Abstract
Identification of highly aggressive tumors from benign phenotypes is an important diagnostic need, with increased expression of hypoxia-inducible factor-1α (HIF-1α) as one factor linked to tumor progression and treatment response. HIF-1α stabilization is closely linked to extracellular pH (pHe) through the regulation of glycolysis, proton transporters and vascular endothelial growth factor (VEGF). Magnetic resonance imaging (MRI)—based pHe measurement has shown promise in differentiating tumor phenotypes. In this study, we evaluated a new protocol designed to enhance tumor acidosis by administering glucose prior to pHe measurements in orthotopic human breast cancer xenografts. To demonstrate the utility of this approach, we used human triple-negative breast cancer (TNBC) MDA-MB-231 cells that were either wild type (231-WT) or genetically engineered to stably express short hairpin RNA (shRNA) against HIF-1α (231-HIF-1α-shRNA) or engineered to overexpress VEGF (231-VEGF). Iopamidol was administered for chemical exchange saturation transfer (CEST) MRI pHe mapping. We observed enhanced differentiation in the pHe maps following glucose administration, with mean pHe of 6.1 ± 0.12 (231-WT), 6.3 ± 0.04 (231-VEGF) and 6.58 ± 0.04 (231-HIF-1α-shRNA). Without glucose stimulation, the corresponding values were 6.31 ± 0.04 (231-WT), 6.30 ± 0.07 (231-VEGF), and 6.55 ± 0.04 (231-HIF-1α-shRNA). These findings were validated by immunoblotting and immunohistochemistry. Collectively our data demonstrate that CEST MRI pHe mapping can effectively differentiates tumors with low HIF-1α expression and that glucose stimulation enhances this differentiation, offering a valuable tool for improved tumor characterization.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-14102-z.
Keywords: Chemical exchange saturation transfer (CEST), pHe, Hypoxia inducible factor-1α (HIF-1α), Iopamidol, Breast cancer
Subject terms: Imaging techniques, Diagnostic markers
Introduction
Tumors display abnormal proliferation of cells often followed by development of hypoxia—a signatory feature of the tumor microenvironment (TME), which is often considered to be a predictive marker of rapid growth, metastases, recurrence, and poor survival rates in solid cancers1–3. Hypoxia arises from an imbalance between oxygen supply and demand due to uncontrolled cell proliferation, triggering the stabilization of hypoxia-inducible factors (HIFs), particularly HIF-1α4,5. This transcription factor enables cancer cells to adapt by shifting energy production from oxidative phosphorylation to glycolysis, even under oxygen-deficient conditions—a phenomenon known as the Warburg effect6,7. Glycolysis produces lactate from pyruvate as a byproduct through lactate dehydrogenase A (LDHA), which, along with other acidic metabolites, is expelled via specialized transporters such as sodium/hydrogen exchanger 1 (NHE-1), vacuolar-type ATPase (V-ATPase), monocarboxylate transporters (MCTs), and carbonic anhydrases (CAs), all upregulated by HIF-1α6,8. This leads to extracellular acidification, further stabilizing HIF-1α, creating a feedback loop that promotes tumor growth, invasion, and treatment resistance5,9. Additionally, HIF-1α drives angiogenesis by upregulating vascular endothelial growth factor (VEGF), which facilitates new blood vessel formation to support tumor growth and metastasis9,10. Therefore, understanding the intricate connections among hypoxia, acidosis and angiogenesis is a major focus in cancer research.
In this context, various methods have been explored to detect tissue hypoxia and oxygen levels. Traditional techniques, such as oxygen electrodes, HIF-1α biopsy analysis, and pimonidazole staining, have had some success but face challenges such as invasiveness, limitations in resolution and the influence of tumor heterogeneity on accuracy11,12. Emerging approaches include electron paramagnetic resonance (EPR) oxygen imaging using trityl radical probes, fluorescent and photoacoustic probes, and nuclear imaging with nitroimidazole-based radiopharmaceuticals for PET or SPECT imaging. While these methods provide valuable insights, they often lack spatial resolution or universal applicability. Oxygen-enhanced MRI (OE-MRI), TOLD MRI, and 19F MRI offer alternative strategies but are limited by sensitivity or signal strength. Thus, no single method currently provides a perfect measure of hypoxia13–22. For research applications, reporters linked to the expression of the hypoxia-responsive elements have also been used, but these do not provide quantitative estimates of hypoxia23,24. This is further discussed in recent review article exploring both current and emerging strategies for hypoxia imaging and their potential clinical translation25.
Indirect detection of hypoxia through downstream pathways, such as measuring acidosis, is a viable and promising approach. Acidosis, initiated and partly driven by HIF-1α upregulation, is associated with tumor growth, metastasis, and treatment resistance26,27. Intracellular or extracellular pH maps can be generated using 31P, 19F, or 1H magnetic resonance spectroscopy (MRS), but have limitations like low spatial resolution, low sensitivity, restricted penetration into deeper tissues, and probe instability28–31. Positron emission tomography (PET) offers low spatial resolution and is restricted to highlighting low pH regions32,33. Due to these limitations in current radiological methods for measuring tumor pHe, the development of new technologies is essential.
Chemical Exchange Saturation Transfer (CEST) is an advanced MRI technique that enables the indirect detection of low-concentration endogenous or exogenous metabolites containing exchangeable protons (e.g., –OH, –NH, or –NH₂ groups). The technique operates by applying a frequency-selective radiofrequency (RF) saturation pulse at the specific resonance frequency of these exchangeable protons. Through chemical exchange with bulk water protons, the saturation is transferred to the water pool, resulting in a reduction of the water signal that depends on the exchange rate and concentration of the solute protons. This frequency-dependent signal attenuation is captured by acquiring images across a range of saturation frequencies, yielding a Z-spectrum, which plots the normalized water signal with saturation (Ssat) relative to the signal without saturation (S₀) as a function of frequency offset. One way to quantify CEST contrast is by isolating the asymmetric CEST effect from symmetric magnetization transfer or direct water saturation, using the asymmetric magnetization transfer ratio (MTRasym):
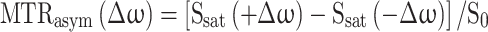 |
where Ssat(+ Δω) is the water signal with saturation pulse applied at frequency + Δω and S₀ is the water signal without saturation. Since proton exchange rates are influenced by pH, particularly in the intermediate exchange regime, CEST contrast is well-suited for non-invasive mapping of extracellular tumor pH (pHe), making it a valuable tool in cancer imaging and metabolic characterization34. Recent CEST MRI pH mapping has been applied to various pathologies, including renal injury35, stroke36, and cancer37–41, where pH disturbances are anticipated. Indeed, studies measuring extracellular tumor pH using exogenous contrast agent-dependent CEST have highlighted its superiority over other techniques, thanks to its signal amplification and concentration-independent pH measurement capabilities. However, its utility is constrained by the limited dynamic range of pH maps34,37. In the present study, leveraging the established link between hypoxia, glycolytic activity, and acidosis in tumors, we hypothesized that glucose-stimulated CEST MRI pHe mapping could enhance tumor differentiation based on their hypoxic status. Specifically, we tested this hypothesis using a 1-h pre-scan intraperitoneal glucose injection to increase the dynamic range of pHe mapping across three breast cancer mouse models: two with differential HIF-1α expression and one with upregulated VEGF expression. Results were compared with those from mice that did not receive glucose stimulation.
Results
In vitro characterization of MDA-MB-231 cells for hypoxia markers, HIF-1α and LDHA
As shown in Fig. 1, we first validated the genetically engineered MDA-MB-231 cells that stably expressed shRNA against HIF-1α, by comparing the expression of HIF-1α under both normoxia and hypoxia. In response to 4 h of hypoxia, 231-WT and 231-VEGF overexpressing cells showed robust HIF-1α expression that was not observed in HIF-1α silenced cells. Additionally, LDHA, a hypoxia-responsive HIF-regulated protein, increased in response to 4 h of hypoxia in 231-WT and 231-VEGF cells but not in 231-HIF-1α-shRNA cells. However, unlike in 231-WT and 231-VEGF, LDHA induction in response to hypoxia was not detected in 231-HIF-1α-shRNA cells suggesting the importance of HIF-1α in regulating the LDHA expression. These studies validated the effectiveness of shRNA against HIF-1α in 231-HIF-1α-shRNA cells in suppressing the HIF-1α-mediated response to hypoxia. Building on these findings, we conducted in vivo mouse studies using tumors derived from these cell lines.
Fig. 1.
Western blots showing HIF-1α and lactate dehydrogenase A (LDHA) from 231-WT, 231-HIF-1α-shRNA and 231-VEGF cells grown under normoxia (20% oxygen) or true hypoxia (1% oxygen) for 4 h. Actin immunoblotting in the bottom row was performed to show equal loading. Merged bright-field and chemiluminescence images of HIF-1α, LDHA and the corresponding actin immunoblots with molecular weight markers are presented in Figure S1 of Supplementary Information.
In vitro CEST MRI pH mapping
For our tumor studies, we used iopamidol, an FDA approved CT contrast agent which is well-suited for CEST MRI pH imaging due to the presence of two pools of exchangeable protons resonating at 4.3 and 5.5 ppm with the chemical structure shown in Fig. 2a. An in vitro pH characterization of our CEST MRI RARE sequence was carried out on iopamidol phantoms adjusted to pH values between 5.8 and 7.3 covering the range of values anticipated in tumors. MTRasym spectra (Fig. 2b) show the impact of pH change on CEST contrast with the 4.3 and 5.5 ppm peaks well-resolved across the pH range of interest using the saturation conditions we intended to apply. Signal differences for pH values between 7.3 and 5.8 were also readily discernible. We fit the ST vs. pH curves to first, second, and third order polynomials and compared the R2 and RMSE which were 0.92, 0.153; 0.94, 0.134; and 0.99, 0.062 for first, second and third order, respectively. Based on this, we concluded that the third order polynomial provides the best fit for these saturation parameters similar to previous studies35,42, with this fit shown in Fig. 2c. For details on CEST methodology, including the calculation of STratio and MTRasym, please refer to the Methodology section. The phantom pH maps generated using this third order polynomial calibration curve show an excellent correlation profile across the tubes in the phantom (Fig. 2d). Based on these data, we concluded the sequence was adequate and we could apply this calibration curve for calculating pH values in vivo.
Fig. 2.
(a) Chemical structure of iopamidol highlighting the amide groups resonating at 4.3 and 5.5 ppm; (b) MTRasym of iopamidol for a series of pH values with saturation frequency (B1) of 3.6 µT; (c) STratio vs pH calibration curve with third order polynomial fitting; (d) calculated pH maps in phantoms using the third order calibration curve.
In vivo CEST MRI pHe mapping
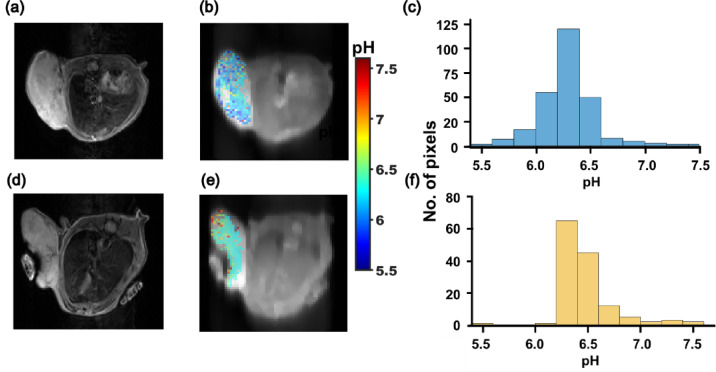
For in vivo studies, we first evaluated whether CEST MRI pH mapping would be sensitive to hypoxia. We acquired CEST MRI images in mice bearing orthotopic transplants of the 231-WT and 231-HIF-1α silenced cells shown in Fig. 1, after intravenous administration of iopamidol. The contrast is evident as an increase in MTRasym values following iopamidol administration compared to pre-administration values, as shown in the representative MTRasym spectra of WT and 231-HIF-1α silenced tumors (Fig. S2, Supplementary Information). As shown in Fig. 3, both tumor types exhibited marked extracellular acidification in their pHe maps, with mean pHe of 6.3 ± 0.04 (231-WT) and 6.5 ± 0.03 (231-HIF-1α-shRNA), and a significant difference (P = 0.003, n = 3) between the two groups. The representative histograms of tumor pixels in these pHe maps (Fig. 3c, f) clearly show the highest percentage of pixels was at pHe value 6.3 and were skewed toward lower pHe in 231-WT tumors, while the highest percentage of pixels was at pHe = 6.5, with the histogram skewed towards higher pHe in HIF-1α silenced tumors. This difference in pHe distribution was promising, although lower than ideal for clinical implementation.
Fig. 3.
Representative T2W images, pHe maps overlays and histograms of all tumor pixels in (a, b, c) 231-WT and (d, e, f) 231-HIF-1α-shRNA.
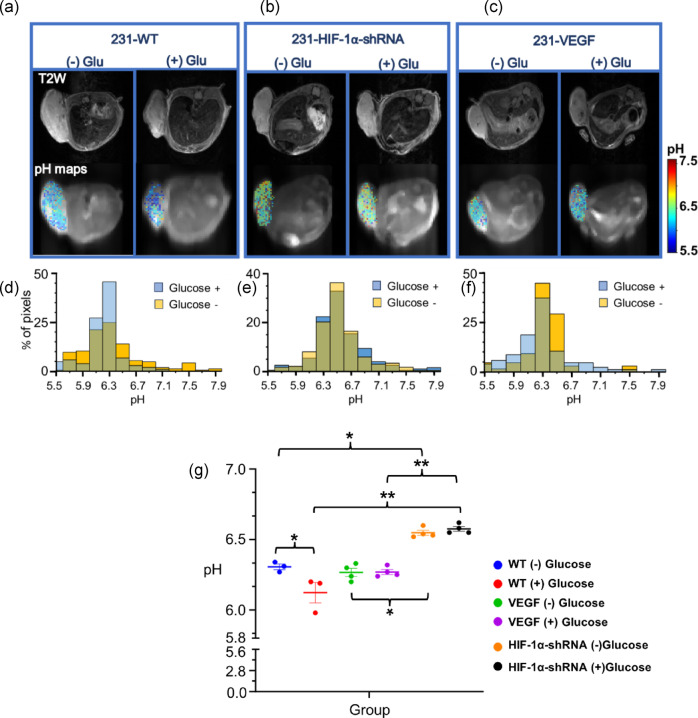
Next, we carried out experiments designed to stimulate tumor metabolism and evaluate its effect on pHe. As elevated glucose consumption is a hallmark of aggressive tumors, we administered 400 μl of 1 M glucose intraperitoneally 1 h prior to CEST MRI and obtained pHe maps to determine if changes in lactate production could further alter these maps. We also included a third tumor model developed with MDA-MB-231 cells overexpressing VEGF, as shown in Fig. 1, as upregulated VEGF expression is a common feature of aggressive tumors. Representative anatomical MR images of the tumors from each group are shown in the top row of Fig. 4, with the second row displaying the corresponding tumor pHe maps from 231-WT, 231-HIF-1α silenced, and 231-VEGF overexpressing before (left) and after glucose administration (right), respectively. As illustrated in Fig. 4a and d, the 231-WT group exhibited a detectable significant decrease in pHe by 0.2 units following glucose administration (P = 0.035, n = 3). In contrast, no changes in mean pHe were observed for the 231-HIF-1α silenced group (Fig. 4b, e) or the VEGF overexpressing group (Fig. 4c, f) post glucose stimulation. The histograms (Fig. 4d–f) further reveal that, prior to administration of glucose, pHe values were skewed toward higher pHe in HIF-1α silenced tumors, whereas the pHe values were skewed toward lower values in 231-WT and 231-VEGF tumors. However, with the glucose stimulation protocol, the mean pHe values for the three tumor models diverged and we were able to differentiate them, with mean pHe values for 231-WT, 231-VEGF and 231-HIF-1α-shRNA being 6.1 ± 0.12, 6.3 ± 0.04 and 6.58 ± 0.04, respectively (Fig. 4a–g). Tukey’s multiple comparison test confirmed that the mean pHe of 231-WT and 231-VEGF tumors was significantly lower (P = 0.002 and 0.007, respectively) than that of the 231-HIF-1α-shRNA group post glucose administration, and the difference between groups showed a lower P value (greater significance) using the glucose stimulation protocol. These findings indicate that glucose administration can enhance the differentiation of tumor types in CEST MRI pHe maps, facilitating better identification of tumors with high HIF-1α expression.
Fig. 4.
Representative T2W images, pHe maps and histograms overlay with and without glucose in (a, d) 231-WT tumor; (b, e) 231-HIF-1α-shRNA tumor and; (c, f) 231-VEGF tumor; (g) plot showing mean pHe values for all tumors bearing mice with and without glucose (Values represent Mean ± S.D., *P < 0.05, **P < 0.01, Tukey’s multiple comparisons test, n = 3 for 231-WT and n = 4 for 231-HIF-1α-shRNA and 231-VEGF).
Tumor perfusion by CEST MRI
Finally, we were interested in determining whether contrast perfusion can provide further differentiating information in these tumor models. The contrast perfusion status of different tumor models was estimated by determining the percentage of tumor pixels enhanced after contrast injection, based on ΔMTRasym (4.3 ppm), using post-20 min and pre-iopamidol administration images. Representative ΔMTRasym (4.3 ppm) maps show the lowest enhancement in 231-WT (Fig. 5a) followed by 231-HIF-1α silenced (Fig. 5b), and highest in 231-VEGF (Fig. 5c) tumors, with the mean percentage of pixels showing positive contrast being 33.7% ± 18, 42.6% ± 5.4 and 55.7% ± 14.8, respectively (Fig. 5d). While the differences observed were modest and not statistically significant, the overall trend was as expected.
Fig. 5.
ΔMTRasym difference maps showing tumor pixels with contrast enhancement at 4.3 ppm post 20 min of i.v. iopamidol administration in (a) 231-WT tumor; (b) 231-HIF-1α-shRNA tumor and; (c) 231-VEGF tumor; (d) plot showing percentage of pixels enhanced in all tumors (Values represent Mean ± S.D., n = 4).
LDHA expression by immunoblots and immunohistochemistry
To provide a molecular basis for our pHe findings with CEST MRI experiments, we performed immunoblot (Western blot) analysis of LDHA protein isolated from tumor tissues and additionally used IHC of formalin-fixed tumor tissues to spatially localize and quantify LDHA expression. Immunoblot data in Fig. 6a of LDHA expression confirmed our in vitro findings (Fig. 1). The quantified band intensities show that LDHA expression is highest in the 231-WT group, followed by 231-VEGF and 231-HIF-1α-shRNA. Statistical analysis revealed a significant decrease in LDHA expression in both the HIF-1α silenced, and VEGF overexpressing groups compared to WT (P < 0.05), while the difference between the HIF-1α silenced and VEGF overexpressing groups was not statistically significant as seen in Fig. 6b. These results were further confirmed by IHC, where LDHA was evident from brown staining around the cell nucleus, consistent with its role as a cytoplasmic protein, as shown in Fig. S4 of Supplementary Information. There was a noticeable decrease in LDHA expression in both viable and necrotic regions of 231-HIF-1α-shRNA tumors (Fig. S4b, d) compared to 231-WT, but it was not statistically significant, which could be attributed to outliers. Interestingly, spatial localization and quantification of LDHA signal from IHC revealed significantly lower expression in 231-VEGF tumors in both viable and necrotic regions (Fig. S4c, d) compared to 231-WT tumors (Fig. S4a, d). The difference between 231-HIF-1α-shRNA and 231-VEGF groups were non-significant as seen with western blot quantification data. These results corroborate our findings of lowest pHe in the 231-WT tumors and higher pHe in the 231-HIF-1α-shRNA tumors.
Fig. 6.
(a) Western blot displaying LDHA protein expression in 231-WT, 231-HIF-1α- shRNA and 231-VEGF tumors. GAPDH was used as a loading control. (b) Quantification of LDHA expression in tumor lysates. Bar graph showing relative LDHA protein expression levels in tumors derived from 231-WT, 231-HIF-1α-shRNA, and 231-VEGF cell lines. Protein expression was quantified from Western blot band intensities and normalized to loading control. (Values represent Mean ± S.E.M., *P < 0.05 and **P < 0.01, 231-WT: n = 9; 231-HIF-1α-shRNA: n = 7; 231-VEGF: n = 4). Merged bright-field and chemiluminescence images of LDHA and the corresponding GAPDH immunoblots are presented in Fig. S3 of Supplementary Information.
NMR metabolomics for tumor lactate quantification
To evaluate whether the metabolic profile, particularly lactate levels, vary with the differential expression of HIF-1α and aligns with the pHe observed in different tumor models, we performed NMR metabolomics. Twenty-five metabolites were identified and quantified based on relative NMR peak intensities: valine, isoleucine, beta-hydroxybutyrate (BHB), alanine, acetate, glutamate, succinate, glutamine, glutathione, choline, phosphocholine, total choline, glycerophosphocholine (GPC), taurine, glycine, aspartate, creatine, myoinositol, lactate, glucose, fumarate, tyrosine, histidine, phenylalanine, and formate. PCA plots with the first three components explaining the maximum variance (98%) as displayed in Figs. S5a and b show distinct clustering of 231-WT and 231-VEGF samples, with the 95% confidence regions marked in colour. However, the samples of 231-HIF-1α-shRNA group did not show a tight clustering, unlike the other two groups indicating greater metabolic variance among these samples.
A supervised multivariate analysis, PLS-DA (supplementary Fig. S5c), was subsequently used to identify the most discriminating metabolites among the three tumor models. Variable importance in projection (VIP) was chosen as criteria for selecting the most important variables of the PLS-DA model (Supplementary Fig. S5d). Since the principal axis 1 (PC 1) showed maximum variance of 72.4%, we used this axis to identify the most significantly contributing metabolites. The most important metabolites with a VIP score above 1 were lactate, total choline, taurine, GPC and glutathione. Lactate, with the highest VIP score of 4, was the most significant metabolite distinguishing the three tumor types, as shown in the VIP score plot. Given its contribution to extracellular acidification of tumors and its prominence in differentiating the tumors, we plotted lactate levels across tumors groups as box plots of NMR peak intensities. The 231-WT showed the highest lactate levels (0.017 ± 0.002 a.u.), followed by 231-HIF-1α-shRNA (0.014 ± 0.003 a.u.), and 231-VEGF (0.0113 ± 0.0016 a.u.). There was a trend towards reduced lactate levels in HIF-1α downregulated group compared to 231-WT, although this was not statistically significant. However, the difference between 231-VEGF and 231-WT was significant (P = 0.013). The difference between 231-HIF-1α-shRNA and 231-VEGF was also not statistically significant.
Discussion
In the present study, we investigated CEST MRI-based pH mapping as a tool to indirectly detect changes in HIF-1α expression in vivo using genetically engineered MDA-MB-231 breast cancer cells with stable downregulation of HIF-1α and compared these to wild-type variant. Acidosis is generally considered a passive outcome of the metabolic reprogramming resulting from the stabilization of HIF-1α, with transcriptional activation of target genes leading to accumulation of lactic acid and other metabolites in the extracellular space. As a result, acidosis has potential to indirectly reflect the hypoxic status of the tumors8. We used triple-negative breast tumor cells, which are aggressive and metastatic43. To the best of our knowledge this is the first study aimed at evaluating the effect of HIF-1α silencing on tumor pHe using CEST MRI. Furthermore, we tested whether stimulating tumor glycolysis through glucose administration would alter pHe maps to better differentiate the tumors. Our results demonstrated that 231-WT tumors expressing HIF-1α have a lower pHe (6.3 ± 0.04) than tumors with HIF-1α silenced (6.5 ± 0.03), and that this difference is enhanced after a 1-h pre-MRI glucose stimulation. The 1-h interval between intraperitoneal glucose administration and CEST MRI imaging was based on the known pharmacokinetics of glucose absorption and metabolism following i.p. injection44. Glucose typically reaches peak systemic concentration (Cmax) within 15–30 min after i.p. administration, and based on this we expect that 1 h is a sufficient time for tumor cells to take up glucose and metabolize it. This metabolic activity in tumors with active HIF-1α signalling leads to the accumulation of glycolytic byproducts, contributing to further extracellular acidification. This is supported by our observation of a greater pHe drop in 231-WT tumors compared to 231-HIF-1α-shRNA tumors following glucose administration. Furthermore, we acknowledge that optimizing the timing of glucose administration relative to imaging may further enhance the sensitivity of pHe detection, and this will be the subject of a future study. This low pHe, and a further drop in 231-WT tumors, is supported by increased expression of HIF-1α dependent LDHA and higher total lactate levels compared to HIF-1α downregulated tumors.
Similar tumor pHe mapping using CEST MRI has been previously reported. Pagel and colleagues observed acidosis with mean pHe values 6.71 ± 0.04 in MCF-7 breast tumors, 6.57 ± 0.06 for lymphomas45, and a range of 6.5 to 7.2 for MDA-MB-231 tumors38, which compare well with our measurements. It has also been demonstrated that pHe mapping by CEST MRI can be useful in monitoring responses to therapeutics such as mTOR inhibitors, metformin, proton pump inhibitors etc., with pHe differences around 0.1–0.4 units depending on the therapeutic46–49. Furthermore, this approach has been used to differentiate metastatic from benign tumors, with more aggressive breast tumors displaying more pronounced acidosis37. However, as Anemone et al. pointed out, it can be challenging to differentiate tumor models based solely on average pHe values. In their study, the use of the percentage of pixels with varying acidity scores improved differentiation. Our glucose stimulation approach could significantly enhance this, potentially improving the sensitivity of pHe mapping to detect therapeutic response. Additionally, this enhancement could further establish pHe mapping as a valuable surrogate for biopsies. While the numerical differences may appear modest, we emphasize that even small shifts in extracellular pH can reflect meaningful biological changes, particularly in the tumor microenvironment. Previous studies have demonstrated that such subtle pH differences can be physiologically and pathologically significant. For instance, Anemone et al. demonstrated that breast tumors of varying aggressiveness had mean pHe differences in the range of ~ 0.1 to 0.2 units, with highly invasive tumors like 4T1 and TS/A having lower pHe (6.79 ± 0.02 and 6.80 ± 0.03) compared to less aggressive TUBO and BALB-neuT tumors (6.84 ± 0.03 and 6.96 ± 0.03)37. Similarly, Stabinska et al. (2022) reported a statistically significant pH difference of only 0.12 units (6.67 ± 0.08 vs. 6.79 ± 0.11) between obstructed and contralateral healthy kidneys, as measured by CEST MRI, indicating that small pH shifts can capture important physiological alterations35.
While the foundational role of HIF-1α in orchestrating metabolic reprogramming leading to acidosis is well established, it is important to consider the interplay of multiple hypoxia-inducible factors. HIF-2α, for example, has been shown to play a complementary role to HIF-1α. While HIF-1α primarily drives the transcriptional response to hypoxia and promotes acidosis, HIF-2α facilitate adaptation to the acidic microenvironment, aiding tumor cells survival50,51. Therefore, silencing HIF-1α alone may result in a partial attenuation of acidosis-related effects, as some compensatory mechanisms via HIF-2α may persist, potentially explaining the modest pHe changes observed in our study.
Interestingly, no difference in mean pHe was observed in tumors overexpressing VEGF after glucose stimulation. VEGF, a downstream target of HIF-1α, promotes angiogenesis and plays a crucial role in tumor aggressiveness and metastasis. Several studies have proposed VEGF as a diagnostic marker and therapeutic target9,10. Although VEGF overexpressing tumors showed marked acidosis similar to 231-WT tumors, no change in pHe was observed after glucose administration. This may be due to an improved perfusion and leaky vasculature in VEGF overexpressing tumors, allowing rapid clearance of lactate and other glycolytic byproducts from the extravascular spaces, which is consistent with higher perfusion and significantly lower total lactate levels observed in these tumors. Additionally, faster acquisition schemes with improved detection sensitivity may be beneficial for detecting pHe changes post-glucose administration in these tumors. However, the presence of acidosis contradicts immunoblot and IHC findings which showed low LDHA expression, implying that other metabolic pathways contribute to acidification in these tumors. Acidosis is a complex phenomenon involving ATP hydrolysis, the pentose phosphate pathway, and serine metabolism alongside glycolysis. Tumors overexpressing VEGF are also metabolically different from 231-WT and 231-HIF-1α-shRNA tumors, as revealed by PCA plots from NMR data. Importantly, the relationship between LDHA expression and tumor acidosis is not strictly linear or directly dependent on extracellular pH. Although both VEGF and LDHA are downstream HIF-1α targets, their expressions do not always correlate. For instance, one study found that LDHA expression was associated with HIF-1α and tumor aggressiveness, but not with VEGF52. Similarly, another study reported that LDHA expression is independently regulated by both oxygen tension and extracellular pH. In their study, LDHA mRNA levels increased significantly with hypoxia and reduction in pHe from7.4 to 6.3 had minimal influence on LDHA transcription under hypoxia53. Furthermore, LDHA transcription is also responsive to intracellular lactate accumulation, which can regulate HIF-1α activity and LDHA expression54. In our study, enhanced perfusion in 231-VEGF tumors may have facilitated more efficient lactate clearance, reducing intracellular lactate buildup and hence lower LDHA expression, despite similar extracellular pH. All these factors could play a role in contradictory results observed in VEGF overexpressing tumors.
We also assessed the impact of differential HIF-1α expression on tumor vascularity to further understand the results observed. Iopamidol, a small molecule contrast agent that leaks into extravascular spaces, is well suited for measuring tumor perfusion and has shown comparable performance to gadolinium-based agents55. The contrast uptake was below 100% for all types of tumors, suggesting that pHe measurements represents only a portion of the tumor, which is heterogenous. 231-WT tumors with high HIF-1α levels showed the lowest contrast uptake, indicating more hypoxic, poor perfused regions as compared to ones lacking HIF-1α. VEGF overexpressing tumors showed the highest perfusion indicating enhanced vascularisation. These findings support the idea that lack of pHe change post-glucose in 231-VEGF tumors might be due to rapid clearance or dilution of acidic metabolites. Indeed, contrast agent perfusion is known to be limited in hypoxic regions, potentially underestimating the extent of pHe changes in such areas. In wild-type tumors, persistent HIF-1α expression drives aberrant, leaky, and disorganized vasculature, contributing to inefficient perfusion and hypoxia. In contrast, silencing HIF-1α has been associated with a more normalized vasculature, characterized by reduced vascular permeability, lower vessel density, and improved perfusion. Additionally, HIF-1α deletion can modulate tumor metabolism, reducing glycolytic flux and lactate production, which in turn may mitigate downstream drivers of acidosis and hypoxia, indirectly supporting better perfusion. Although we observed a trend toward higher perfusion in the HIF-1αsilenced tumors compared to wild-type, this difference was not statistically significant, possibly due to compensatory upregulation of HIF-2α, inter-tumoral variability, or suboptimal scan timing. Future studies with increased sample size may clarify this.
Hypoxia and HIF-1α have potential as biomarkers for tumor subtyping and predicting metastasis. In fact, a meta-analysis by The Cancer Genome Atlas Network found increased expression of HIF-1α target genes in basal breast cancer, an aggressive and resistant subtype56. Similarly, high HIF-1α levels correlate with progression, lymph node metastasis, vascular invasion, and VEGF expression in gastric cancer57. Currently, hypoxia or HIF-1α signature is assessed via tumor biopsy which is invasive and not definitive. We investigated the possibility of using pHe mapping by CEST MRI as a non-invasive surrogate for measuring HIF-1α status. Although differences in pHe were observed based on HIF-1α levels, further investigations are needed for direct correlation given the complexity of tumor physiology. Pixelwise analyses of pHe maps based on varying acidity levels and their correlation with HIF-1α expression in immunohistochemistry can improve the accuracy by accounting for heterogeneity. Nevertheless, given the role of hypoxia in cancer progression and increased sensitivity of our method combined with spatiotemporal resolution, glucose stimulated pHe mapping could be a valuable tool for radiogenic tumor characterization. In the same vein, pH maps could be utilized to identify aggressive regions in heterogenous tumors, potentially assisting in directing image-guided biopsy and tumor ablation.
In conclusion, we have demonstrated that 231-WT tumors with high HIF-1α expression are more acidic and less perfused, with pHe responding to glycolytic stimulus, compared to HIF-1α silenced tumors. Our study reinforces that tumor pHe is closely linked to hypoxia. CEST MRI-based pHe mapping could serve as a non-invasive method to assess HIF-1α expression, offering clinical utility when biopsies are not feasible. However further investigation and validation is required for broader application.
Methods
Materials and reagents
Unless otherwise noted, all compounds, chemicals and solvents were purchased from Sigma-Aldrich (St. Louis, MO, USA) or Bracco Imaging (Colleretto Giacossa, Italy).
Phantom preparation for in vitro CEST MRI
Iopamidol phantoms at 25 mM concentration were prepared in phosphate buffered saline (PBS) to measure the CEST MR contrast at different pH values. The solutions were titrated to achieve pH values 5.8, 6.1, 6.3, 6.5, 6.7, 7.0 and 7.3 as measured by pH electrode.
Cell lines and tumors
Cloning and generation of MDA-MB-231 cells stably expressing shRNA against HIF-1α or VEGF using lentiviral transduction were performed as previously described58–60. All cell lines were grown in RPMI 1640 medium (Mediatech, Manassas, VA, USA) supplemented with 10% fetal bovine serum (FBS), and maintained at 37 °C in a CO2 incubator. The cells were evaluated for HIF-1α downregulation and lactate dehydrogenase A (LDHA) in response to true hypoxia condition by incubating them in hypoxic chambers and validating their expression by immunoblotting (details mentioned under ‘Protein Isolation, Immunoblotting and Immunohistochemistry section) before inoculation into animals. Tumors were obtained by inoculating 2 × 106 MDA-MB-231 wild-type (231-WT) or MDA-MB-231 HIF-1α shRNA (231-HIF-1α-shRNA) or MDA-MB-231 VEGF (231-VEGF) cells in the second right side mammary fat pad of 4–6 weeks old female severe combined immunodeficient (SCID) mice. Tumors were imaged at volumes of 200–300 mm3 reached within 6–8 weeks following inoculation. At the end of the MR imaging studies, tumors were harvested and partly fixed in formalin for histology and with the remaining tissue processed for molecular and metabolic studies. All animal studies were done in compliance with a protocol approved by the Animal Care and Use Committee of the Johns Hopkins University School of Medicine with all methods performed in accordance with the relevant guidelines and regulations. These studies are reported in accordance with ARRIVE guidelines.
Protein isolation, immunoblotting and immunohistochemistry
Protein isolation and immunoblotting was performed as previously described61. Briefly, total protein from the cells was obtained by lysing the cells with radioimmunoprecipitation assay buffer (RIPA) supplemented with protease inhibitors. In the case of tumor tissue, 30–40 mg of snap-frozen and homogenized tissue was resuspended in 500 μl of RIPA. Resuspended tissue was sonicated and incubated at 4 °C on ice. Further, cell and tumor tissue-derived total protein were quantified. About 60 μg of protein, either from cells or tumor tissue was resolved on 7–15% gradient-polyacrylamide gel. Separated proteins on the gel was transferred to a nitrocellulose membrane. Following the transfer of proteins, the membrane was blocked with 5% non-fat milk for 2 h and incubated with specific antibodies overnight. For HIF-1α and LDHA detection, a mouse monoclonal anti-HIF-1α antibody (BD-Bioscience, 1:1000 dilution) and a rabbit polyclonal antibody to detect LDHA (GeneTex, Irvine CA, 1:5000 dilution). A mouse monoclonal antibody against actin or GAPDH (SIGMA, St. Louis, MO, 1:50,000 dilution) was used as a loading control. Horseradish peroxidase-conjugated secondary antibodies were used at 1:2000 dilution. Blots were visualized using the SuperSignal West Pico Chemiluminescent substrate kit (Thermo Scientific, Rockford, IL, USA). Quantification of western blot bands was done by ImageJ software (version 1.53 m, National Institutes of Health, USA; https://imagej.nih.gov/ij/). A region of interest (ROI) of consistent size was manually drawn over each LDHA band to measure its intensity. A similar-sized ROI was also drawn in an area of the blot devoid of any bands to estimate background intensity. The background-subtracted intensity for each LDHA band was then calculated. This procedure was repeated for the corresponding GAPDH loading control bands using the same ROI dimensions and background subtraction approach. For each sample, the normalized band intensity was obtained by calculating the ratio of the background-subtracted LDHA signal to the background-subtracted GAPDH signal. The mean normalized intensity for each experimental group was then calculated and plotted for comparison.
For localization of LDHA by IHC, formalin-fixed, paraffin- embedded tumor tissues were sectioned at 5 µm thickness and deparaffinized followed by antigen retrieval performed by boiling the slides immersed in citrate buffer (pH 6.0) for 20 min in the steamer. Tissue sections underwent typical blocking steps to quench peroxidase, alkaline phosphatase activities, endogenous biotin and avidin, and for non-specific binding of proteins before addition of the primary antibody. A rabbit polyclonal antibody against LDHA (1:250 dilution, Genetex, cat.no 101416) was used. Sections were incubated with the antibody overnight at 4 °C. The following day, sections were incubated with horseradish peroxidase conjugated with anti-rabbit IgG (VECTASTAIN rabbit kit, PK-4001) and ABC reagent (VECTASTAIN) following manufacturer’s recommendation. Finally, slides were stained with 3,3′-diaminobenzidine (DAB) and counterstained with hematoxylin. High-resolution digital scans of the stained sections were obtained using ScanScope (Aperio, Vista, CA). Quantification was performed with the ImageScope software using the Positive Pixel Count V9 algorithm supplied by the manufacturer. The number of strongly positive pixels (NSP) was normalized to the area of the section in µm2. Analyses were performed using the entire histological section, with entire viable and necrotic regions in the section.
CEST MR imaging
For in vitro CEST experiments, iopamidol phantoms prepared at 25 mM concentration and adjusted to pH values 5.8, 6.1, 6.4, 6.7, 7.0, 7.3 were imaged at 11.7 T Bruker vertical bore spectrometer (Bruker Biospin GmbH, Ettlingen, Germany) using a modified single-shot rapid acquisition with refocusing echoes (RARE) sequence (TR = 6500 ms, effective TE = 3.49 s, RARE factor = 33, centric encoding, slice thickness = 1 mm, FOV = 30 × 16 mm, matrix size = 64 × 64, No. of averages = 1). The CEST saturation module was continuous wave (CW) irradiation with B1 = 6 µT and saturation time = 4 s. We incremented this over 61 frequency offsets evenly distributed from -8 ppm to 8 ppm (step size = 133 Hz) around the water resonance (0 ppm) as we have employed previously35. Temperature was set to 310 K and maintained throughout the experiments using a warm-air feedback system.
The in vivo CEST MRI experiments were conducted on an 11.7 T Bruker horizontal bore animal scanner (Bruker Biospin GmbH, Ettlingen, Germany) using an eight-channel mouse body phased-array receive coil. A 31-gauge needle catheter was placed into a lateral tail vein of each mouse for intravenous injection before placing them into scanner. The animals were positioned on an imaging cradle and anesthetized with 0.5–2% isoflurane. The respiratory rate was continuously monitored, and the mouse body temperature was maintained at 37 °C using a water-heated animal bed. The tumor was located by collecting twenty-one axial slices using a multi-slice T2W RARE sequence with the following parameters: TE/TR: 66/4000 ms, matrix size: 128 × 128 and RARE factor: 8. A single axial slice of 1.5 mm thickness across the centre of tumor was selected for CEST imaging. To obtain B0 inhomogeneity maps, the water saturation shift referencing (WASSR) approach was used62 with images collected at 42 frequency offsets between − 1.5 and 1.5 ppm using 10 saturation pulses with a duration of 300 ms, an inter-pulse delay of 10 μs and B1 = 1.2 μT. CEST images were acquired using a single-shot RARE sequence with centric encoding, parameters: TR/TE = 6.5 s/3.5 ms preceded by a B1 = 3.6 µT, Tsat = 4 s saturation pulse and a fat-suppression module. A series of 73 frequency offsets were acquired in the range of ± 7.5 ppm relative to water peak. We used an acquisition matrix of 64 × 64 for a field of view of 3.2 × 2.0 cm2 with a slice thickness = 1.5 mm with the acquisition time for each Z-spectrum being 8 min. The CEST acquisition was repeated nine times, once before and eight times after intravenous injection of iopamidol contrast agent (dose = 4 g iodine/kg bodyweight). Total scan time was 64 min. To assess the effect of glucose stimulation on tumor pH, all mice were re-imaged 24 h after the initial scan to ensure clearance of any residual contrast agent. A 400 μL dose of 1 M glucose solution in saline was administered intraperitoneally 1 h prior to image acquisition. This interval allowed sufficient time for systemic absorption, metabolic processing, and potential alterations in the tumor microenvironment.
CEST-MRI data analysis and pH mapping
Image post-processing and CEST data analysis were performed using custom-written MATLAB (Mathworks, Natick, MA, USA) scripts. The processing was based on calculating the saturation transfer (ST) ratio at 4.3 and 5.5 ppm as described previously35. Pixel-by-pixel Z-spectra were calculated from the CEST data by plotting the water signal after saturation (Sz) normalized by the signal without saturation (S0) as a function of saturation frequency, Δω. Pixelwise B0 inhomogeneity corrections were performed using the spline interpolation method by shifting the interpolated Z-spectrum to adjust the bulk water resonance to zero frequency. Magnetization ratio (MTR) was calculated from each Z spectra using MTR = 1–Sz/S0 followed by calculation of MTRasym by subtracting the MTR values at positive frequency offsets (Δω) from the MTR values at negative frequency offsets (− Δω) with respect to water frequency through:
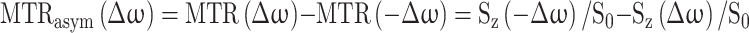 |
The ratio of CEST contrast at two different frequencies of interest (i.e. 4.3 and 5.5 ppm) is termed the saturation transfer ratio (STratio) which is quantified pixel by pixel through the ratio of MTRasym using:
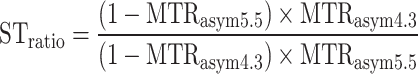 |
A region of interest (ROI) was drawn, and the mean STratio versus pH plot was obtained and fit to a polynomial function to generate a pH calibration curve. Using this calibration equation, the pH for each pixel in the phantom was then calculated.
For in vivo data, the Z spectra were first normalized and B0 corrected. The post-injection MTRasym at 4.3 and 5.5 ppm was quantified for each CEST acquisitions for pHe mapping. Pre-injection MTRasym maps were then subtracted from the post-injection MTRasym images to remove endogenous CEST signals and obtain ΔMTRasym at each frequency followed by calculation of STratio using
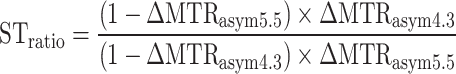 |
The pHe value for each voxel was determined using the calibration curve between ratiometric values of the two frequencies and pH mentioned above. To obtain a mean pHe for the tumor ROI of each mouse, we averaged the pHe values obtained for all the post-contrast time points.
For determining perfusion, MTRasym (4.3 ppm) was calculated followed by subtraction of pre injection from 20 min post-injection MTRasym and depicted as ΔMTRasym above the threshold (1%) in all pixels of the manually defined tumor region of interest (ROI). The average ΔMTRasym % of all pixels was used as measure of amount of contrast perfusion in tumor region.
NMR metabolomics for tumor lactate quantification
The water-soluble fraction was extracted from tumor tissue using a dual-phase extraction method63. Briefly, the homogenized tumor was mixed with 4 mL of ice-cold methanol and vigorously vortexed. After keeping samples on ice for 10 min, 4 mL of chloroform were added, vortexed vigorously and kept on ice for an additional 10 min. Ultrasonication under ice-cold conditions was performed for 5 min with a 1-s pulse interval to make sure all the tissues dissolved completely. Finally, 4 mL of water was added to samples followed by vortex again. All procedures were performed on ice and samples were stored at 4 °C overnight for phase separation and then centrifuged at 15,000×g at 4 °C for 10 min. The top layer which is also the aqueous phase containing water-soluble metabolites was collected. Methanol in the aqueous phase was then evaporated under nitrogen gas, and any water remaining in the aqueous phase was lyophilized. Dried aqueous phase extracts were re-suspended in 0.6 mL deuterated water (D2O) for NMR. TSP (3-(trimethylsilyl) propionic 2,2,3,3-d4 acid sodium salt) dissolved in D2O was used as an internal standard.
Fully relaxed and water supressed one dimensional 1H NMR spectra of aqueous phase extracts were acquired on a Bruker Avance 750 spectrometer (Bruker BioSpin Corp.) equipped with 5 mm probe using following parameters: a spectral width of 15,495.86 Hz, an acquisition time of 2.11 s, a relaxation delay (RD) of 10.0 s, a scan accumulation of 32 times, 90° flip angle, 8 dummy scans, and data points of 64 K. Spectral processing (phase and baseline correction and, referenced to singlet peak of TSP at δ 0.00) and quantification were performed using TOPSPIN 3.5 software. Characterization of the metabolites was performed based on previous literature data and libraries like Human Metabolome Database (HMDB) and Biological Magnetic Resonance Data Bank (BMRB)64,65. For quantitative analysis of metabolites, integrals of resonances were determined and normalized to tumor weight and compared to the TSP standard to obtain relative concentrations in arbitrary units (A.U.).
Multivariate analysis including principal component analysis (PCA), partial least squares-discriminate analysis (PLS-DA), and variable importance in projection scores (VIP) was conducting for targeted metabolomics to visualize the clustering between samples using MetaboAnalyst software (version 6.0). Metabolites with VIP values higher than 1.00 were considered significant.
Statistical analysis
Unpaired student’s t-test was used for calculating statistical significance of protein data, lactate levels, pHe values from first round of experiments with only two groups and MR perfusion data. ANOVA analysis coupled with Tukey’s post-hoc correction was performed to analyse CEST MR pHe data and western blot quantification data using GraphPad Prism 7 software (GraphPad Inc., San Diego, CA, USA) to determine statistically significant differences between the measurements of pHe values among the three groups of breast cancers under different conditions explored. For all tests, a P value < 0.05 was considered statistically significant.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by NIH grants R01EB030376, R01CA82337, R50CA243562 and P41EB024495.
Author contributions
A.S. performed all the MRI experiments, data processing, tumor characterizations and prepared all the figures. J.S. assisted with the MRI data processing, B.K. developed the cell lines, assisted with tumor inoculation and characterizations. A.S., Z.B. and M.T.M. designed the study, interpreted the data and drafted the manuscript. F.S., S.N., and J.W.M.B. assisted in manuscript editing. All authors reviewed the manuscript.
Funding
This work was supported by NIH grants R01CA285792A1, R01EB030376, R01CA082337, R50CA243562 and P41EB024495.
Data availability
The MRI datasets generated during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vaupel, P. & Mayer, A. Hypoxia in cancer: Significance and impact on clinical outcome. Cancer Metastasis Rev.26, 225–239. 10.1007/s10555-007-9055-1 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Nordsmark, M. et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother. Oncol.77, 18–24. 10.1016/j.radonc.2005.06.038 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Hockel, M. et al. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res.56, 4509–4515 (1996). [PubMed] [Google Scholar]
- 4.Nakazawa, M. S., Keith, B. & Simon, M. C. Oxygen availability and metabolic adaptations. Nat. Rev. Cancer16, 663–673. 10.1038/nrc.2016.84 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corbet, C. & Feron, O. Tumour acidosis: From the passenger to the driver’s seat. Nat. Rev. Cancer17, 577–593. 10.1038/nrc.2017.77 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Semenza, G. L. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene29, 625–634. 10.1038/onc.2009.441 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warburg, O. On respiratory impairment in cancer cells. Science124, 269–270 (1956). [PubMed] [Google Scholar]
- 8.Chiche, J., Brahimi-Horn, M. C. & Pouysségur, J. Tumour hypoxia induces a metabolic shift causing acidosis: A common feature in cancer. J. Cell Mol. Med.14, 771–794. 10.1111/j.1582-4934.2009.00994.x (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang, J. S., Gillies, R. D. & Gatenby, R. A. Adaptation to hypoxia and acidosis in carcinogenesis and tumor progression. Semin. Cancer Biol.18, 330–337. 10.1016/j.semcancer.2008.03.011 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukumura, D. et al. Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in Vivo1. Can. Res.61, 6020–6024 (2001). [PubMed] [Google Scholar]
- 11.Nordsmark, M., Bentzen, S. M. & Overgaard, J. Measurement of human tumour oxygenation status by a polarographic needle electrode. An analysis of inter- and intratumour heterogeneity. Acta Oncol.33, 383–389. 10.3109/02841869409098433 (1994). [DOI] [PubMed] [Google Scholar]
- 12.Varia, M. A. et al. Pimonidazole: A novel hypoxia marker for complementary study of tumor hypoxia and cell proliferation in cervical carcinoma. Gynecol. Oncol.71, 270–277. 10.1006/gyno.1998.5163 (1998). [DOI] [PubMed] [Google Scholar]
- 13.Tsai, Y. T. et al. Real-time noninvasive monitoring of in vivo inflammatory responses using a pH ratiometric fluorescence imaging probe. Adv. Healthc. Mater.3, 221–229. 10.1002/adhm.201200365 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Wang, L. et al. Evaluating tumor metastatic potential by imaging intratumoral acidosis via pH-activatable near-infrared fluorescent probe. Int. J. Cancer136, E107-116. 10.1002/ijc.29153 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Chen, Q. et al. A self-assembled albumin-based nanoprobe for in vivo ratiometric photoacoustic pH imaging. Adv. Mater.27, 6820–6827. 10.1002/adma.201503194 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Miao, Q., Lyu, Y., Ding, D. & Pu, K. Semiconducting oligomer nanoparticles as an activatable photoacoustic probe with amplified brightness for in vivo imaging of pH. Adv. Mater.28, 3662–3668. 10.1002/adma.201505681 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Rasey, J. S., Martin, G. V. & Krohn, K. A. Imaging of Hypoxia: Tracer Developments 85–117 (Springer, 1999). [Google Scholar]
- 18.Zanzonico, P. et al. Iodine-124-labeled iodo-azomycin-galactoside imaging of tumor hypoxia in mice with serial microPET scanning. Eur. J. Nucl. Med. Mol. Imaging31, 117–128 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Koch, C. J. & Evans, S. M. Non-invasive PET and SPECT imaging of tissue hypoxia using isotopically labeled 2-nitroimidazoles. In Oxygen Transport To Tissue XXIII: Oxygen Measurements in the 21st Century: Basic Techniques and Clinical Relevance 285–292 (2003). [DOI] [PubMed]
- 20.Rickard, A. G. et al. Evaluating tumor hypoxia radiosensitization via electron paramagnetic resonance oxygen imaging (EPROI). Mol. Imaging Biol.26, 435–447. 10.1007/s11307-023-01855-0 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Connor, J. P. et al. Oxygen-enhanced MRI accurately identifies, quantifies, and maps tumor hypoxia in preclinical cancer models. Can. Res.76, 787–795 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mason, R. P., Shukla, H. & Antich, P. P. In vivo oxygen tension and temperature: simultaneous determination using 19F NMR spectroscopy of perfluorocarbon. Magn. Reson. Med.29, 296–302 (1993). [DOI] [PubMed] [Google Scholar]
- 23.Krishnamachary, B., Danhier, P., Kakkad, S., Bharti, S. K. & Bhujwalla, Z. M. Hypoxia-induced reporter genes with different half-lives. Methods Mol. Biol.1790, 113–125. 10.1007/978-1-4939-7860-1_9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danhier, P. et al. Combining optical reporter proteins with different half-lives to detect temporal evolution of hypoxia and reoxygenation in tumors. Neoplasia17, 871–881. 10.1016/j.neo.2015.11.007 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brender, J. R., Saida, Y., Devasahayam, N., Krishna, M. C. & Kishimoto, S. Hypoxia imaging as a guide for hypoxia-modulated and hypoxia-activated therapy. Antioxid. Redox Signal.36, 144–159. 10.1089/ars.2021.0176 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang, X., Lin, Y. & Gillies, R. J. Tumor pH and its measurement. J. Nucl. Med.51, 1167–1170 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estrella, V. et al. Acidity generated by the tumor microenvironment drives local invasion. Can. Res.73, 1524–1535 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anemone, A., Consolino, L., Arena, F., Capozza, M. & Longo, D. L. Imaging tumor acidosis: A survey of the available techniques for mapping in vivo tumor pH. Cancer Metastasis Rev.38, 25–49. 10.1007/s10555-019-09782-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillies, R. J., Liu, Z. & Bhujwalla, Z. 31P-MRS measurements of extracellular pH of tumors using 3-aminopropylphosphonate. Am. J. Physiol.267, C195-203. 10.1152/ajpcell.1994.267.1.C195 (1994). [DOI] [PubMed] [Google Scholar]
- 30.Huang, X. et al. Multi-chromatic pH-activatable 19F-MRI nanoprobes with binary ON/OFF pH transitions and chemical-shift barcodes. Angewandte Chemie (Int. Ed. Engl.)52, 8074–8078. 10.1002/anie.201301135 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.García-Martín, M. L. et al. Mapping extracellular pH in rat brain gliomas in vivo by 1H magnetic resonance spectroscopic imaging: Comparison with maps of metabolites. Cancer Res.61, 6524–6531 (2001). [PubMed] [Google Scholar]
- 32.Vāvere, A. L. et al. A novel technology for the imaging of acidic prostate tumors by positron emission tomography. Cancer Res.69, 4510–4516. 10.1158/0008-5472.can-08-3781 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flavell, R. R. et al. Caged [(18)F]FDG glycosylamines for imaging acidic tumor microenvironments using positron emission tomography. Bioconjug. Chem.27, 170–178. 10.1021/acs.bioconjchem.5b00584 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMahon, M. T., Gilad, A., Bulte, J. W. M. & van Zijl, P. C. M. Chemical Exchange Saturation Transfer Imaging: Advances and Applications (Pan Stanford Publishing Pte. Ltd., 2017). [Google Scholar]
- 35.Stabinska, J. et al. Noninvasive assessment of renal dynamics and pH in a unilateral ureter obstruction model using DCE MR-CEST urography. Magn. Reson. Med.89, 343–355 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun, P. Z., Cheung, J. S., Wang, E. & Lo, E. H. Association between pH-weighted endogenous amide proton chemical exchange saturation transfer MRI and tissue lactic acidosis during acute ischemic stroke. J. Cereb. Blood Flow Metab.31, 1743–1750. 10.1038/jcbfm.2011.23 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anemone, A. et al. Tumour acidosis evaluated in vivo by MRI-CEST pH imaging reveals breast cancer metastatic potential. Br. J. Cancer124, 207–216. 10.1038/s41416-020-01173-0 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen, L. Q. et al. Evaluations of extracellular pH within in vivo tumors using acidoCEST MRI. Magn. Reson. Med.72, 1408–1417. 10.1002/mrm.25053 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen, L. Q. et al. Evaluations of tumor acidosis within in vivo tumor models using parametric maps generated with Acido CEST MRI. Mol. Imag. Biol.17, 488–496. 10.1007/s11307-014-0816-2 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang, Y. et al. Noninvasive detection of extracellular pH in human benign and malignant liver tumors using CEST MRI. Front. Oncol.10, 578985. 10.3389/fonc.2020.578985 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jardim-Perassi, B. V. et al. Intraperitoneal delivery of iopamidol to assess extracellular pH of orthotopic pancreatic tumor model by CEST-MRI. Contrast Media Mol. Imaging2023, 1944970. 10.1155/2023/1944970 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pavuluri, K. et al. Noninvasive monitoring of chronic kidney disease using pH and perfusion imaging. Sci. Adv.5, eaaw8357 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arroyo-Crespo, J. J. et al. Characterization of triple-negative breast cancer preclinical models provides functional evidence of metastatic progression. Int. J. Cancer145, 2267–2281. 10.1002/ijc.32270 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker-Samuel, S. et al. In vivo imaging of glucose uptake and metabolism in tumors. Nat. Med.19, 1067–1072. 10.1038/nm.3252 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moon, B. F. et al. A comparison of iopromide and iopamidol, two acidoCEST MRI contrast media that measure tumor extracellular pH. Contrast Media Mol. Imaging10, 446–455. 10.1002/cmmi.1647 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akhenblit, P. J. et al. Assessing metabolic changes in response to mTOR inhibition in a mantle cell lymphoma xenograft model using AcidoCEST MRI. Mol. Imaging10.1177/1536012116645439 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Irrera, P. et al. In vivo MRI-CEST tumor pH imaging detects resistance to proton pump inhibitors in human prostate cancer murine models. Cancers14, 4916 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McVicar, N., Li, A. X., Meakin, S. O. & Bartha, R. Imaging chemical exchange saturation transfer (CEST) effects following tumor-selective acidification using lonidamine. NMR Biomed.28, 566–575. 10.1002/nbm.3287 (2015). [DOI] [PubMed] [Google Scholar]
- 49.Anemone, A. et al. In vivo evaluation of tumour acidosis for assessing the early metabolic response and onset of resistance to dichloroacetate by using magnetic resonance pH imaging. Int. J. Oncol.51, 498–506. 10.3892/ijo.2017.4029 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Davis, L. et al. Targeting HIF-2α in the tumor microenvironment: Redefining the role of HIF-2α for solid cancer therapy. Cancers14, 1259 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moreno Roig, E. et al. HIF-1α and HIF-2α differently regulate the radiation sensitivity of NSCLC cells. Cells8, 45 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koukourakis, M. I., Giatromanolaki, A., Sivridis, E., Gatter, K. C. & Harris, A. L. Pyruvate dehydrogenase and pyruvate dehydrogenase kinase expression in non small cell lung cancer and tumor-associated stroma. Neoplasia7, 1–6. 10.1593/neo.04373 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sørensen, B. S. et al. Influence of oxygen concentration and pH on expression of hypoxia induced genes. Radiother. Oncol.76, 187–193. 10.1016/j.radonc.2005.06.037 (2005). [DOI] [PubMed] [Google Scholar]
- 54.Valvona, C. J., Fillmore, H. L., Nunn, P. B. & Pilkington, G. J. The regulation and function of lactate dehydrogenase A: therapeutic potential in brain Tumor. Brain Pathol.26, 3–17. 10.1111/bpa.12299 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anemone, A., Consolino, L. & Longo, D. L. MRI-CEST assessment of tumour perfusion using X-ray iodinated agents: Comparison with a conventional Gd-based agent. Eur. Radiol.27, 2170–2179. 10.1007/s00330-016-4552-7 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Comprehensive molecular portraits of human breast tumours. Nature490, 61–70. 10.1038/nature11412 (2012). [DOI] [PMC free article] [PubMed]
- 57.Nam, S. & Lee, Y. HIF1A protein expression is correlated with clinical features in gastric cancer: An updated systematic review and meta-analysis. Sci. Rep.14, 13736. 10.1038/s41598-024-63019-6 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shah, T. et al. HIF isoforms have divergent effects on invasion, metastasis, metabolism and formation of lipid droplets. Oncotarget6, 28104–28119. 10.18632/oncotarget.4612 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krishnamachary, B. et al. Hypoxia regulates CD44 and its variant isoforms through HIF-1α in triple negative breast cancer. PLoS ONE7, e44078. 10.1371/journal.pone.0044078 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pathak, A. P. et al. In vivo “MRI phenotyping” reveals changes in extracellular matrix transport and vascularization that mediate VEGF-driven increase in breast cancer metastasis. PLoS ONE8, e63146. 10.1371/journal.pone.0063146 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goggins, E. et al. Hypoxia inducible factors modify collagen I fibers in MDA-MB-231 triple negative breast cancer xenografts. Neoplasia20, 131–139. 10.1016/j.neo.2017.11.010 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim, M., Gillen, J., Landman, B. A., Zhou, J. & Van Zijl, P. C. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn. Reson. Med.61, 1441–1450 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Glunde, K., Raman, V., Mori, N. & Bhujwalla, Z. M. RNA interference-mediated choline kinase suppression in breast cancer cells induces differentiation and reduces proliferation. Cancer Res.65, 11034–11043. 10.1158/0008-5472.can-05-1807 (2005). [DOI] [PubMed] [Google Scholar]
- 64.Wishart, D. S. et al. HMDB: the Human Metabolome Database. Nucleic Acids Res.35, D521-526. 10.1093/nar/gkl923 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ulrich, E. L. et al. BioMagResBank. Nucleic Acids Res.36, D402-408. 10.1093/nar/gkm957 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The MRI datasets generated during the current study are available from the corresponding author on reasonable request.