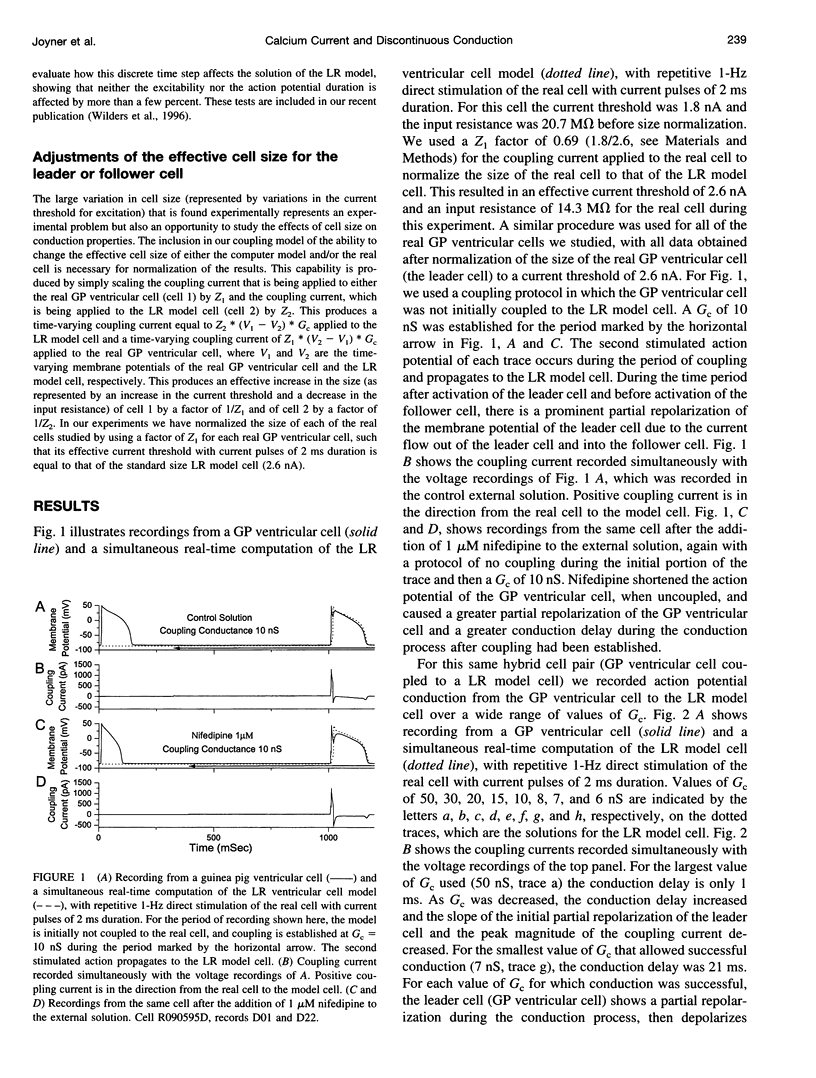
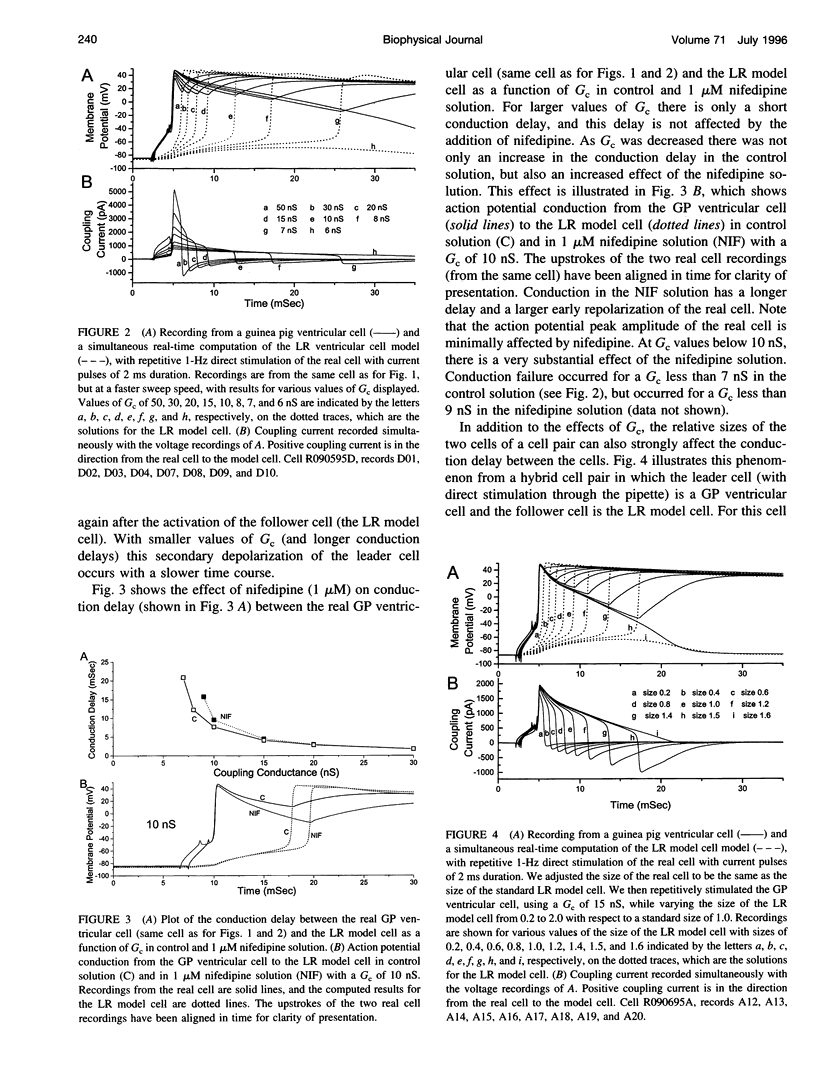
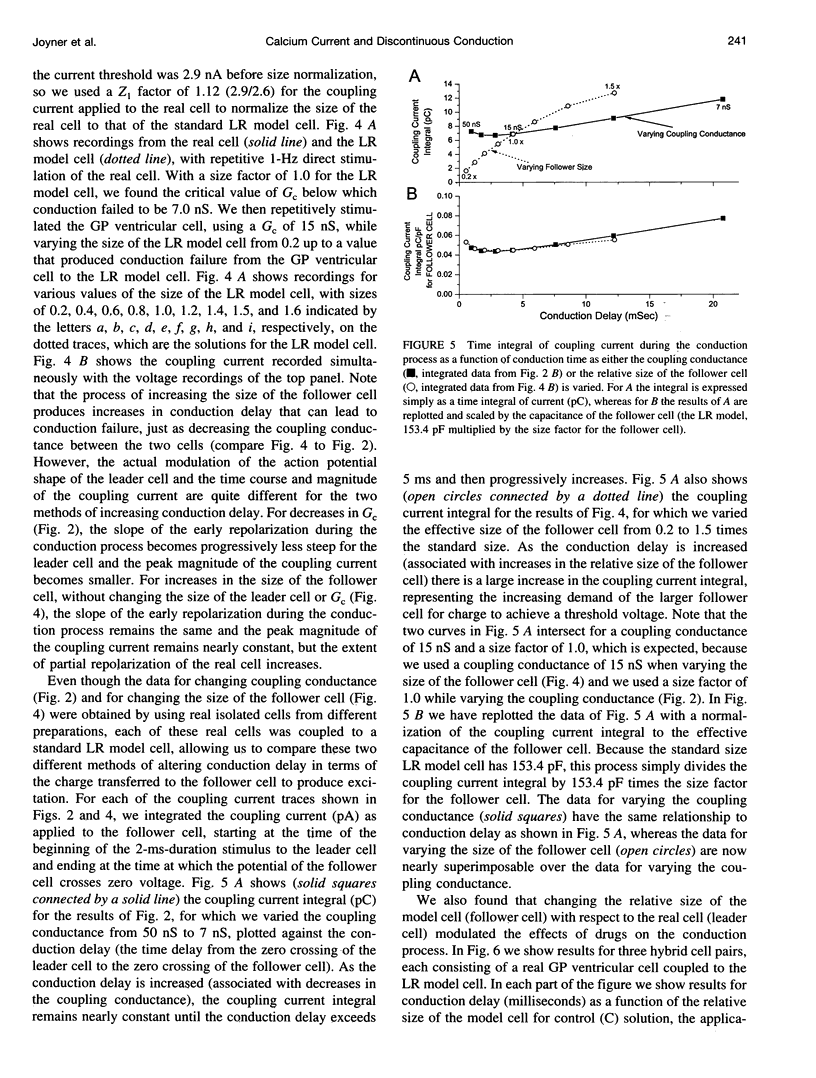
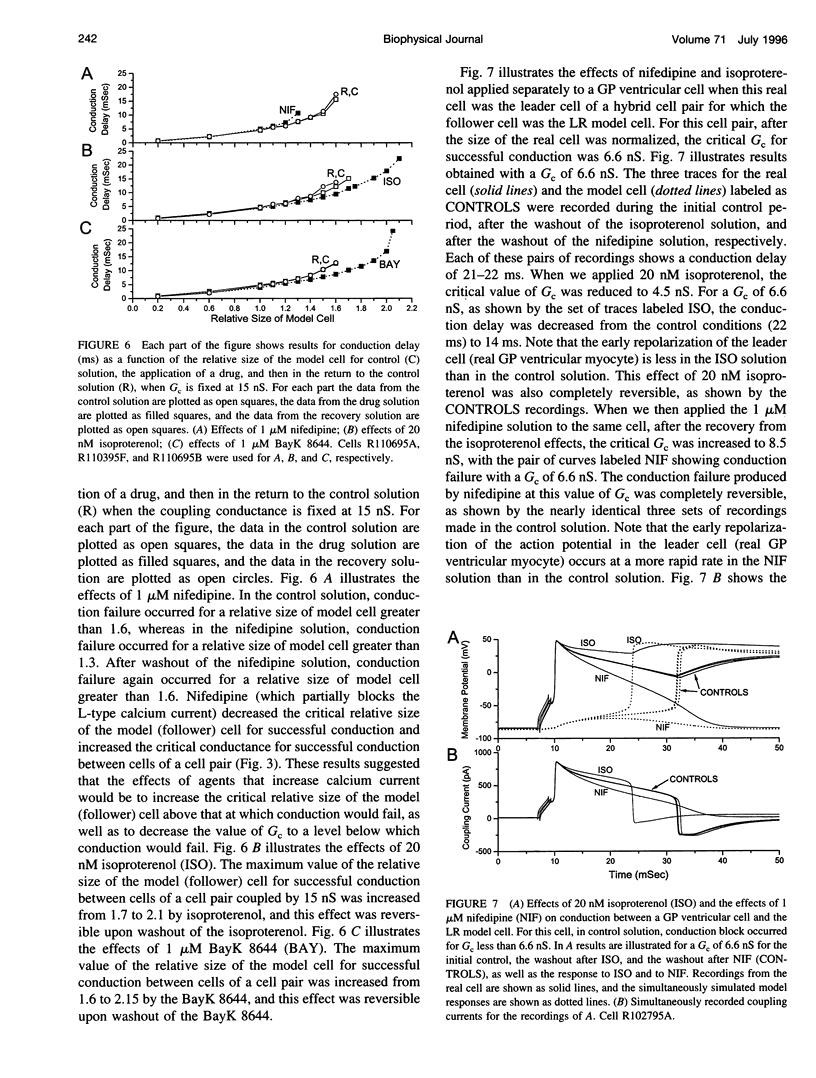
Abstract
We have used pairs of cardiac cells (i.e., one real guinea pig ventricular cell and a real-time simulation of a numerical model of a guinea pig ventricular cell) to evaluate the effects on action potential conduction of a variable coupling conductance in combination with agents that either increase or decrease the magnitude of the L-type calcium current. For the cell pairs studied, we applied a direct repetitive stimulation to the real cell, making it the "leader" cell of the cell pair. We have demonstrated that significant delays in action potential conduction for a cell pair can occur either with a decreased value of coupling conductance or with an asymmetry in size such that the follower cell is larger than the leader cell. In both conditions we have shown that isoproterenol, applied to the real cell at very low concentrations, can reversibly decrease the critical coupling conductance (below which action potential conduction fails) for a cell pair with fixed cell sizes, or, for a fixed value of coupling conductance, increase the maximum allowable asymmetry in cell size for successful conduction. For either of these effects, we were able to show that treatment of the real cell with BayK 8644, which more specifically increases the magnitude of the L-type calcium current, was able to mimic the actions of isoproterenol. Treatment of the leader cell of the cell pair (the real cell) with nifedipine, which selectively lowers the magnitude of the L-type calcium current, had effects opposite those of isoproterenol or BayK 8644. The actions of nifedipine, isoproterenol, and BayK 8644 are all limited to conditions in which the conduction delay is on the order of 5 ms or more, whether this delay is caused by limited coupling conductance or by asymmetry in size of the cells. This limitation is consistent with the time course of the L-type calcium current and suggests that the effects of calcium channel blockers or beta-adrenergic blocking drugs, in addition to being selective for regions of the heart that depend on the L-type calcium current for the upstroke of the action potential, would also be somewhat selective for regions of the heart that have discontinuous conduction, either normally or because of some pathological condition.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahinski A., Nairn A. C., Greengard P., Gadsby D. C. Chloride conductance regulated by cyclic AMP-dependent protein kinase in cardiac myocytes. Nature. 1989 Aug 31;340(6236):718–721. doi: 10.1038/340718a0. [DOI] [PubMed] [Google Scholar]
- Beeler G. W., Reuter H. Reconstruction of the action potential of ventricular myocardial fibres. J Physiol. 1977 Jun;268(1):177–210. doi: 10.1113/jphysiol.1977.sp011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett P. B., Begenisich T. B. Catecholamines modulate the delayed rectifying potassium current (IK) in guinea pig ventricular myocytes. Pflugers Arch. 1987 Sep;410(1-2):217–219. doi: 10.1007/BF00581919. [DOI] [PubMed] [Google Scholar]
- Boyden P. A., Pinto J. M. Reduced calcium currents in subendocardial Purkinje myocytes that survive in the 24- and 48-hour infarcted heart. Circulation. 1994 Jun;89(6):2747–2759. doi: 10.1161/01.cir.89.6.2747. [DOI] [PubMed] [Google Scholar]
- De Mello W. C., van Loon P. Further studies on the influence of cyclic nucleotides on junctional permeability in heart. J Mol Cell Cardiol. 1987 Aug;19(8):763–771. doi: 10.1016/s0022-2828(87)80387-5. [DOI] [PubMed] [Google Scholar]
- Drummond G. I., Severson D. L. Cyclic nucleotides and cardiac function. Circ Res. 1979 Feb;44(2):145–153. doi: 10.1161/01.res.44.2.145. [DOI] [PubMed] [Google Scholar]
- El-Sherif N. Reentrant ventricular arrhythmias in the late myocardial infarction period. 6. Effect of the autonomic system. Circulation. 1978 Jul;58(1):103–110. doi: 10.1161/01.cir.58.1.103. [DOI] [PubMed] [Google Scholar]
- Gardner P. I., Ursell P. C., Fenoglio J. J., Jr, Wit A. L. Electrophysiologic and anatomic basis for fractionated electrograms recorded from healed myocardial infarcts. Circulation. 1985 Sep;72(3):596–611. doi: 10.1161/01.cir.72.3.596. [DOI] [PubMed] [Google Scholar]
- Gillis A. M., Kohlhardt M. Voltage-dependent Vmax blockade in Na+-dependent action potentials after beta 1- and H2-receptor stimulation in mammalian ventricular myocardium. Can J Physiol Pharmacol. 1988 Oct;66(10):1291–1296. doi: 10.1139/y88-211. [DOI] [PubMed] [Google Scholar]
- Gilmour R. F., Jr, Heger J. J., Prystowsky E. N., Zipes D. P. Cellular electrophysiologic abnormalities of diseased human ventricular myocardium. Am J Cardiol. 1983 Jan 1;51(1):137–144. doi: 10.1016/s0002-9149(83)80024-1. [DOI] [PubMed] [Google Scholar]
- Harvey R. D., Hume J. R. Autonomic regulation of a chloride current in heart. Science. 1989 May 26;244(4907):983–985. doi: 10.1126/science.2543073. [DOI] [PubMed] [Google Scholar]
- Jeck C., Pinto J., Boyden P. Transient outward currents in subendocardial Purkinje myocytes surviving in the infarcted heart. Circulation. 1995 Aug 1;92(3):465–473. doi: 10.1161/01.cir.92.3.465. [DOI] [PubMed] [Google Scholar]
- Joyner R. W. Modulation of repolarization by electrotonic interactions. Jpn Heart J. 1986 Nov;27 (Suppl 1):167–183. [PubMed] [Google Scholar]
- Joyner R. W., Sugiura H., Tan R. C. Unidirectional block between isolated rabbit ventricular cells coupled by a variable resistance. Biophys J. 1991 Nov;60(5):1038–1045. doi: 10.1016/S0006-3495(91)82141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienzle M. G., Tan R. C., Ramza B. M., Young M. L., Joyner R. W. Alterations in endocardial activation of the canine papillary muscle early and late after myocardial infarction. Circulation. 1987 Oct;76(4):860–874. doi: 10.1161/01.cir.76.4.860. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M., Fleckenstein A. Inhibition of the slow inward current by nifedipine in mammalian ventricular myocardium. Naunyn Schmiedebergs Arch Pharmacol. 1977 Jul;298(3):267–272. doi: 10.1007/BF00500899. [DOI] [PubMed] [Google Scholar]
- Kumar R., Joyner R. W. Calcium currents of ventricular cell pairs during action potential conduction. Am J Physiol. 1995 Jun;268(6 Pt 2):H2476–H2486. doi: 10.1152/ajpheart.1995.268.6.H2476. [DOI] [PubMed] [Google Scholar]
- Lue W. M., Boyden P. A. Abnormal electrical properties of myocytes from chronically infarcted canine heart. Alterations in Vmax and the transient outward current. Circulation. 1992 Mar;85(3):1175–1188. doi: 10.1161/01.cir.85.3.1175. [DOI] [PubMed] [Google Scholar]
- Luo C. H., Rudy Y. A dynamic model of the cardiac ventricular action potential. I. Simulations of ionic currents and concentration changes. Circ Res. 1994 Jun;74(6):1071–1096. doi: 10.1161/01.res.74.6.1071. [DOI] [PubMed] [Google Scholar]
- Luo C. H., Rudy Y. A dynamic model of the cardiac ventricular action potential. II. Afterdepolarizations, triggered activity, and potentiation. Circ Res. 1994 Jun;74(6):1097–1113. doi: 10.1161/01.res.74.6.1097. [DOI] [PubMed] [Google Scholar]
- Luo C. H., Rudy Y. A model of the ventricular cardiac action potential. Depolarization, repolarization, and their interaction. Circ Res. 1991 Jun;68(6):1501–1526. doi: 10.1161/01.res.68.6.1501. [DOI] [PubMed] [Google Scholar]
- Matsuura H., Ehara T., Imoto Y. An analysis of the delayed outward current in single ventricular cells of the guinea-pig. Pflugers Arch. 1987 Dec;410(6):596–603. doi: 10.1007/BF00581319. [DOI] [PubMed] [Google Scholar]
- Ono K., Kiyosue T., Arita M. Isoproterenol, DBcAMP, and forskolin inhibit cardiac sodium current. Am J Physiol. 1989 Jun;256(6 Pt 1):C1131–C1137. doi: 10.1152/ajpcell.1989.256.6.C1131. [DOI] [PubMed] [Google Scholar]
- Schubert B., Vandongen A. M., Kirsch G. E., Brown A. M. Inhibition of cardiac Na+ currents by isoproterenol. Am J Physiol. 1990 Apr;258(4 Pt 2):H977–H982. doi: 10.1152/ajpheart.1990.258.4.H977. [DOI] [PubMed] [Google Scholar]
- Sugiura H., Joyner R. W. Action potential conduction between guinea pig ventricular cells can be modulated by calcium current. Am J Physiol. 1992 Nov;263(5 Pt 2):H1591–H1604. doi: 10.1152/ajpheart.1992.263.5.H1591. [DOI] [PubMed] [Google Scholar]
- Tan R. C., Joyner R. W. Electrotonic influences on action potentials from isolated ventricular cells. Circ Res. 1990 Nov;67(5):1071–1081. doi: 10.1161/01.res.67.5.1071. [DOI] [PubMed] [Google Scholar]
- Tsien R. W. Cyclic AMP and contractile activity in heart. Adv Cyclic Nucleotide Res. 1977;8:363–420. [PubMed] [Google Scholar]
- Ursell P. C., Gardner P. I., Albala A., Fenoglio J. J., Jr, Wit A. L. Structural and electrophysiological changes in the epicardial border zone of canine myocardial infarcts during infarct healing. Circ Res. 1985 Mar;56(3):436–451. doi: 10.1161/01.res.56.3.436. [DOI] [PubMed] [Google Scholar]
- Walsh K. B., Begenisich T. B., Kass R. S. Beta-adrenergic modulation in the heart. Independent regulation of K and Ca channels. Pflugers Arch. 1988 Feb;411(2):232–234. doi: 10.1007/BF00582323. [DOI] [PubMed] [Google Scholar]
- Wilde A. A., Kléber A. G. Effect of norepinephrine and heart rate on intracellular sodium activity and membrane potential in beating guinea pig ventricular muscle. Circ Res. 1991 May;68(5):1482–1489. doi: 10.1161/01.res.68.5.1482. [DOI] [PubMed] [Google Scholar]
- Wilders R., Kumar R., Joyner R. W., Jongsma H. J., Verheijck E. E., Golod D., van Ginneken A. C., Goolsby W. N. Action potential conduction between a ventricular cell model and an isolated ventricular cell. Biophys J. 1996 Jan;70(1):281–295. doi: 10.1016/S0006-3495(96)79569-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa K., Kaibara M., Ohara M., Kameyama M. An improved method for isolating cardiac myocytes useful for patch-clamp studies. Jpn J Physiol. 1990;40(1):157–163. doi: 10.2170/jjphysiol.40.157. [DOI] [PubMed] [Google Scholar]
- Yazawa K., Kameyama M. Mechanism of receptor-mediated modulation of the delayed outward potassium current in guinea-pig ventricular myocytes. J Physiol. 1990 Feb;421:135–150. doi: 10.1113/jphysiol.1990.sp017937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuanetti G., Hoyt R. H., Corr P. B. Beta-adrenergic-mediated influences on microscopic conduction in epicardial regions overlying infarcted myocardium. Circ Res. 1990 Aug;67(2):284–302. doi: 10.1161/01.res.67.2.284. [DOI] [PubMed] [Google Scholar]
- de Bakker J. M., van Capelle F. J., Janse M. J., Tasseron S., Vermeulen J. T., de Jonge N., Lahpor J. R. Slow conduction in the infarcted human heart. 'Zigzag' course of activation. Circulation. 1993 Sep;88(3):915–926. doi: 10.1161/01.cir.88.3.915. [DOI] [PubMed] [Google Scholar]


