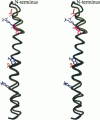
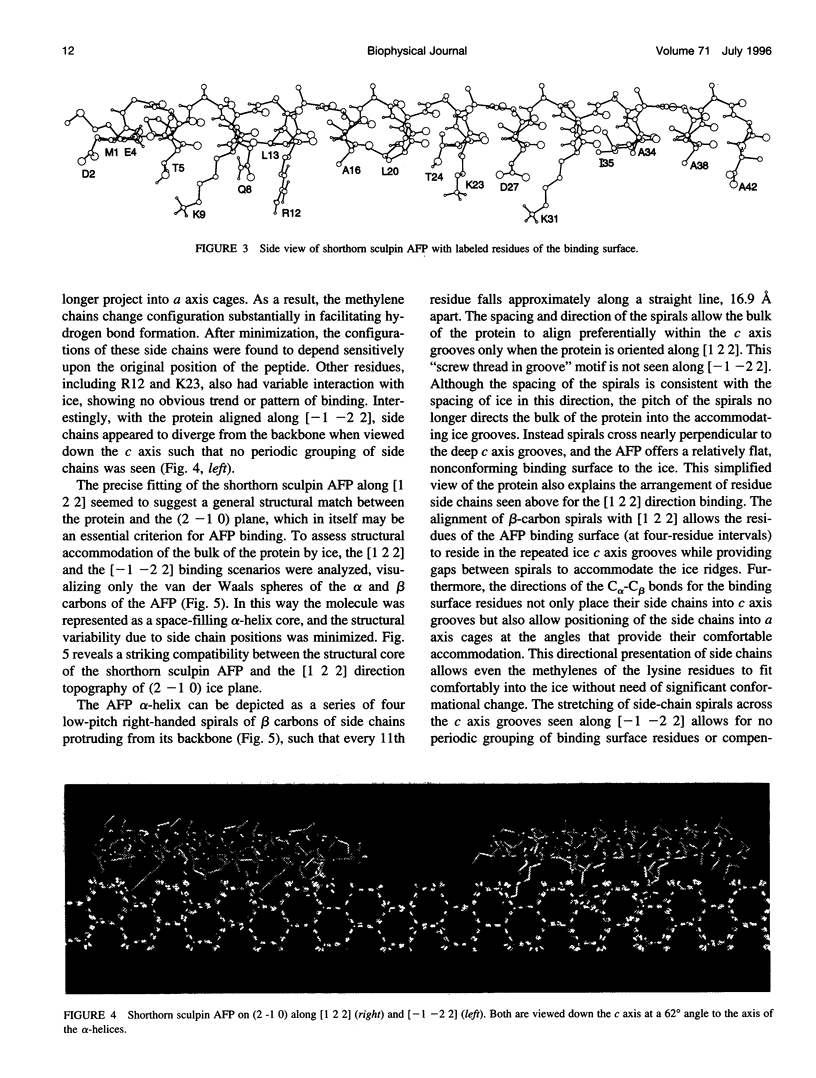
Abstract
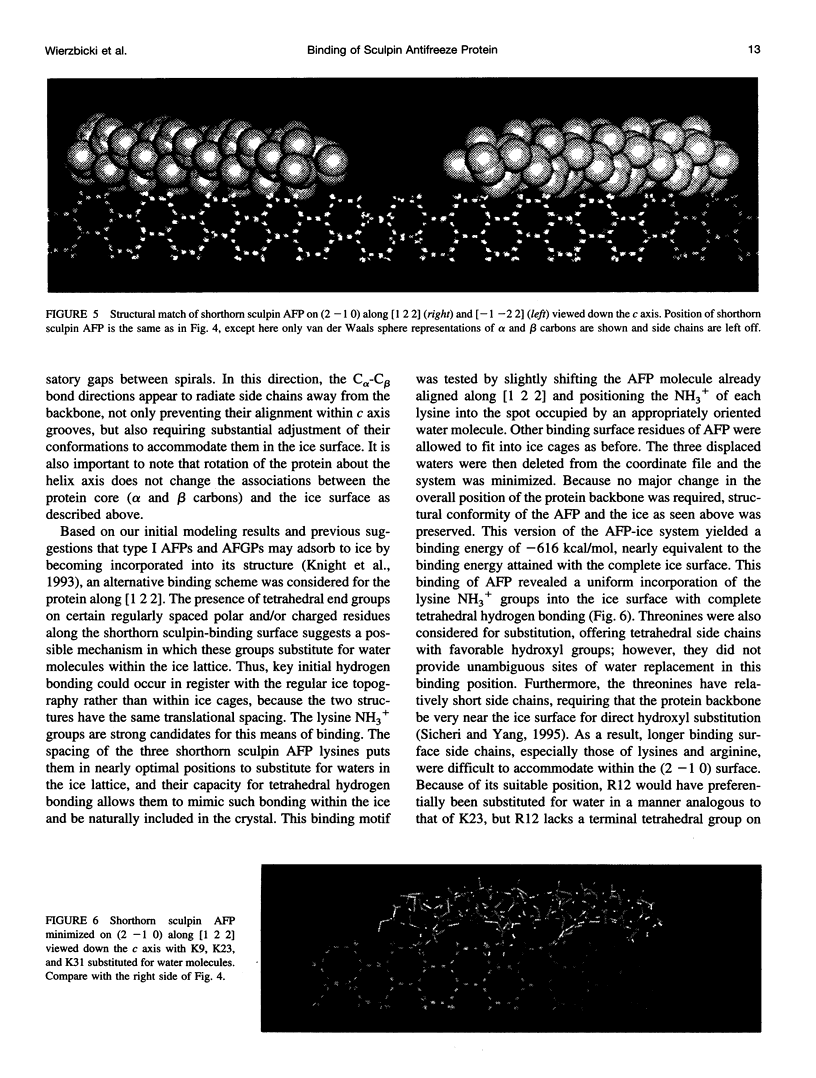
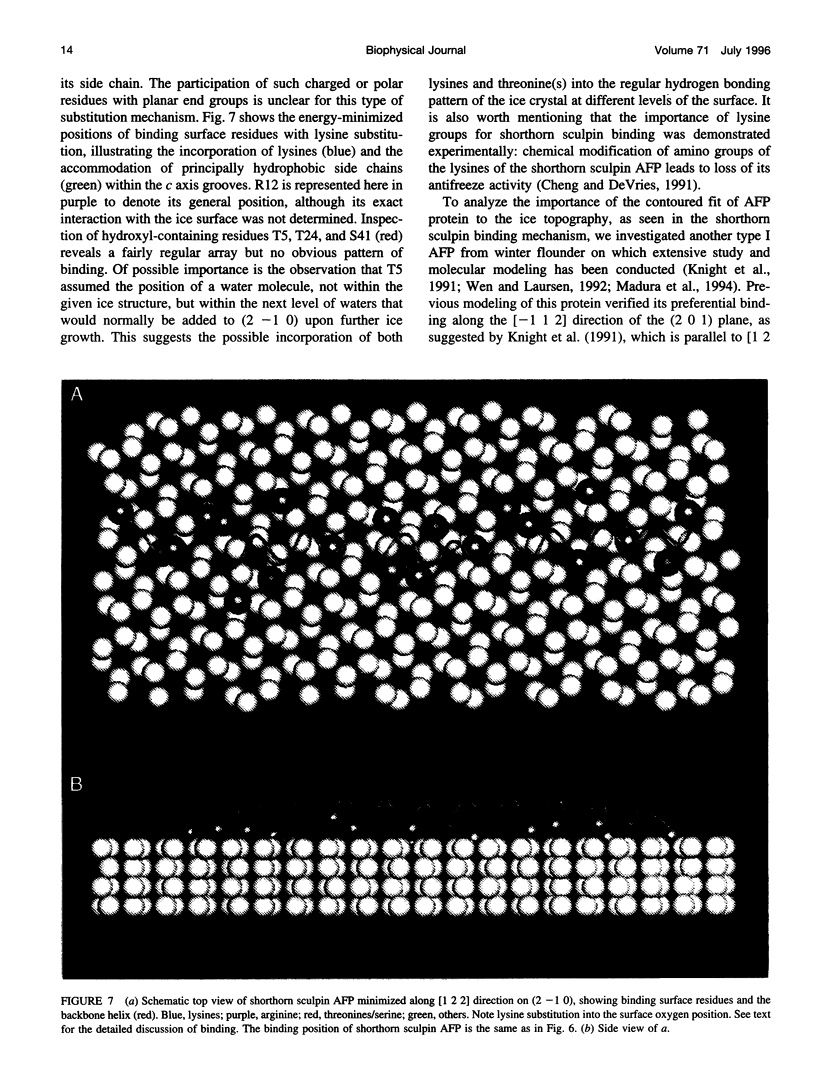
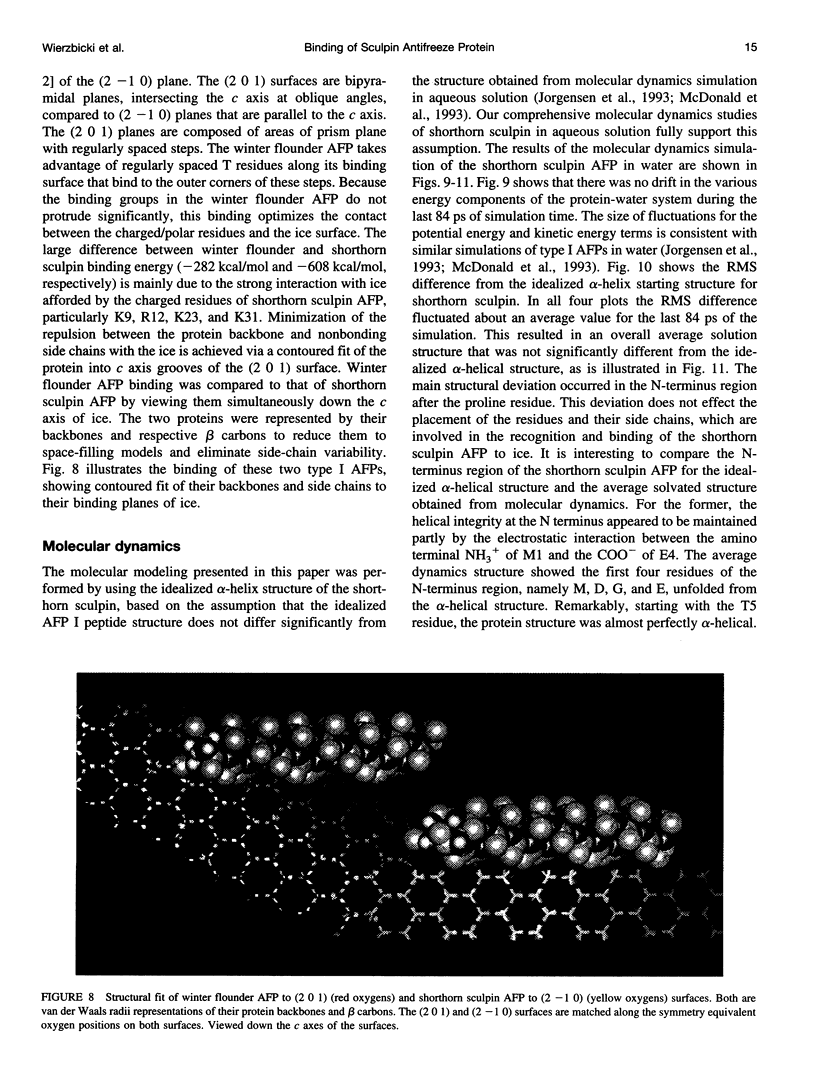
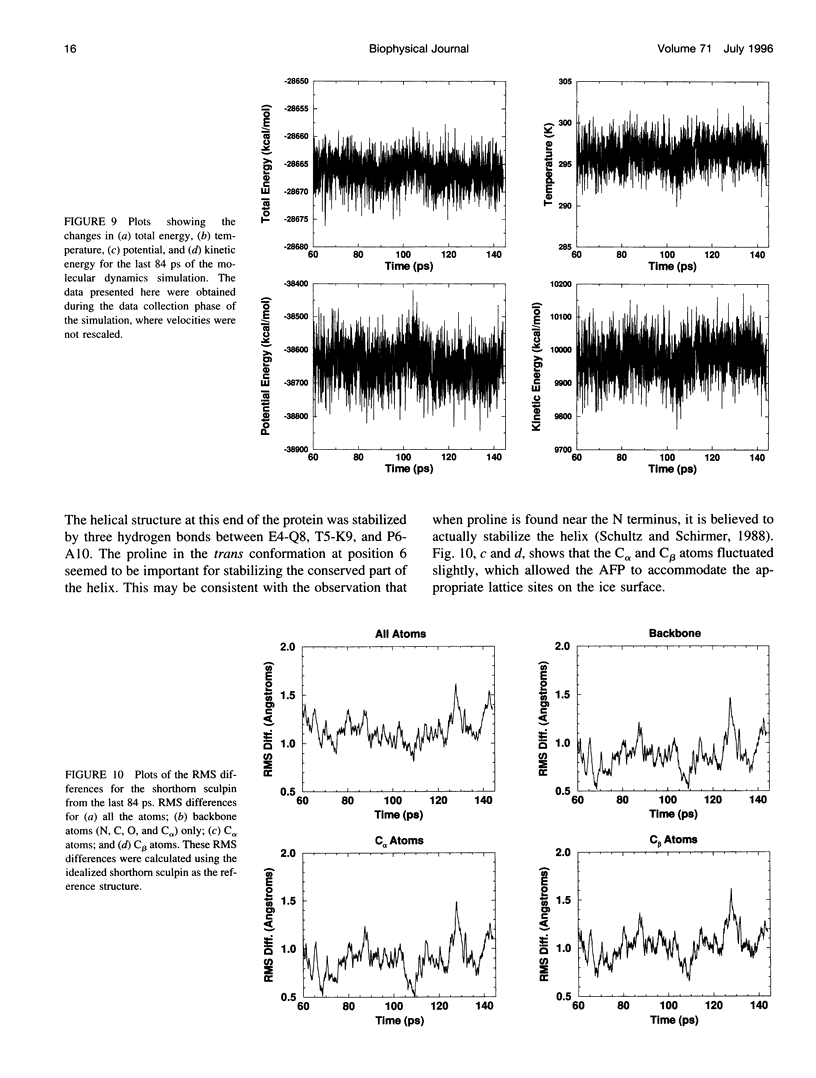
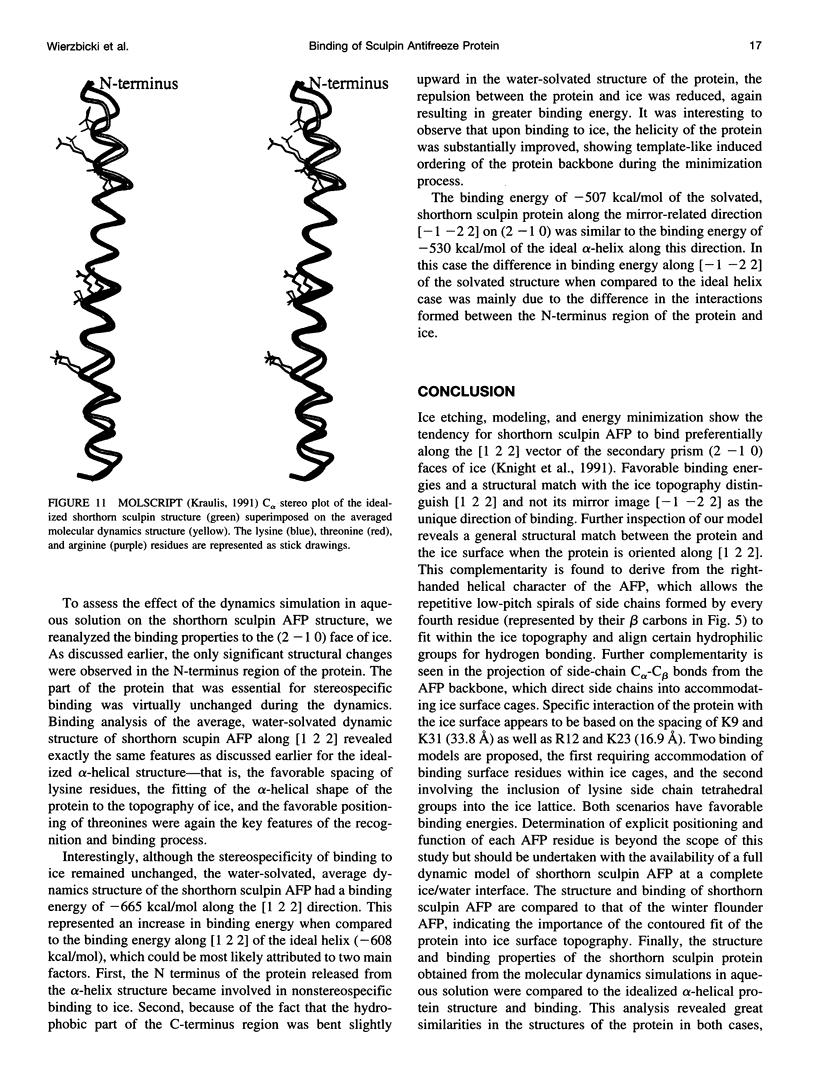
In this paper we report the results of our studies on the stereospecific binding of shorthorn sculpin antifreeze protein (AFP) to (2 -1 0) secondary prism faces of ice. Using ice crystal growth and etching techniques together with molecular modeling, molecular dynamics, and energy minimization, we explain the nature of preferential binding of shorthorn sculpin AFP along the [1 2 2] direction on (2- 1 0) planes. In agreement with ice etching studies, the mechanism of preferential binding suggested by molecular modeling explains why the binding of shorthorn sculpin AFP occurs along [1 2 2] and not along its mirror symmetry-related direction [-1 -2 2] on (2 -1 0). This binding mechanism is based on the protein-crystal surface enantioselective recognition that utilizes both alpha-helical protein backbone matching to the (2 -1 0) surface topography and matching of side chains of polar/charged residues with specific water molecule positions in the ice surface. The mechanisms of winter flounder and shorthorn sculpin antifreeze binding to ice are compared.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davies P. L., Hew C. L. Biochemistry of fish antifreeze proteins. FASEB J. 1990 May;4(8):2460–2468. doi: 10.1096/fasebj.4.8.2185972. [DOI] [PubMed] [Google Scholar]
- Hew C. L., Yang D. S. Protein interaction with ice. Eur J Biochem. 1992 Jan 15;203(1-2):33–42. doi: 10.1111/j.1432-1033.1992.tb19824.x. [DOI] [PubMed] [Google Scholar]
- Jorgensen H., Mori M., Matsui H., Kanaoka M., Yanagi H., Yabusaki Y., Kikuzono Y. Molecular dynamics simulation of winter flounder antifreeze protein variants in solution: correlation between side chain spacing and ice lattice. Protein Eng. 1993 Jan;6(1):19–27. doi: 10.1093/protein/6.1.19. [DOI] [PubMed] [Google Scholar]
- Knight C. A., Cheng C. C., DeVries A. L. Adsorption of alpha-helical antifreeze peptides on specific ice crystal surface planes. Biophys J. 1991 Feb;59(2):409–418. doi: 10.1016/S0006-3495(91)82234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight C. A., Driggers E., DeVries A. L. Adsorption to ice of fish antifreeze glycopeptides 7 and 8. Biophys J. 1993 Jan;64(1):252–259. doi: 10.1016/S0006-3495(93)81361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald S. M., Brady J. W., Clancy P. Molecular dynamics simulations of a winter flounder "antifreeze" polypeptide in aqueous solution. Biopolymers. 1993 Oct;33(10):1481–1503. doi: 10.1002/bip.360331002. [DOI] [PubMed] [Google Scholar]
- Raymond J. A., DeVries A. L. Adsorption inhibition as a mechanism of freezing resistance in polar fishes. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2589–2593. doi: 10.1073/pnas.74.6.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicheri F., Yang D. S. Ice-binding structure and mechanism of an antifreeze protein from winter flounder. Nature. 1995 Jun 1;375(6530):427–431. doi: 10.1038/375427a0. [DOI] [PubMed] [Google Scholar]
- Wen D., Laursen R. A. A model for binding of an antifreeze polypeptide to ice. Biophys J. 1992 Dec;63(6):1659–1662. doi: 10.1016/S0006-3495(92)81750-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicki A., Sikes C. S., Madura J. D., Drake B. Atomic force microscopy and molecular modeling of protein and peptide binding to calcite. Calcif Tissue Int. 1994 Feb;54(2):133–141. doi: 10.1007/BF00296064. [DOI] [PubMed] [Google Scholar]
- Wierzbicki A., Sikes C. S., Sallis J. D., Madura J. D., Stevens E. D., Martin K. L. Scanning electron microscopy and molecular modeling of inhibition of calcium oxalate monohydrate crystal growth by citrate and phosphocitrate. Calcif Tissue Int. 1995 Apr;56(4):297–304. doi: 10.1007/BF00318050. [DOI] [PubMed] [Google Scholar]