. Proc. Biol. Sci. B 273, 2305–2312 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palumbi S. R., Barshis D. J., Traylor-Knowles N., Bay R. A., Mechanisms of reef coral resistance to future climate change. Science 344, 895–898 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Claar D. C., Starko S., Tietjen K. L., Epstein H. E., Cunning R., Cobb K. M., Baker A. C., Gates R. D., Baum J. K., Dynamic symbioses reveal pathways to coral survival through prolonged heatwaves. Nat. Commun. 11, 6097 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liew Y. J., Zoccola D., Li Y., Tambutté E., Venn A. A., Michell C. T., Cui G., Deutekom E. S., Kaandorp J. A., Voolstra C. R., Forêt S., Allemand D., Tambutté S., Aranda M., Epigenome-associated phenotypic acclimatization to ocean acidification in a reef-building coral. Sci. Adv. 4, eaar8028 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin B., Li Y., Robertson K. D., DNA methylation: Superior or subordinate in the epigenetic hierarchy? Genes Cancer 2, 607–617 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang X., Li S., Ni P., Gao Y., Jiang B., Zhou Z., Zhan A., Rapid response to changing environments during biological invasions: DNA methylation perspectives. Mol. Ecol. 26, 6621–6633 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Casariego J. A., Ladd M. C., Shantz A. A., Lopes C., Cheema M. S., Kim B., Roberts S. B., Fourqurean J. W., Ausio J., Burkepile D. E., Eirin-Lopez J. M., Coral epigenetic responses to nutrient stress: Histone H2A. X phosphorylation dynamics and DNA methylation in the staghorn coral Acropora cervicornis. Ecol. Evol. 8, 12193–12207 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hackerott S., Virdis F., Flood P. J., Souto D. G., Paez W., Eirin-Lopez J. M., Relationships between phenotypic plasticity and epigenetic variation in two Caribbean Acropora corals. Mol. Ecol. 32, 4814–4828 (2023). [DOI] [PubMed] [Google Scholar]
- 38.Kenkel C. D., Meyer E., Matz M. V., Gene expression under chronic heat stress in populations of the mustard hill coral (Porites astreoides) from different thermal environments. Mol. Ecol. 22, 4322–4334 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Moghaddam S., Shokri M. R., Tohidfar M., The enhanced expression of heat stress-related genes in scleractinian coral ‘Porites harrisoni’ during warm episodes as an intrinsic mechanism for adaptation in ‘the Persian Gulf’. Coral Reefs 40, 1013–1028 (2021). [Google Scholar]
- 40.Scucchia F., Zaslansky P., Boote C., Doheny A., Mass T., Camp E. F., The role and risks of selective adaptation in extreme coral habitats. Nat. Commun. 14, 4475 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoadley K. D., Lockridge G., McQuagge A., Pahl K. B., Lowry S., Wong S., Craig Z., Petrik C., Klepac C., Muller E. M., A phenomic modeling approach for using chlorophyll-a fluorescence-based measurements on coral photosymbionts. Front. Mar. Sci. 10, 1092202 (2023). [Google Scholar]
- 42.Voolstra C. R., Buitrago-López C., Perna G., Cárdenas A., Hume B. C. C., Rädecker N., Barshis D. J., Standardized short-term acute heat stress assays resolve historical differences in coral thermotolerance across microhabitat reef sites. Glob. Chang. Biol. 26, 4328–4343 (2020). [DOI] [PubMed] [Google Scholar]
- 43.Evensen N. R., Parker K. E., Oliver T. A., Palumbi S. R., Logan C. A., Ryan J. S., Klepac C. N., Perna G., Warner M. E., Voolstra C. R., The Coral Bleaching Automated Stress System (CBASS): A low-cost, portable system for standardized empirical assessments of coral thermal limits. Limnol. Oceanogr. Methods 21, 421–434 (2023). [Google Scholar]
- 44.S. Wright, Variability Within and Among Natural Populations (Chicago: University of Chicago Press, 1978). [Google Scholar]
- 45.Caruso C., Hughes K., Drury C., Selecting heat-tolerant corals for proactive reef restoration. Front. Mar. Sci. 8, 632027 (2021). [Google Scholar]
- 46.Dixon G. B., Bay L. K., Matz M. V., Bimodal signatures of germline methylation are linked with gene expression plasticity in the coral Acropora millepora. BMC Genomics 15, 1109 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanvet C., Camp E. F., Sutton J., Houlbrèque F., Thouzeau G., Rodolfo-Metalpa R., Corals adapted to extreme and fluctuating seawater pH increase calcification rates and have unique symbiont communities. Ecol. Evol. 13, e10099 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alderdice R., Voolstra C. R., Nuñez Lendo C. I., Boote C., Suggett D. J., Edmondson J., Goyen S., Haydon T., Camp E. F., Loss of coral thermotolerance following year-long in situ nursery propagation with a consecutively high summer heat-load. Coral Reefs 43, 919–933 (2024). [Google Scholar]
- 49.van Hooidonk R., Maynard J., Tamelander J., Gove J., Ahmadia G., Raymundo L., Williams G., Heron S. F., Planes S., Local-scale projections of coral reef futures and implications of the Paris Agreement. Sci. Rep. 6, 39666 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bartels N., Dilernia N. J., Howlett L., Camp E. F., Stress event for “super corals” in Great Barrier Reef mangrove lagoon. Mar. Biodivers. 53, 64 (2023). [Google Scholar]
- 51.Cornwell B., Armstrong K., Walker N. S., Lippert M., Nestor V., Golbuu Y., Palumbi S. R., Widespread variation in heat tolerance and symbiont load are associated with growth tradeoffs in the coral Acropora hyacinthus in Palau. eLife 13, e64790 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones A. M., Berkelmans R., Tradeoffs to thermal acclimation: Energetics and reproduction of a reef coral with heat tolerant Symbiodinium type-D. J. Mar. Sci. 1, 185890 (2011). [Google Scholar]
- 53.Cunning R., Parker K. E., Johnson-Sapp K., Karp R. F., Wen A. D., Williamson O. M., Bartels E., D’Alessandro M., Gilliam D. S., Hanson G., Levy J., Lirman D., Maxwell K., Million W. C., Moulding A. L., Moura A., Muller E. M., Nedimyer K., Reckenbeil B., van Hooidonk R., Dahlgren C., Kenkel C., Parkinson J. E., Baker A. C., Census of heat tolerance among Florida’s threatened staghorn corals finds resilient individuals throughout existing nursery populations. Proc. Biol. Sci. B 288, 20211613 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Australian Institute of Marine Science (AIMS), Sea Water Temperature Observing System (AIMS Temperature Logger Program) (2017); 10.25845/5b4eb0f9bb848. [DOI]
- 55.Haydon T. D., Matthews J. L., Seymour J. R., Raina J. B., Seymour J. E., Chartrand K., Camp E. F., Suggett D. J., Metabolomic signatures of corals thriving across extreme reef habitats reveal strategies of heat stress tolerance. Proc. Biol. Sci. B 290, 20221877 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.E. J. Howells, L. K. Bay, R. A. Bay, “Identifying, monitoring, and managing adaptive genetic variation in reef-building corals under rapid climate warming,” in Coral Reef Conservation and Restoration in the Omics Age (Springer, 2022), pp. 55–70. [Google Scholar]
- 57.Drury C., Manzello D., Lirman D., Genotype and local environment dynamically influence growth, disturbance response and survivorship in the threatened coral, Acropora cervicornis. PLOS ONE 12, e0174000 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Humanes A., Lachs L., Beauchamp E. A., Bythell J. C., Edwards A. J., Golbuu Y., Martinez H. M., Palmowski P., Treumann A., van der Steeg E., van Hooidonk R., Guest J. R., Within-population variability in coral heat tolerance indicates climate adaptation potential. Proc. Biol. Sci. B 289, 20220872 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson D. F., Oxidative phosphorylation: Regulation and role in cellular and tissue metabolism. J. Physiol. 595, 7023–7038 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feijão E., Cruz de Carvalho R., Duarte I. A., Matos A. R., Cabrita M. T., Novais S. C., Lemos M. F. L., Caçador I., Marques J. C., Reis-Santos P., Fluoxetine arrests growth of the model diatom Phaeodactylum tricornutum by increasing oxidative stress and altering energetic and lipid metabolism. Front. Microbiol. 11, 534732 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Polapa F., Werorilangi S., Ali S. M., Jompa J., Physiological responses of scleractinian corals in marginal habitat. Biodiversitas 22, (2021). [Google Scholar]
- 62.Maor-Landaw K., Karako-Lampert S., Ben-Asher H. W., Goffredo S., Falini G., Dubinsky Z., Levy O., Gene expression profiles during short-term heat stress in the red sea coral Stylophora pistillata. Glob. Chang. Biol. 20, 3026–3035 (2014). [DOI] [PubMed] [Google Scholar]
- 63.Farah M. E., Sirotkin V., Haarer B., Kakhniashvili D., Amberg D. C., Diverse protective roles of the actin cytoskeleton during oxidative stress. Cytoskeleton 68, 340–354 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chakravarti L. J., Buerger P., Levin R. A., van Oppen M. J. H., Gene regulation underpinning increased thermal tolerance in a laboratory-evolved coral photosymbiont. Mol. Ecol. 29, 1684–1703 (2020). [DOI] [PubMed] [Google Scholar]
- 65.DeSalvo M. K., Voolstra C. R., Sunagawa S., Schwarz J. A., Stillman J. H., Coffroth M. A., Szmant A. M., Medina M., Differential gene expression during thermal stress and bleaching in the Caribbean coral Montastraea faveolata. Mol. Ecol. 17, 3952–3971 (2008). [DOI] [PubMed] [Google Scholar]
- 66.Kenkel C. D., Aglyamova G., Alamaru A., Bhagooli R., Capper R., Cunning R., deVillers A., Haslun J. A., Hédouin L., Keshavmurthy S., Kuehl K. A., Mahmoud H., McGinty E. S., Montoya-Maya P. H., Palmer C. V., Pantile R., Sánchez J. A., Schils T., Silverstein R. N., Squiers L. B., Tang P. C., Goulet T. L., Matz M. V., Development of gene expression markers of acute heat-light stress in reef-building corals of the genus Porites. PLOS ONE 6, e26914 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seneca F. O., Forêt S., Ball E. E., Smith-Keune C., Miller D. J., van Oppen M. J., Patterns of gene expression in a scleractinian coral undergoing natural bleaching. Marine Biotechnol. 12, 594–604 (2010). [DOI] [PubMed] [Google Scholar]
- 68.Wright R. M., Kenkel C. D., Dunn C. E., Shilling E. N., Bay L. K., Matz M. V., Intraspecific differences in molecular stress responses and coral pathobiome contribute to mortality under bacterial challenge in Acropora millepora. Sci. Rep. 7, 2609 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.J. S. Torday, V. K. Rehan, Evolutionary Biology: Cell-Cell Communication, and Complex Disease (John Wiley & Sons, 2012). [Google Scholar]
- 70.Runchel C., Matsuzawa A., Ichijo H., Mitogen-activated protein kinases in mammalian oxidative stress responses. Antioxid. Redox Signal. 15, 205–218 (2011). [DOI] [PubMed] [Google Scholar]
- 71.Courtial L., Picco V., Grover R., Cormerais Y., Rottier C., Labbe A., Pagès G., Ferrier-Pagès C., The c-Jun N-terminal kinase prevents oxidative stress induced by UV and thermal stresses in corals and human cells. Sci. Rep. 7, 45713 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bellantuono A. J., Granados-Cifuentes C., Miller D. J., Hoegh-Guldberg O., Rodriguez-Lanetty M., Coral thermal tolerance: Tuning gene expression to resist thermal stress. PLOS ONE 7, e50685 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnston E. C., Forsman Z. H., Flot J. F., Schmidt-Roach S., Pinzón J. H., Knapp I. S., Toonen R. J., A genomic glance through the fog of plasticity and diversification in Pocillopora. Sci. Rep. 20, 5991 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Putnam H. M., Davidson J. M., Gates R. D., Ocean acidification influences host DNA methylation and phenotypic plasticity in environmentally susceptible corals. Evol. Appl. 9, 1165–1178 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ishida-Castañeda J., Iguchi A., Sakai K., Changes in DNA methylation in response to heat stress and symbiotic conditions in coral primary polyps. Mar. Ecol. Prog. Ser. 714, 113–117 (2023). [Google Scholar]
- 76.Ros M., Suggett D. J., Edmondson J., Haydon T., Hughes D. J., Kim M., Guagliardo P., Bougoure J., Pernice M., Raina J. B., Camp E. F., Symbiont shuffling across environmental gradients aligns with changes in carbon uptake and translocation in the reef-building coral Pocillopora acuta. Coral Reefs 40, 595–607 (2021). [Google Scholar]
- 77.Cunning R., Baker A. C., Thermotolerant coral symbionts modulate heat stress-responsive genes in their hosts. Mol. Ecol. 29, 2940–2950 (2020). [DOI] [PubMed] [Google Scholar]
- 78.Cziesielski M. J., Schmidt-Roach S., Aranda M., The past, present, and future of coral heat stress studies. Ecol. Evol. 9, 10055–10066 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith H., Epstein H., Torda G., The molecular basis of differential morphology and bleaching thresholds in two morphs of the coral Pocillopora acuta. Sci. Rep. 7, 10066 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sampayo E. M., Ridgway T., Franceschinis L., Roff G., Hoegh-Guldberg O., Dove S., Coral symbioses under prolonged environmental change: Living near tolerance range limits. Sci. Rep. 2, 36271 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Torda G., Donelson J. M., Aranda M., Barshis D. J., Bay L., Berumen M. L., Bourne D. G., Cantin N., Foret S., Matz M., Miller D. J., Moya A., Putnam H. M., Ravasi T., van Oppen M. J. H., Thurber R. V., Vidal-Dupiol J., Voolstra C. R., Watson S.-A., Whitelaw E., Willis B. L., Munday P. L., Rapid adaptive responses to climate change in corals. Nat. Clim. Change 1, 627–636 (2017). [Google Scholar]
- 82.Great Barrier Reef Marine Park Authority, Low Isles Management Plan for Low Islets (Low Island and Woody Island) and Reef (Great Barrier Reef Marine Park Authority, 1993). [Google Scholar]
- 83.Deloitte Access Economics, Economic Contribution of the Great Barrier Reef (Great Barrier Reef Marine Park Authority, 2013). [Google Scholar]
- 84.Stephens T. G., Lee J., Jeong Y., Yoon H. S., Putnam H. M., Majerová E., Bhattacharya D., High-quality genome assembles from key Hawaiian coral species. GigaScience 11, giac098 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Conesa A., Madrigal P., Tarazona S., Gomez-Cabrero D., Cervera A., McPherson A., Szcześniak M. W., Gaffney D. J., Elo L. L., Zhang X., Mortazavi A., A survey of best practices for RNA-seq data analysis. Genome Biol. 17, 13 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Anders S., Pyl P. T., Huber W., HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.M. Kay, L. Elkin, J. Higgins, J. Wobbrock, ARTool: Aligned rank transform for nonparametric factorial ANOVAs, Zenodo (2025); 10.5281/zenodo.594511. [DOI]
- 88.Gruber B., Unmack P. J., Berry O. F., Georges A., dartr: An R package to facilitate analysis of SNP data generated from reduced representation genome sequencing. Mol. Ecol. Resour. 18, 691–699 (2018). [DOI] [PubMed] [Google Scholar]
- 89.Yu L., Li T., Li L., Lin X., Li H., Liu C., Guo C., Lin S., SAGER: A database of symbiodiniaceae and algal genomic resource. Database 2020, baaa051 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Klepac C. N., Petrik C. G., Karabelas E., Owens J., Hall E. R., Muller E. M., Assessing acute thermal assays as a rapid screening tool for coral restoration. Sci. Rep. 14, 1898 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S4
Tables S1 to S7