Abstract
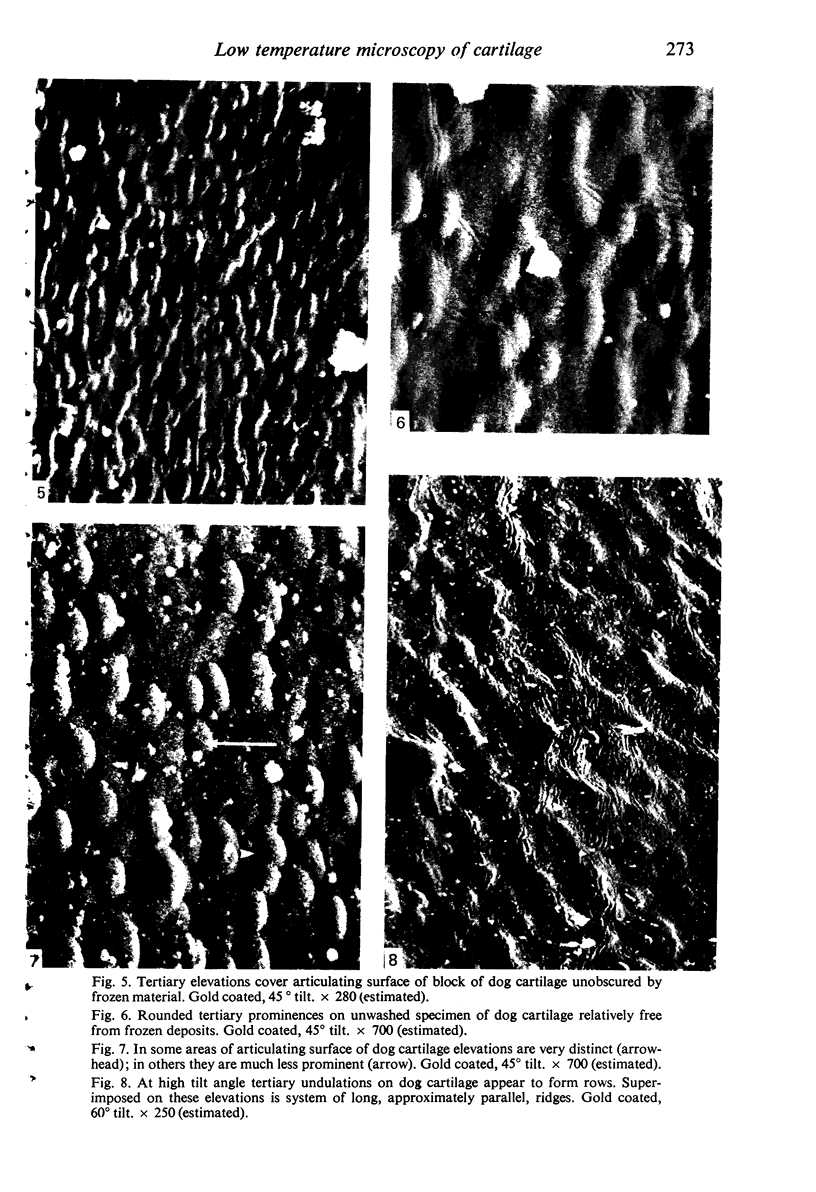
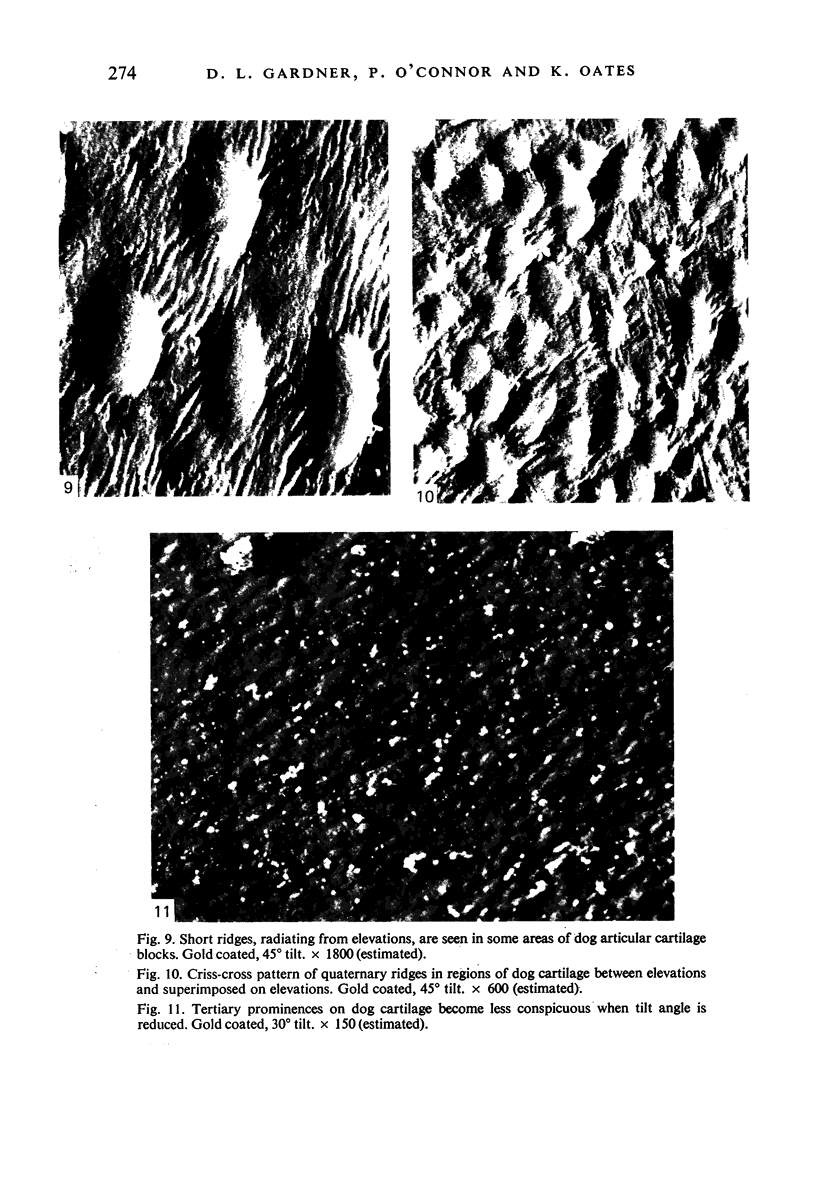
Fifty seven blocks of cartilage excised from the femoral condyles of 20 beagle dogs, and whole lower ends of 5 guinea-pig femora, were examined at -195 degrees (78 K), by scanning electron microscopy. The unfixed tissue, taken into slushy nitrogen at -210 degrees (63 K), was not exposed to atmospheric air after quenching and remained fully hydrated throughout long periods of observation. Images susceptible to analysis were obtained from washed and from unwashed cartilage surfaces. Preliminary coating with gold or with aluminium, known to be possible without exposing cold cartilage surfaces to changes in temperature likely to cause water loss by sublimation, was valuable in minimising charging and in facilitating the recording of electron images at higher magnifications. Although examination was possible without coating, the resultant images were of low resolution. Microscopy revealed a pattern of secondary surface irregularities of tertiary elevations closely resembling those seen by the conventional scanning electron microscopy of fixed, dehydrated hyaline cartilage. However, the pattern of tertiary surface structures was predominantly that of elevations, not of hollows. Quaternary surface ridges were common on the surfaces of excised dog cartilage blocks and were not seen on the surfaces of guinea-pig cartilage which remained on the femoral condyles.
Full text
PDF

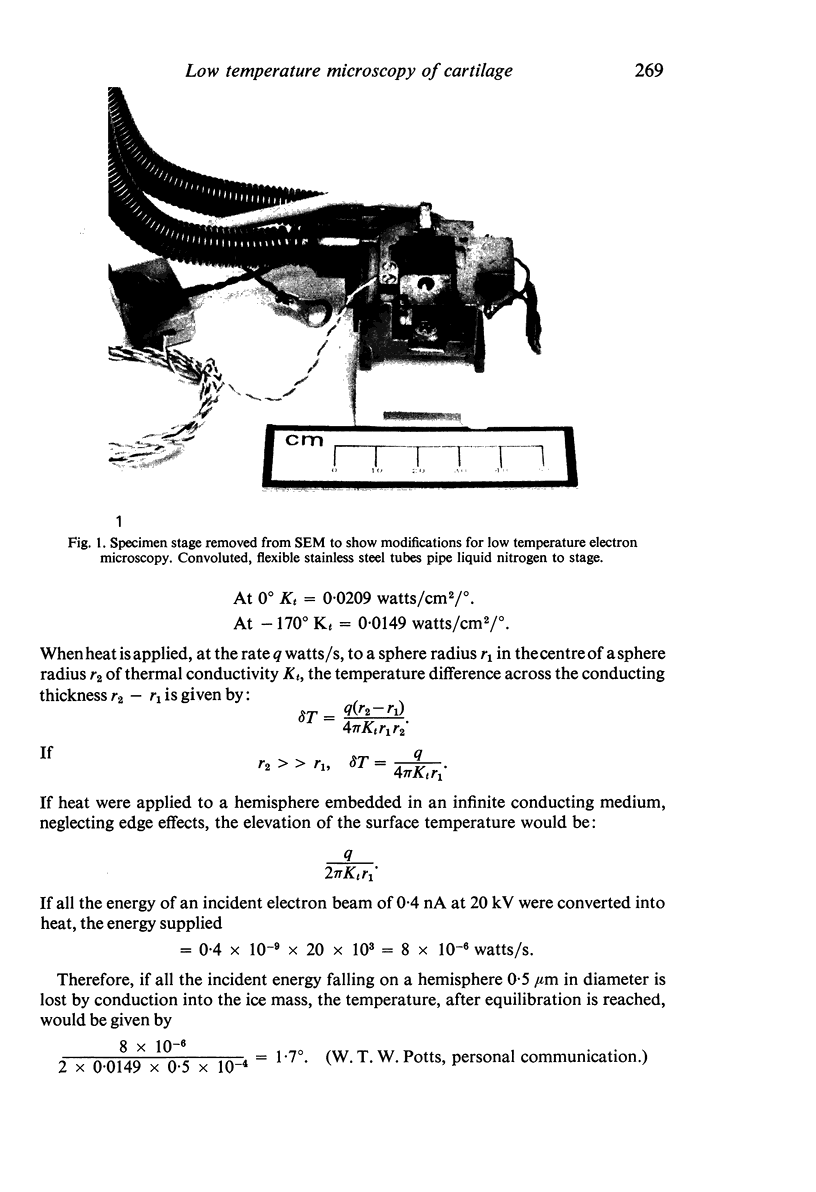
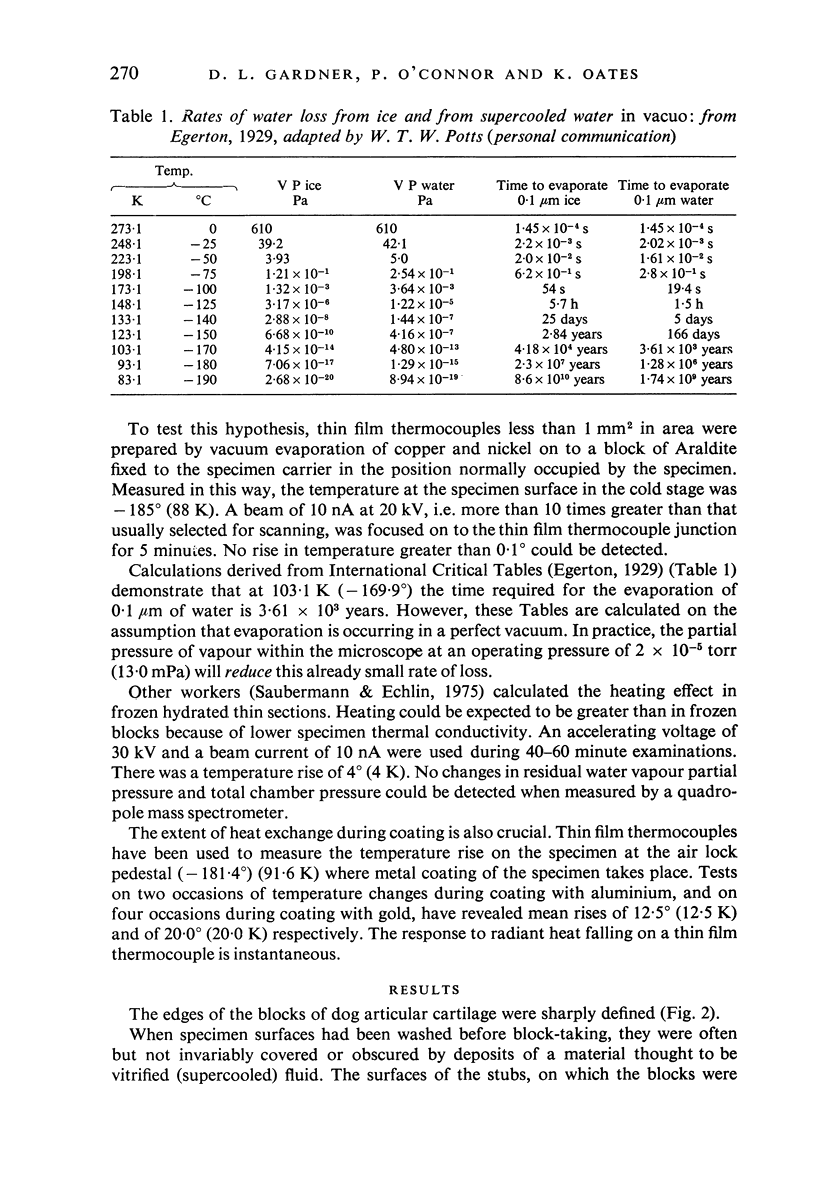
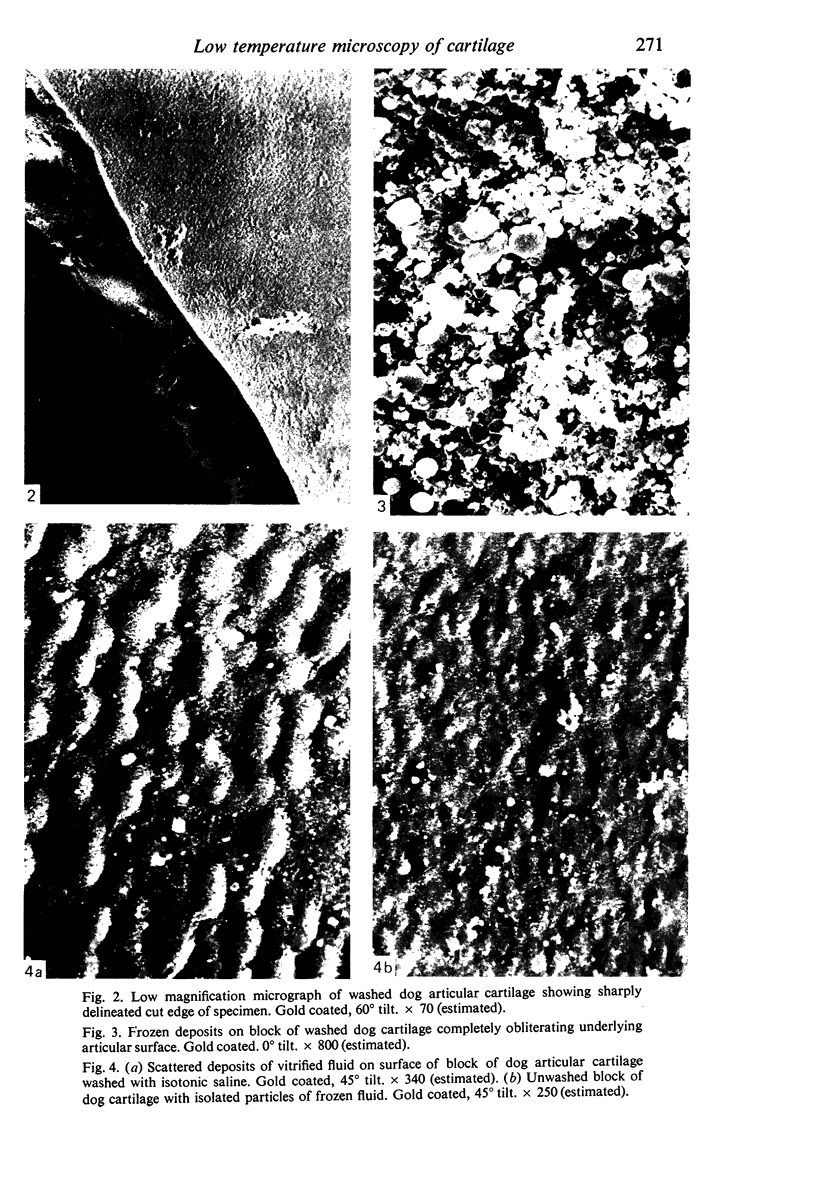









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aspden R. M., Hukins D. W. The lamina splendens of articular cartilage is an artefact of phase contrast microscopy. Proc R Soc Lond B Biol Sci. 1979 Nov 30;206(1162):109–113. doi: 10.1098/rspb.1979.0094. [DOI] [PubMed] [Google Scholar]
- Bald W. B., Robards A. W. A device for the rapid freezing of biological specimens under precisely controlled and reproducible conditions. J Microsc. 1978 Jan;112(1):3–15. doi: 10.1111/j.1365-2818.1978.tb01150.x. [DOI] [PubMed] [Google Scholar]
- Clarke I. C. Human articular surface contours and related surface depression frequency studies. Ann Rheum Dis. 1971 Jan;30(1):15–23. doi: 10.1136/ard.30.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke I. C. Surface characteristics of human articular cartilage--a scanning electron microscope study. J Anat. 1971 Jan;108(Pt 1):23–30. [PMC free article] [PubMed] [Google Scholar]
- Fell H. B. Synoviocytes. J Clin Pathol Suppl (R Coll Pathol) 1978;12:14–24. [PMC free article] [PubMed] [Google Scholar]
- Gardner D. L., O'Connor P., Oates K. Surface structure of mammalian articular cartilage viewed at low temperature. Lancet. 1979 Sep 8;2(8141):538–538. doi: 10.1016/s0140-6736(79)91605-2. [DOI] [PubMed] [Google Scholar]
- Gardner D. L. The influence of microscopic technology on knowledge of cartilage surface structure. Ann Rheum Dis. 1972 Jul;31(4):235–258. doi: 10.1136/ard.31.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner D. L., Woodward D. Scanning electron microscopy and replica studies of articular surfaces of guinea-pig synovial joints. Ann Rheum Dis. 1969 Jul;28(4):379–391. doi: 10.1136/ard.28.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner D. L., McGillivray D. C. Living articular cartilage is not smooth. The structure of mammalian and avian joint surfaces demonstrated in vivo by immersion incident light microscopy. Ann Rheum Dis. 1971 Jan;30(1):3–9. doi: 10.1136/ard.30.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadially F. N., Ghadially J. A., Oryschak A. F., Yong N. K. Experimental production of ridges on rabbit articular cartilage: a scanning electron microscope study. J Anat. 1976 Feb;121(Pt 1):119–132. [PMC free article] [PubMed] [Google Scholar]
- Ghadially F. N., Moshurchak E. M., Thomas I. Humps on young human and rabbit articular cartilage. J Anat. 1977 Nov;124(Pt 2):425–435. [PMC free article] [PubMed] [Google Scholar]
- Inoue H., Kodama T., Fujita T. Scanning electron microscopy of normal and rheumatoid articular cartilages. Arch Histol Jpn. 1969 Aug;30(5):425–435. doi: 10.1679/aohc1950.30.425. [DOI] [PubMed] [Google Scholar]
- Longfield M. D., Dowson D., Walker P. S., Wright V. "Boosted lubrication" of human joints by fluid enrichment and entrapment. Biomed Eng. 1969 Nov;4(11):517–522. [PubMed] [Google Scholar]
- Longmore R. B., Gardner D. L. Development with age of human articular cartilage surface structure. A survey by interference microscopy of the lateral femoral condyle. Ann Rheum Dis. 1975 Feb;34(1):26–37. doi: 10.1136/ard.34.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longmore R. B., Gardner D. L. The surface structure of ageing human articular cartilage: a study by reflected light interference microscopy (RLIM). J Anat. 1978 Jun;126(Pt 2):353–365. [PMC free article] [PubMed] [Google Scholar]
- MacCONAILL M. A. The movements of bones and joints; the mechanical structure of articulating cartilage. J Bone Joint Surg Br. 1951 May;33B(2):251–257. [PubMed] [Google Scholar]
- McCall J. G. Scanning electron microscopy of articular surfaces. Lancet. 1968 Nov 30;2(7579):1194–1194. doi: 10.1016/s0140-6736(68)91680-2. [DOI] [PubMed] [Google Scholar]
- Muir H. Proteoglycans of cartilage. J Clin Pathol Suppl (R Coll Pathol) 1978;12:67–81. [PMC free article] [PubMed] [Google Scholar]
- Nielsen E. H., Bytzer P. High resolution scanning electron microscopy of elastic cartilage. J Anat. 1979 Dec;129(Pt 4):823–831. [PMC free article] [PubMed] [Google Scholar]
- Redler I., Zimny M. L. Scanning electron microscopy of normal and abnormal articular cartilage and synovium. J Bone Joint Surg Am. 1970 Oct;52(7):1395–1404. [PubMed] [Google Scholar]
- Saubermann A. J., Echlin P. The preparation, examination and analysis of frozen hydrated tissue sections by scanning transmission electron microscopy and x-ray microanalysis. J Microsc. 1975 Nov;105(2):155–191. doi: 10.1111/j.1365-2818.1975.tb04048.x. [DOI] [PubMed] [Google Scholar]
- Sayles R. S., Thomas T. R., Anderson J., Haslock I., Unsworth A. Measurement of the surface microgeometry of articular cartilage. J Biomech. 1979;12(4):257–267. doi: 10.1016/0021-9290(79)90068-x. [DOI] [PubMed] [Google Scholar]
- Wilson N. H., Gardner D. L. Influence of aqueous fixation on articular surface morphology. A reflected light interference microscope study. J Pathol. 1980 Aug;131(4):333–338. doi: 10.1002/path.1711310405. [DOI] [PubMed] [Google Scholar]