Abstract
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the most effective salvage strategy for B-cell acute lymphoblastic leukemia (B-ALL). In this study, we explored the efficacy and safety of cladribine and medium-dose cytarabine intensified busulfan plus cyclophosphamide conditioning regimen (CBAC) for high-risk B-ALL patients at complete remission (CR) after chemotherapy undergoing allo-HSCT. And compared it with patients historical received traditional total body irradiation plus cyclophosphamide (TBI-Cy) regimen. The 3-year non-relapse mortality (NRM), cumulative incidence of relapse (CIR), disease-free survival (DFS) and overall survival (OS) of CBAC and TBI-Cy group were 15.0% vs. 11.1% (p = 0.576), 17.3% vs. 35.7% (p = 0.077), 67.7% vs. 53.2% (p = 0.235) and 74.3% vs. 66.8% (p = 0.482). Overall cohort multivariate analysis indicated TBI-Cy increased the risk of relapse after transplantation compared with CBAC (HR: 2.544, p = 0.049). Subgroup analysis revealed that among cytogenetic high-risk patients, the CBAC group showed a trend of higher 3-year DFS (75.1% vs 52.6%, p = 0.073). Among patients with minimal residual disease positive (MRD+) at transplantation, the CBAC group showed higher 3-year DFS (60.0% vs 11.1%, p = 0.018). In conclusion, CBAC conditioning regimen may reduce relapse without increasing NRM, improving the prognosis of high-risk B-ALL patients, especially those with cytogenetic high-risk factors or MRD+ at transplantation.
Keywords: Allogeneic hematopoietic stem cell transplantation, B-cell acute lymphoblastic leukemia, Conditioning regimen, Cladribine
Introduction
Adult B-cell acute lymphoblastic leukemia (B-ALL) is a common hematological malignant with high heterogeneity, rapid progression and high incidence of relapse [1, 2]. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is still the most effective salvage therapy for B-ALL patients, although the curative effect has improved with the introduction of target agents and immunotherapy [3].
In early clinical practice, conditioning regimens based on total body irradiation (TBI) are classical myeloablative regimens [4]. However, studies have shown that high doses of TBI increase the risk of secondary tumors and treatment-related mortality [5]. Consequently, chemotherapy-based conditioning regimens, such as the classic busulfan plus cyclophosphamide (BuCy) regimen, have been developed. There have been a series of studies with different opinions on the effects of TBI based and chemotherapy-based regimen for ALL patients. An open-label, multicenter, phase III trial showed BuCy regimen demonstrated comparable effects compared with the TBI-Cy regimen in adult standard-risk B-ALL in first complete remission (CR1) [6]. Some other studies also suggested comparable therapeutic efficacy between TBI-based and chemotherapy-based regimens in ALL patients [7, 8]. However, several studies supported that TBI-based regimens can improve overall survival (OS), disease-free survival (DFS) and reduce the risk of relapse [9–11], especially for patients that cannot achieve MRD negativity prior to allo-HSCT [12, 13]. Therefore, chemotherapy-based conditioning regimens require further development and refinement for some B-ALL patients. Scholars have tried to intensify chemotherapy-based conditioning regimen to improve the prognosis of B-ALL patients. While idarubicin (IDA) combined with BuCy did not benefit high-risk B-ALL patients [14], and other agents, such as fludarabine (Flu), thiotepa (TT) were also added to BuCy regimen, the results showed high transplant-related mortality (TRM) but the recurrence rate did not decrease [15]. Although the conditioning regimen is constantly improving, relapse is still the leading cause of death in B-ALL patients after allo-HSCT [16, 17]. Currently, there is no consensus on the optimal conditioning regimen for B-ALL patients undergoing HSCT. Exploring new conditioning regimens to reduce relapse without increasing TRM is of great significance for improving the prognosis of high-risk ALL patients.
Cladribine (2-chloro-2’-deoxyadenosine, CLAD) is a new generation purine analogue can be activated by intracellular phosphorylation, interfering with DNA repair and synthesis, thereby killing proliferative and non-proliferative lymphocytes and monocytes by promoting cellular apoptosis [18]. CLAD was initially used to treat hairy cell leukemia [19], and subsequent clinical practice gradually confirmed the anti-tumor activity of CLAD in other hematologic malignancies, such as indolent lymphoma, chronic lymphocytic leukemia (CLL), acute myeloid leukemia (AML) and so on [20], and even showed stronger anti-tumor effect than another purine analogue fludarabine, according to clinical trails’ data [21, 22]. CLAD also has a synergistic effect with cytarabine (Ara-C) [23, 24]. As a potent anti-tumor agent, CLAD's application has extended from combination chemotherapy to pre-transplant conditioning. In the clinical application of refractory/recurrent AML patients, CLAD intensified conditioning regimen can reduce the recurrence after HSCT [17, 25]. However, there is currently a lack of clinical data exploring the role of CLAD intensified conditioning regimens in B-ALL patients.
In consideration of the synergistic anti-leukemia effect of CALD and Ara-C, our center added CLAD and medium-dose Ara-C on the basis of BuCy regimen (CBAC) to intensify the conditioning. This study aims to investigate the efficacy of this conditioning regimen and compare it with traditional TBI-Cy regimen.
Material and methods
Patients’ data
Clinical data of 45 high-risk B-ALL patients in CR who underwent allo-HSCT after receiving CBAC conditioning regimen at Xiangya Hospital, Central South University, from July 2018 to October 2022 were retrospectively reviewed. Additionally, data of 38 high-risk B-ALL patients in CR who underwent traditional TBI-Cy conditioning regimen from January 2013 to October 2022 were collected as a control group. Follow-up was conducted until February 28, 2023. The study was approved by the Ethics Committee of Xiangya Hospital, Central South University on September 6, 2024, and informed consent was waived (the ethics approval number: 2024091079).
Transplantation regimens
Two conditioning regimens were used: CBAC regimen and TBI-Cy regimen.
CBAC regimen: CLAD (5 mg/m2/d, days −8 to −4), Ara-C (2 g/m2/d, days −8 to −4), Bu (0.8 mg/kg, Q6h, days −8 to −5), cyclophosphamide (CTX) (1.8 g/m2/d, days −3 to −2). The medication sequence involved intravenous infusion of CLAD over 3 h, followed by Ara-C through continuous intravenous drip over 3 h, with a 4-h interval.
TBI-Cy regimen: TBI (4.5 Gy, days −5 to −4,) CTX (60 mg/kg, days −3 to −2).
Patients underwent HLA-matched sibling donor (MSD) HSCT all received graft versus host disease (GVHD) prevention regimen based on cyclosporin A (CsA) combined with mycophenolate mofetil (MMF) and short-term methotrexate (MTX), as previously described [26]. Haploidentical or unrelated donor transplant recipients received antithymocyte globulin (ATG) + CsA + MMF + short-term MTX regimen for GVHD prevention, as previously published [27].
Methylprednisolone 2 mg/kg/day intravenously was administered for treatment of grade II-IV acute graft versus host disease (aGVHD). For patients resistant or dependent on methylprednisolone, or in the presence of severe infections, second-line treatments such as basiliximab, ruxolitinib, and MTX were promptly initiated.
Post-transplant monitoring
Bone marrow aspirations were performed at least once a month in the first 6 months after transplantation and then every 2 months in the second 6 months, every 2–3 months in the second year, and every 3–6 months thereafter to evaluate the remission status and chimerism. And the frequency of bone marrow aspirations could be adjusted as needed based on the patient's clinical condition. Patients with Philadelphia chromosome-positive (Ph+) B-ALL received tyrosine kinase inhibitor (TKI) prophylactic treatment around 2 months post-transplant, continuing for 2 years, with adjustments for severe aGVHD or serious infections. In the presence of MRD post-transplant, patients were treated with reduction or discontinuation of immunosuppressive agents, donor lymphocyte infusion (DLI), or, for those without DLI, CD19 bispecific antibodies or chemotherapy. Treatment strategies for post-transplant relapse were chosen based on individual patient conditions, including CD19 bispecific antibodies or chemotherapy combined with DLI, chimeric antigen receptor T-cell therapy (CAR-T), or second transplantation.
Endpoint events and definitions
The diagnosis of B-ALL followed the 2016 WHO standards [28]. High risk patients met at least one of the following criteria: (1) cytogenetic high risk; (2) MRD high risk. Cytogenetic risk stratification for B-ALL followed the 2021.V2 version of NCCN guidelines [29]. MRD high risk was defined as failure to achieve CR after 3–4 weeks of induction chemotherapy, or MRD positive after CR [30]. MRD detection involved multiparameter flow cytometry (MFC) on bone marrow samples, with a sensitivity level of 1/10,000 (0.01%) cells. For specific fusion genes such as BCR/ABL, real-time quantitative polymerase chain reaction (RT-PCR) was used to assess MRD, with BCR-ABL < 0.01% considered negative [31].
Neutrophil engraftment was defined as an absolute neutrophil count (ANC) > 0.5 × 109/L sustained for at least 3 days, and platelet engraftment was defined as a platelet count (PLT) > 20 × 109/L sustained for 7 days without platelet transfusion. cytomegalovirus (CMV) reactivation was defined as two consecutive blood samples with CMV DNA levels greater than 1000 copies/ml. Epstein-Barr virus (EBV) reactivation was defined as two consecutive blood samples with EBV DNA levels greater than 10,000 copies/ml. The diagnosis and grading criteria of GVHD were implemented with reference to earlier publications [32–34]. Relapse was defined on the basis of bone marrow histology analysis with more than 5% blasts or extramedullary relapse. non-relapse mortality (NRM) was defined as death due to any cause without previous disease progression or relapse. Overall survival (OS) was calculated from the date of transplantation to the date of death due to any cause, and surviving patients were censored at the last follow-up examination. DFS was defined as survival in continuous complete remission without relapse.
Statistics and analysis
The χ2 or Fisher’s exact test was used to compare categorical variables. A non-parametric test (Mann–Whitney U‐test) was employed to compare continuous variables. Three-year OS or DFS was calculated using the Kaplan–Meier outcome curve and compared by log-rank test. Considering the competing risks, the cumulative incidence rate of NRM and relapse were calculated by the Gray test. Competing events were defined as follows: relapse as the competing event for NRM; and death as the competing event for relapse.
Univariate analysis included factors that might affect prognosis, such as patient age (≥ 35 vs. < 35), gender, cytogenetic risk stratification, MRD risk stratification, MRD status at HSCT, achievement of CR1 before HSCT, donor source (haploidentical vs. matched sibling vs. unrelated), aGVHD, and chronic graft versus host disease (cGVHD). Factors with p < 0.10 were included in the multivariate analysis, and a stepwise regression method was used to construct a Cox proportional hazards model to assess the impact of multiple covariates on OS and DFS. For competing risk events, the Fine-Gray model was used for proportional hazard regression analysis. Cumulative incidence rate was calculated using R 4.2.0, and other data were analyzed using SPSS 26.0. All tests were two-sided, and p < 0.05 was considered to indicate statistically significant differences.
Results
Patient characteristics
The data were collected for a total of 83 high-risk B-ALL patients who achieved CR before HSCT, divided into CBAC group and TBI-Cy group, as shown in Table 1. All patients received allo-HSCT after at least 3 cycles of chemotherapy. Patients in CBAC group predominantly underwent haploidentical-HSCT (64.4%), while in the TBI-Cy group mainly underwent MSD HSCT (52.6%). The CBAC group had a higher proportion of MRD high risk patients (71.1% vs. 39.5%, p = 0.004). The two groups were similar in terms of age, gender, cytogenetic risk stratification, MRD status at HSCT, pre-HSCT remission status, infused mononuclear cell (MNC) count, and infused CD34+ cell count (Table 1).
Table 1.
Patients’ characteristics
| Characteristic/Group | CBAC (N = 45) | TBI-Cy(N = 38) | P value |
|---|---|---|---|
| Median age, year (range) | 28 (14–48) | 26 (14–46) | 0.696 |
| Gender, n (%) | 0.846 | ||
| Male | 27 (60.0%) | 22 (57.9%) | |
| Female | 18 (40.0%) | 16 (42.1%) | |
| Cytogenetic risk stratification, n (%) | 0.652 | ||
| Standard risk | 16 (35.6%) | 16 (42.1%) | |
| High risk | 29 (64.4%) | 22 (57.9%) | |
| MRD risk stratification, n (%) | 0.004 | ||
| Standard risk | 13 (28.9%) | 23 (60.5%) | |
| High risk | 32 (71.1%) | 15 (39.5%) | |
| Disease status at allo-HSCT, n (%) | 0.202 | ||
| CR1 | 34 (75.6%) | 34 (89.5%) | |
| CR2 | 10 (21.7%) | 3 (7.9%) | |
| CR3 | 1 (2.2%) | 1 (2.6%) | |
| MRD at allo-HSCT, n (%) | 0.791 | ||
| Negative | 36(80.0%) | 29 (76.3%) | |
| Positive | 9 (20.0%) | 9 (23.7%) | |
| Donor source | < 0.05 | ||
| Matched sibling | 10 (22.2) | 20 (52.6%) | |
| Unrelated | 6 (13.3) | 10 (26.3%) | |
| Haploidentical | 29 (64.4) | 8 (21.1%) | |
| Mononuclear cells, × 108/L, median (range) | 9.11 (3.79–25.23) | 8.675 (2.89–13.72) | 0.207 |
| CD34+ cells, × 106/L, median (range) | 5.11 (0.58–14.12) | 5.015 (1.24–7.73) | 0.373 |
MRD minimal residual disease; allo-HSCT allogeneic hematopoietic stem cell transplantation; CR complete remission
Hematopoietic reconstitution and transplant-related complications
The median time of neutrophil engraftment in both CBAC group and TBI-Cy group was 13 (10–22) days (p = 0.889), and platelet engraftment occurred at a median time of 19 (10–38) days, 13 (10–31) days, respectively, with complete donor chimerism achieved by day 30 post-HSCT.
In CBAC and TBI-Cy group, the incidence of aGVHD, grade II-IV aGVHD, III-IV grade aGVHD were 46.7% vs. 50.0%, 31.1% vs. 34.2%, 6.7% vs. 7.9%, and the incidence of cGVHD, moderate/severe cGVHD were 42.2% vs. 50.0%, 15.6% vs. 15.8%, respectively, with no significant difference between the two groups.
Within the first 100 days post-HSCT, CMV reactivation occurred in 28.9% and 42.1% of CBAC and TBI-Cy groups, and the EBV reactivation rates were 33.3% and 42.1%, respectively. The bacterial infection rates were 42.2% and 52.6%, and the fungal infection rates were 8.9% and 15.8%, respectively. There was no statistical difference in early infection between the two groups (p > 0.05). (Table 2).
Table 2.
Outcomes after allo-HSCT
| Outcome | CBAC | TBI-Cy | P value |
|---|---|---|---|
| Number of patients | 45 | 38 | |
| Neutrophil engraftment, day, median (range) | 13 (10–22) | 13 (10–22) | 0.889 |
| Platelet engraftment, day, median (range) | 19 (10–38) | 13 (10–31) | 0.010 |
| aGVHD, n (%) | 0.947 | ||
| Grade I-II | 18 (40.0) | 16 (42.1) | |
| Grade III-IV | 3 (6.7) | 3 (7.9) | |
| cGVHD, n (%) | 0.999 | ||
| Mild cGVHD | 12 (26.7) | 10 (26.3) | |
| Moderate/severe cGVHD | 7 (15.6) | 6 (15.8) | |
| Relapse, n (%) | 6 (13.3) | 13 (34.2) | 0.024 |
| Median time to relapse, days, median (range) | 284.5 (129 - 623) | 261 (82 - 737) | 0.930 |
| Infections at < 100 d, n (%) | |||
| Cytomegalovirus disease/reactivation | 13 (28.9) | 16 (42.1) | 0.208 |
| EBV | 15 (33.3) | 16 (42.1) | 0.410 |
| Bacterial | 19 (42.2) | 20 (52.6) | 0.344 |
| Fungal | 4 (8.9) | 5 (13.2) | 0.533 |
| Other | 4 (8.9) | 6 (15.8) | 0.336 |
| Death, n (%) | 9 (20.0) | 5 (13.2) | 0.407 |
| Cause of death, n | |||
| Relapse | 3 | 8 | |
| GVHD | 1 | 2 | |
| Infection | 4 | 2 | |
| Others | 1 | 0 |
aGVHD acute graft versus host disease; cGVHD chronic graft versus host disease; EBV Epstein-Barr virus
Prognosis of patients in both groups
The median follow-up time in the CBAC group was 507 (106–1685) days, and by the last follow-up, 36 of the 45 patients were alive. There were 3 patients died of relapse, and 6 patients died of transplant-related complications (1 of GVHD, 4 of severe infections, and 1 of diffuse alveolar hemorrhage), with a median time to death of 240 (106–670) days. And 6 patients relapsed, with a median time to relapse of 284.5 (129–623) days after HSCT.
In the TBI-Cy group, the median follow-up time was 2356 (126–3680) days, and by the last follow-up, 26 of the 38 patients were alive. There were 8 patients died of relapse, and 4 patients died of transplant-related complications (2 of GVHD, 2 of severe infections), with a median time to death of 274.5 (130–635) days. A total of 13 patients relapsed, with a median time of 261 (82–737) days after HSCT.
The cumulative incidence of 3-year NRM in the CBAC group was 15.0% (95% CI: 5.8–28.1%), whereas it was 11.1% (95% CI: 3.4–23.9%) in the TBI-Cy group (p = 0.576). The 3-year cumulative incidence of relapse (CIR), 3-year DFS and 3-year OS in the CBAC group and TBI-Cy group were17.3% vs. 35.7% (p = 0.077), 67.7% vs. 53.2% (p = 0.235), 74.3% vs. 66.8% (p = 0.482), respectively (Fig. 1).
Fig. 1.
Cumulative incidence of 3-year relapse A, non-relapse mortality B, overall survival C and disease-free survival D of CBAC and TBI-Cy group
Multivariate analysis of total cohort showed that MRD+ at transplantation was adverse prognostic factor for relapse (HR = 2.710, p = 0.044), TBI-Cy regimen increased the risk of relapse after transplantation compared with CBAC regimen (HR: 2.544, p = 0.049). And achieving ≥ CR2 at HSCT (HR = 7.103, p = 0.001), grade III-IV aGVHD (HR = 13.612, p < 0.05) were adverse prognostic factors for DFS, and were also risk factors for OS (≥ CR2: HR = 4.628, p = 0.002; grade III-IV aGVHD: HR = 7.803, p = 0.001). Grade III-IV aGVHD also increased the risk of NRM compared with grade 0-II aGVHD (HR = 6.240, p = 0.003). (Table 3).
Table 3.
Univariate/multivariate analysis
| Outcomes | Univariate P value | Multivariate | ||
|---|---|---|---|---|
| HR | 95%CI | P value | ||
| Relapse | ||||
| Age (≥ 35 vs. < 35) | 0.887 | / | / | / |
| Gender (male vs. female) | 0.775 | / | / | / |
| Cytogenetic risk stratification (high risk vs. standard risk) | 0.511 | / | / | / |
| MRD risk stratification (high risk vs. standard risk) | 0.929 | / | / | / |
| MRD at HSCT (positive vs. negative) | 0.014 | 2.710 | 1.027 - 7.170 | 0.044 |
| Disease status at allo-HSCT (≥ CR2 vs. CR1) | 0.080 | 2.247 | 0.748 - 6.760 | 0.150 |
| Donor (MSD vs. Haplo vs. MUD) | 0.325 | / | / | / |
| aGVHD (III-IV vs. 0-II) | 0.494 | / | / | / |
| cGVHD (with vs. without) | 0.519 | / | / | / |
| Conditioning regimens (TBI-Cy vs. CBAC) | 0.077 | 2.544 | 1.004 - 6.430 | 0.049 |
| NRM | ||||
| Age (≥ 35 vs. < 35) | 0.482 | / | / | / |
| Gender (male vs. female) | 0.449 | / | / | / |
| Cytogenetic risk stratification (high risk vs. standard risk) | 0.463 | / | / | / |
| MRD risk stratification (high risk vs. standard risk) | 0.335 | / | / | / |
| MRD at HSCT (positive vs. negative) | 0.523 | / | / | / |
| Disease status at allo-HSCT (≥ CR2 vs. CR1) | 0.274 | / | / | / |
| Donor (MSD vs. Haplo vs. MUD) | 0.332 | / | / | / |
| aGVHD (III-IV vs. 0-II) | 0.003 | 6.240 | 1.860 - 21.000 | 0.003 |
| cGVHD (with vs. without) | 0.288 | / | / | / |
| Conditioning regimens (TBI-Cy vs. CBAC) | 0.576 | / | / | / |
| DFS | ||||
| Age (≥ 35 vs. < 35) | 0.585 | / | / | / |
| Gender (male vs. female) | 0.846 | / | / | / |
| Cytogenetic risk stratification (high risk vs. standard risk) | 0.247 | / | / | / |
| MRD risk stratification (high risk vs. standard risk) | 0.394 | / | / | / |
| MRD at HSCT (positive vs. negative) | 0.004 | 2.445 | 0.889 - 6.725 | 0.083 |
| Disease status at allo-HSCT (≥ CR2 vs. CR1) | 0.014 | 7.103 | 2.225 - 22.370 | 0.001 |
| Donor (reference: MSD) | 0.085 | / | / | / |
| Haplo | 0.512 | 0.170 - 1.542 | 0.234 | |
| MUD | 2.356 | 0.654 - 8.488 | 0.190 | |
| aGVHD (III-IV vs. 0-II) | 0.004 | 13.612 | 3.432 - 54.130 | < 0.05 |
| cGVHD (with vs. without) | 0.822 | / | / | / |
| Conditioning regimens (TBI-Cy vs. CBAC) | 0.235 | / | / | / |
| OS | / | / | / | |
| Age (≥ 35 vs. < 35) | 0.701 | / | / | / |
| Gender (male vs. female) | 0.486 | / | / | / |
| Cytogenetic risk stratification (high risk vs. standard risk) | 0.408 | / | / | / |
| MRD risk stratification (high risk vs. standard risk) | 0.502 | / | / | / |
| MRD at HSCT (positive vs. negative) | 0.147 | / | / | / |
| Disease status at allo-HSCT (≥ CR2 vs. CR1) | 0.004 | 4.628 | 1.780 - 12.036 | 0.002 |
| Donor (MSD vs. Haplo vs. MUD) | 0.175 | / | / | / |
| aGVHD (0-II garde vs. III-IV grade) | 0.002 | 7.803 | 2.369 - 25.700 | 0.001 |
| cGVHD (with vs. without) | 0.354 | / | / | / |
| Conditioning regimens (TBI-Cy vs. CBAC) | 0.482 | / | / | / |
NRM non-relapse mortality; DFS disease-free survival; OS Overall survival; MRD minimal residual disease; allo-HSCT, allogeneic hematopoietic stem cell transplantation; CR complete remission; MSD HLA-matched sibling donor; MUD HLA-matched unrelated donor; aGVHD acute graft versus host disease; cGVHD chronic graft versus host disease
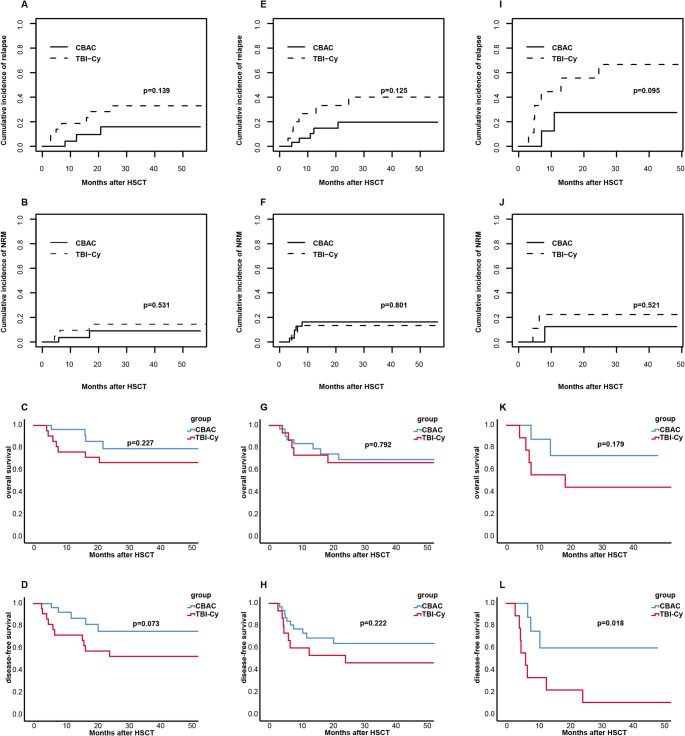
Subsequently, the impact of different conditioning regimens on prognosis in patients with cytogenetic high risk, MRD high risk, and MRD+ at HSCT was analyzed. (Fig. 2).
Cytogenetic high risk: the 3-year OS, DFS, CIR and NRM in CBAC group and TBI-Cy group were 79.1% vs. 66.7% (p = 0.227), 75.1% vs. 52.6% (p = 0.073), 15.9% vs. 33.0% (p = 0.139), 9.0%vs. 14.4% (p = 0.531), respectively.
MRD high risk: the 3-year OS, DFS, CIR and NRM in CBAC group and TBI-Cy group were 69.4% vs. 66.7% (p = 0.792), 64.0% vs. 46.7% (p = 0.222), 19.7% vs. 40.0% (p = 0.125), 16.3% vs. 13.3% (p = 0.801), respectively.
MRD+ at HSCT: the 3-year OS, DFS, CIR and NRM in CBAC group and TBI-Cy group were 72.9% vs. 44.4% (p = 0.179), 60.0% vs. 11.1% (p = 0.018), 27.5% vs. 66.7% (p = 0.095), 12.5% vs. 22.2% (p = 0.521), respectively.
Fig. 2.
Cumulative incidence of 3-year relapse, non-relapse mortality, overall survival and disease-free survival of CBAC and TBI-Cy group in cytogenetic high risk patients A-D, MRD high risk patients E–H and patients with MRD+ at HSCT I-L
Prognosis of patients with special types of ALL in the CBAC group
Ph+, Philadelphia chromosome-like (Ph-like), and Ikaros family zinc-finger 1(IKZF1) alterations are currently discussed as special types of B-ALL. This study further analyzed the prognosis of patients with these cytogenetic abnormalities in the CBAC group.
There were eight Ph+ B-ALL patients achieved CR1 before HSCT, of which three patients had IKZF1 alterations, two patients had positive BCR-ABL quantification at the time of HSCT. The 3-year OS, DFS, NRM, and CIR of these patients were 87.5%, 75.0%, 12.5% and 12.5%, respectively. Among the seven Ph-like patients, two patients complicated with IKZF1 alteration, five patients were in CR1 and two patients were in CR2 at HSCT, all of them survived disease-free. The 3-year OS, DFS, NRM, and CIR of these patients were 100.0%, 100.0%, 0% and 0%, respectively. There were ten patients had IKZF1 alteration, all patients survived disease-free until the end of follow-up except one patient died of several intestinal GVHD, with 3-year OS, DFS, NRM, and CIR of 90.0%, 80.0%, 10.0%, and 10.0%, respectively.
Discussion
In this study, the results showed successful engraftment of neutrophil and platelet, and there was no significant difference in neutrophils engraftment time between the two groups. Compared with the TBI-Cy group, later platelet engraftment (19 days vs. 13 days, P = 0.010) may be associated with the relatively high proportion of haplo-HSCT in CBAC group.
We analyzed the occurrence of aGVHD, grade III-IV aGVHD, cGVHD and moderate/severe cGVHD, early post-HSCT (within 100 days) CMV, EBV, bacterial and fungal infections of the two groups. The results showed no significant differences between the two groups, consistent with previous studies [6, 35]. Furthermore, the 3-year NRM of CBAC group was 15.0%, which was similar to that in the TBI-Cy group. Overall, the CBAC conditioning regimen demonstrated good safety.
Relapse is a crucial factor influencing prognosis. Earlier studies indicated that the recurrence rate of B-ALL patients after HSCT was about 40–60% [36, 37]. In recent years, CIR is approximately 20%−33%. Patients with high-risk factors such as cytogenetic risk, MRD+ at HSCT, ≥ CR2, and receiving reduced-intensity or non-myeloablative conditioning still experience high risk of relapse, especially for patients with MRD+ and ≥ CR2, the incidence rate of relapse can be as high as 40–60% [4, 6, 38–43]. In our study, the 3-year CIR in CBAC and TBI-Cy group were 17.3% vs. 35.7%, the CBAC group showed a trend towards reduced CIR (p = 0.077). And multivariate analysis suggested that the CBAC conditioning regimen was an independent favorable prognostic factor for lower relapse (HR = 2.544, p = 0.049). In CBAC and TBI-Cy group, the 3-year DFS, OS were 67.7% vs. 53.2% (p = 0.235), 74.3% vs. 66.8% (p = 0.482), CBAC group showed better survival. In subgroup analysis, for patients with cytogenetic high-risk factors, CBAC group trended to show higher 3-year DFS than TBI-Cy group (75.1% vs. 52.6%, p = 0.073). And for patients with MRD+ at HSCT, the 3-year DFS in the CBAC group was significantly higher than that in the TBI-Cy group (60.0% vs. 11.1%, p = 0.018). In general, CBAC conditioning regimen may benefit B-ALL patients with cytogenetic high-risk factors and overcome the adverse effects of MRD+ on prognosis to some extent. According to transplant conditioning intensity (TCI) score [44], the score of TBI-Cy is 4, while it is 5.5 in CBAC, which showed advantages in eliminating malignant cells. The better curative effect of CBAC group may be related to the intensify conditioning regimen.
Subsequently, we further analyzed the impact of several cytogenetic abnormalities in B-ALL, such as Ph+, Ph-like and IKZF alterations, on prognosis. In our study, among 8 Ph+ patients, only one patient with IKZF1 deletion mutation died of severe intestinal GVHD, and 1 Ph+ patient with central nervous system relapse got CR until follow-up endpoint after receiving multiple lumbar punctures and intrathecal chemotherapy, TKI and DLI therapy. Survival analysis showed that the 3-year OS, DFS, and CIR of Ph+ patients were 87.5%, 75.0%, and 12.5%, respectively. Ph-like is also a crucial subgroup of high-risk cytogenetic factors. In our study, all Ph-like patients survived without relapse after HSCT. IKZF1 alteration is a common genetic mutation in B-ALL, accounting for approximately 30% to 40% in adult ALL [45]. In present study, among ten patients with IKZF1 alterations, the 3-year OS, DFS, and CIR were 90.0%, 80.0%, and 10.0%, respectively. Generally speaking, for the patients with Ph+, Ph-like and IKZF1 alterations, the anti-leukemia effect of CBAC regimen seems to overcome the impact of these high-risk factors on prognosis to some extent, which seems to be better than that reported in previous literature [31, 39, 46]. But the number of cases is too small, so it is necessary to further expand the sample size to analyze the curative effect.
In conclusion, CLAD and intermediate-dose cytarabine intensified BuCy conditioning regimen appears to be effective in high-risk B-ALL patients without increasing transplant-related mortality. It may be a potential alternative to TBI-based conditioning regimens to some extent. However, larger sample sizes and longer follow-up periods are needed for further confirmation. Further randomized trials are warranted to confirm these results.
Acknowledgements
The authors thank all of the physicians and nurses for their unevaluated contribution to this study and the patients for participating in this research.
Author contributions
YJ. X, Y. C and TT. C designed the study, and TT. C wrote the manuscript under the guidance of YJ. X and Y. C, J. P, Y. L and SH. Y performed the data collection and statistical analysis. All authors read and critically revised the manuscript for intellectual content and approved the final manuscript.
Funding
This work was supported by the China Anti-cancer Association—Hengrui TPO Receptor Agonist Research Fund (CORP-253).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval
The study was approved by the Ethics Committee of Xiangya Hospital, Central South University, and informed consent was waived.
Disclosure
N/A.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yan Chen, Email: yanchen2018@csu.edu.cn.
Yajing Xu, Email: xyyajingxu@csu.edu.cn.
References
- 1.McNeer JL, Bleyer A (2018) Acute lymphoblastic leukemia and lymphoblastic lymphoma in adolescents and young adults. Pediatr Blood Cancer 65(6):e26989 [DOI] [PubMed] [Google Scholar]
- 2.Bispo JAB, Pinheiro PS, Kobetz EK (2020) Epidemiology and Etiology of Leukemia and Lymphoma. Cold Spring Harb Perspect Med 10(6):a034819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bazarbachi A et al (2022) 20-year steady increase in survival of adult patients with relapsed philadelphia-positive acute lymphoblastic leukemia post allogeneic hematopoietic cell transplantation. Clin Cancer Res 28(5):1004–1012 [DOI] [PubMed] [Google Scholar]
- 4.Giebel S et al (2017) Improving results of allogeneic hematopoietic cell transplantation for adults with acute lymphoblastic leukemia in first complete remission: an analysis from the acute leukemia working party of the European society for blood and marrow transplantation. Haematologica 102(1):139–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohty M, Malard F, Savani BN (2015) High-dose total body irradiation and myeloablative conditioning before allogeneic hematopoietic cell transplantation: time to rethink? Biol Blood Marrow Transplant 21(4):620–624 [DOI] [PubMed] [Google Scholar]
- 6.Zhang H et al (2023) Busulfan plus cyclophosphamide versus total body irradiation plus cyclophosphamide for adults acute B lymphoblastic leukemia: an open-label, multicenter Phase III trial. J Clin Oncol 41(2):343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang YH et al (2022) Busulfan-containing conditioning regimens in allogeneic hematopoietic stem cell transplantation for acute lymphoblastic leukemia: a Taiwan observational study. Cancer Rep (Hoboken) 5(3):e1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitsuhashi K et al (2016) Comparison of cyclophosphamide combined with total body irradiation, oral busulfan, or intravenous busulfan for allogeneic hematopoietic cell transplantation in adults with acute lymphoblastic leukemia. Biol Blood Marrow Transplant 22(12):2194–2200 [DOI] [PubMed] [Google Scholar]
- 9.Eroglu C et al (2013) Comparison of total body irradiation plus cyclophosphamide with busulfan plus cyclophosphamide as conditioning regimens in patients with acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplant. Leuk Lymphoma 54(11):2474–2479 [DOI] [PubMed] [Google Scholar]
- 10.Khimani F et al (2021) Impact of total body irradiation-based myeloablative conditioning regimens in patients with acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplantation: systematic review and meta-analysis. Transplant Cell Ther 27(7):620.e1-620.e9 [DOI] [PubMed] [Google Scholar]
- 11.Rehman MEU et al (2023) Total body irradiation versus chemotherapy conditioning in pediatric acute lymphoblastic leukemia patients undergoing hematopoietic stem cell transplant: a systematic review and meta-analysis. Clin Lymphoma Myeloma Leuk 23(4):249–258 [DOI] [PubMed] [Google Scholar]
- 12.Friend BD et al (2020) The impact of total body irradiation-based regimens on outcomes in children and young adults with acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplantation. Pediatr Blood Cancer 67(2):e28079 [DOI] [PubMed] [Google Scholar]
- 13.Lv M et al (2025) Total body irradiation versus chemotherapy myeloablative conditioning in B-cell acute lymphoblastic leukaemia patients with first complete remission. Sci Rep 15(1):10079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang J et al (2016) Idarubicin-intensified BUCY2 conditioning regimen improved survival in high-risk acute myeloid, but not lymphocytic leukemia patients undergoing allogeneic hematopoietic stem cell transplantation: A retrospective comparative study. Leuk Res 46:61–68 [DOI] [PubMed] [Google Scholar]
- 15.Dholaria B et al (2021) Improved outcomes of haploidentical hematopoietic cell transplantation with total body irradiation-based myeloablative conditioning in acute lymphoblastic leukemia. Transplant Cell Ther 27(2):171.e1-171.e8 [DOI] [PubMed] [Google Scholar]
- 16.D’Souza A et al (2020) Current use of and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant 26(8):e177–e182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J et al (2018) A new intensive conditioning regimen for allogeneic hematopoietic stem cell transplantation in patients with refractory or relapsed acute myeloid leukemia. Medicine (Baltimore) 97(17):e0228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carson DA et al (1983) Specific toxicity of 2-chlorodeoxyadenosine toward resting and proliferating human lymphocytes. Blood 62(4):737–743 [PubMed] [Google Scholar]
- 19.Goodman GR et al (2003) Extended follow-up of patients with hairy cell leukemia after treatment with cladribine. J Clin Oncol 21(5):891–896 [DOI] [PubMed] [Google Scholar]
- 20.Sigal DS et al (2010) Beyond hairy cell: the activity of cladribine in other hematologic malignancies. Blood 116(16):2884–2896 [DOI] [PubMed] [Google Scholar]
- 21.Wyczechowska D, Fabianowska-Majewska K (2003) The effects of cladribine and fludarabine on DNA methylation in K562 cells. Biochem Pharmacol 65(2):219–225 [DOI] [PubMed] [Google Scholar]
- 22.Park H et al (2016) Comparison of cladribine- and fludarabine-based induction chemotherapy in relapsed or refractory acute myeloid leukaemia. Ann Hematol 95(11):1777–1786 [DOI] [PubMed] [Google Scholar]
- 23.Armentrout SA, Burns CP (1974) Cytosine arabinoside as a single agent in the therapy of adult acute leukemia. Am J Med Sci 268(3):163–168 [DOI] [PubMed] [Google Scholar]
- 24.Gandhi V, Plunkett W (1988) Modulation of arabinosylnucleoside metabolism by arabinosylnucleotides in human leukemia cells. Cancer Res 48(2):329–334 [PubMed] [Google Scholar]
- 25.Sun Y et al (2021) A novel intensive conditioning regimen for allogeneic hematopoietic stem cell transplantation in the treatment of relapsed/refractory acute myeloid leukemia. Neoplasma 68(6):1351–1358 [DOI] [PubMed] [Google Scholar]
- 26.Cheng T et al (2022) Comparison of outcomes of haploidentical peripheral blood stem cell transplantation supported by third-party cord blood versus human. Leukocyte antigen-matched sibling peripheral blood stem cell transplantation in hematologic malignancy patients. Front Oncol 12:922120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng C et al (2022) Haploidentical peripheral blood stem cell transplantation combined with unrelated cord blood in hematologic malignancy patients: a report of 80 cases. Front Immunol 13:980464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arber DA et al (2016) The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127(20):2391–2405 [DOI] [PubMed] [Google Scholar]
- 29.Brown PA et al (2021) Acute lymphoblastic leukemia, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 19(9):1079–1109 [DOI] [PubMed] [Google Scholar]
- 30.Gökbuget N, Hoelzer D (2009) Treatment of adult acute lymphoblastic leukemia. Semin Hematol 46(1):64–75 [DOI] [PubMed] [Google Scholar]
- 31.Lyu M et al (2021) Comparison of autologous and allogeneic stem cell transplantation for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Hematology 26(1):65–74 [DOI] [PubMed] [Google Scholar]
- 32.Schoemans HM et al (2018) EBMT-NIH-CIBMTR Task force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transplant 53(11):1401–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SJ et al (2015) Measuring therapeutic response in chronic graft-versus-host disease. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 response criteria working group report. Biol Blood Marrow Transplant 21(6):984–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jagasia MH et al (2015) National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant 21(3):389-401.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao YL et al (2021) Integrating CAR T-cell therapy and transplantation: comparisons of safety and long-term efficacy of allogeneic hematopoietic stem cell transplantation after CAR T-cell or chemotherapy-based complete remission in B-cell acute lymphoblastic leukemia. Front Immunol 12:605766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta V, Richards S, Rowe J (2013) Allogeneic, but not autologous, hematopoietic cell transplantation improves survival only among younger adults with acute lymphoblastic leukemia in first remission: an individual patient data meta-analysis. Blood 121(2):339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Messori A et al (2013) Acute lymphoblastic leukemia in first complete remission: temporal trend of outcomes in studies comparing allogeneic transplant with autologous transplant or chemotherapy. Ann Hematol 92(9):1221–1228 [DOI] [PubMed] [Google Scholar]
- 38.Czerw T et al (2018) Anti-thymocyte globulin improves survival free from relapse and graft-versus-host disease after allogeneic peripheral blood stem cell transplantation in patients with Philadelphia-negative acute lymphoblastic leukemia: an analysis by the acute leukemia working party of the EBMT. Cancer 124(12):2523–2533 [DOI] [PubMed] [Google Scholar]
- 39.Aldoss I et al (2022) Outcomes of allogeneic hematopoietic cell transplantation in adults with fusions associated with Ph-like ALL. Blood Adv 6(17):4936–4948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen Z et al (2018) Influence of pre-transplant minimal residual disease on prognosis after Allo-SCT for patients with acute lymphoblastic leukemia: systematic review and meta-analysis. BMC Cancer 18(1):755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang YJ et al (2020) Haploidentical donor is preferred over matched sibling donor for pre-transplantation MRD positive ALL: a phase 3 genetically randomized study. J Hematol Oncol 13(1):27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y et al (2016) Haploidentical versus matched-sibling transplant in adults with Philadelphia-Negative high-risk acute lymphoblastic leukemia: a biologically phase III randomized study. Clin Cancer Res 22(14):3467–3476 [DOI] [PubMed] [Google Scholar]
- 43.Han LJ et al (2017) Haploidentical transplantation compared with matched sibling and unrelated donor transplantation for adults with standard-risk acute lymphoblastic leukaemia in first complete remission. Br J Haematol 179(1):120–130 [DOI] [PubMed] [Google Scholar]
- 44.Spyridonidis A et al (2020) Redefining and measuring transplant conditioning intensity in current era: a study in acute myeloid leukemia patients. Bone Marrow Transplant 55(6):1114–1125 [DOI] [PubMed] [Google Scholar]
- 45.Marke R, van Leeuwen FN, Scheijen B (2018) The many faces of IKZF1 in B-cell precursor acute lymphoblastic leukemia. Haematologica 103(4):565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang BQ et al (2022) Prognostic significance of IKZF1 gene deletions in patients with B-cell acute lymphoblastic leukemia. Zhonghua Xue Ye Xue Za Zhi 43(3):235–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.