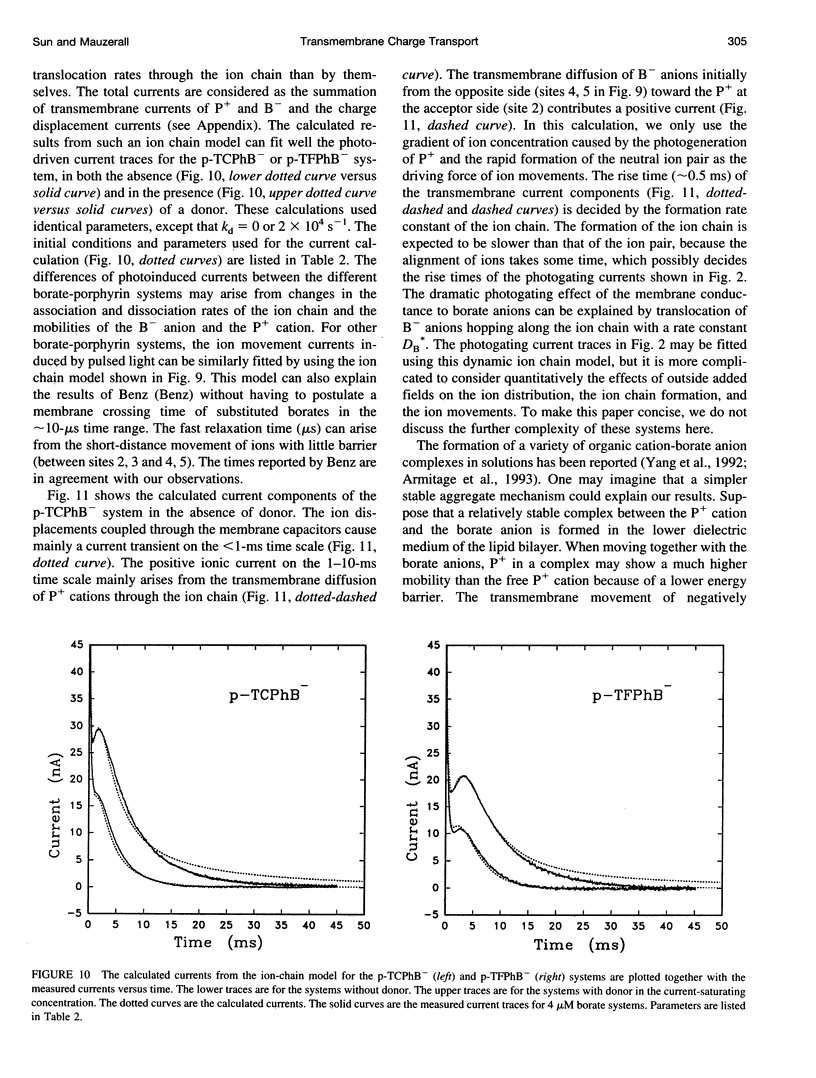
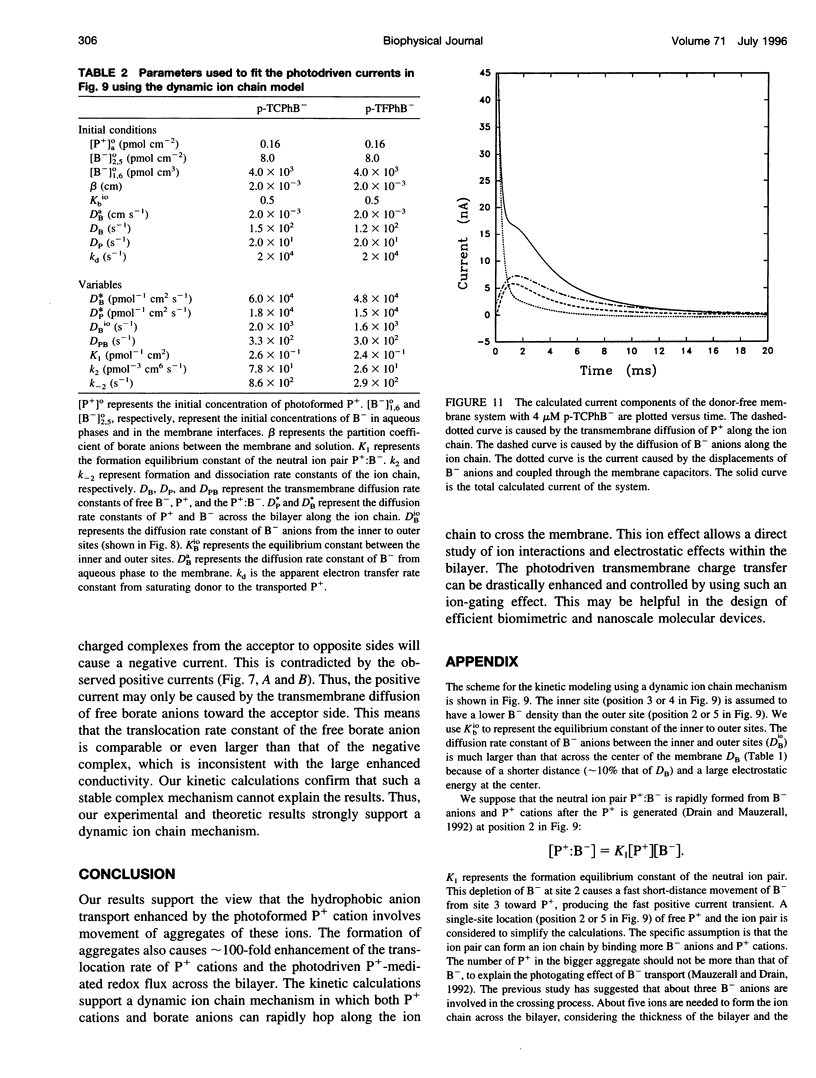
Abstract
The conductivity across a lipid bilayer by tetraphenylborate anion is increased 10-fold on the photoformation of lipophilic porphyrin cations. The cations alone have negligible conductivity. This nonlinear photogenerated increase of ion conductivity is termed the photogating effect. Substitution of H by Cl in the para position of tetraphenylborate leads to a 100-fold enhancement of conductivity, whereas the dark conductivities for this and other substituted borates are the same. Moreover, the halo-substituted borates show a large enhancement of conductivity in the low concentration range (10(-8) M), whereas that of tetraphenylborate is small and space charge is negligible. The enhanced ion conductivity has great structural sensitivity to the structure of the anion, the cation, and the lipid, whereas the partition coefficient of all the borates and the concentration of photoformed cations are only slightly affected. The photogated ion transport has a twofold larger activation energy than transport in the dark. Time-resolved photocurrents and voltages demonstrate that the translocation rate of the porphyrin cation is also enhanced 100-fold by the Cl-borate anion but only 10-fold by the H-borate anion. For these reasons the nonlinear gating effect cannot be explained by electrostatics alone, but requires an ion chain or ion aggregate mechanism. Kinetic modeling of the photoinduced current with a mixed cation-anion ion chain can fit the data well. The photogating effect allows the direct study of ion interactions within the bilayer.
Full text
PDF

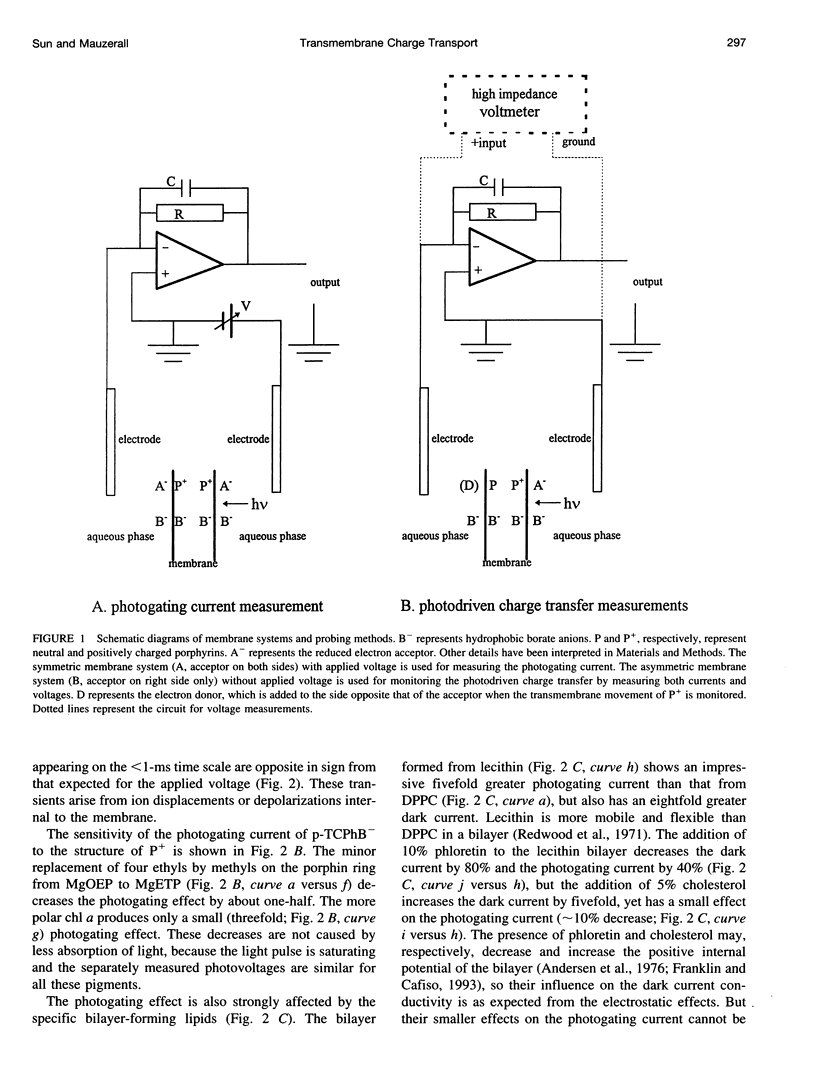
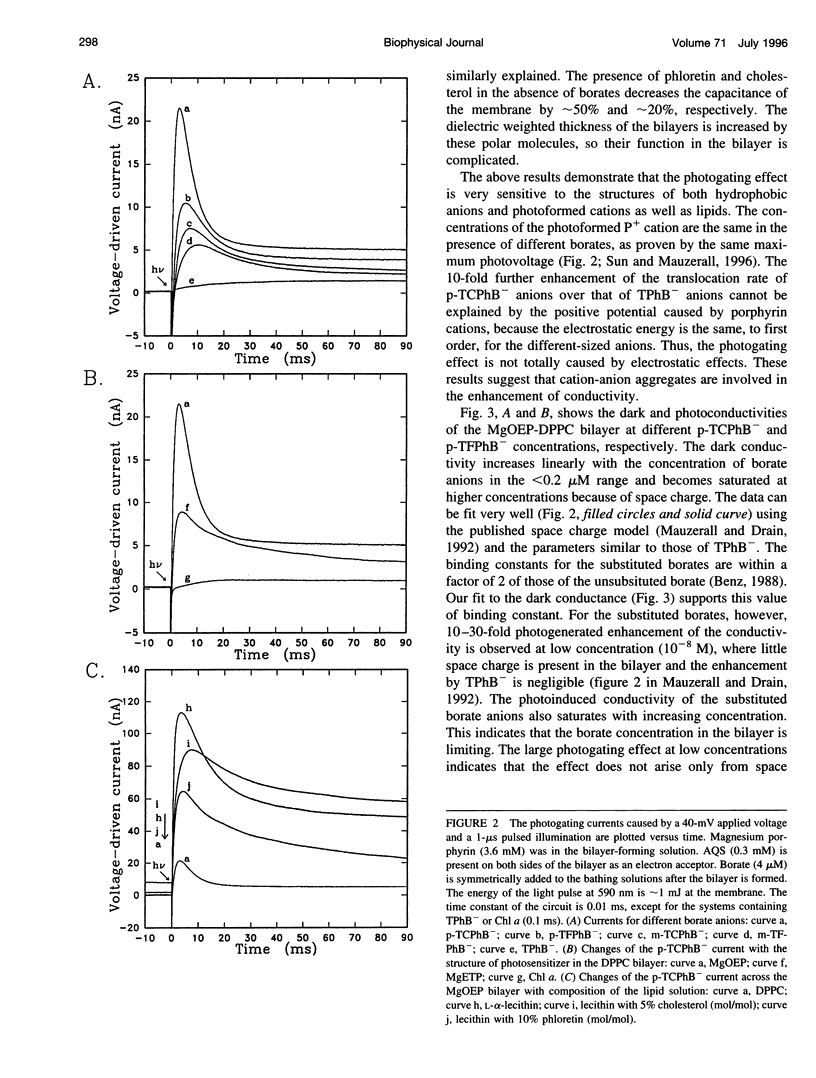
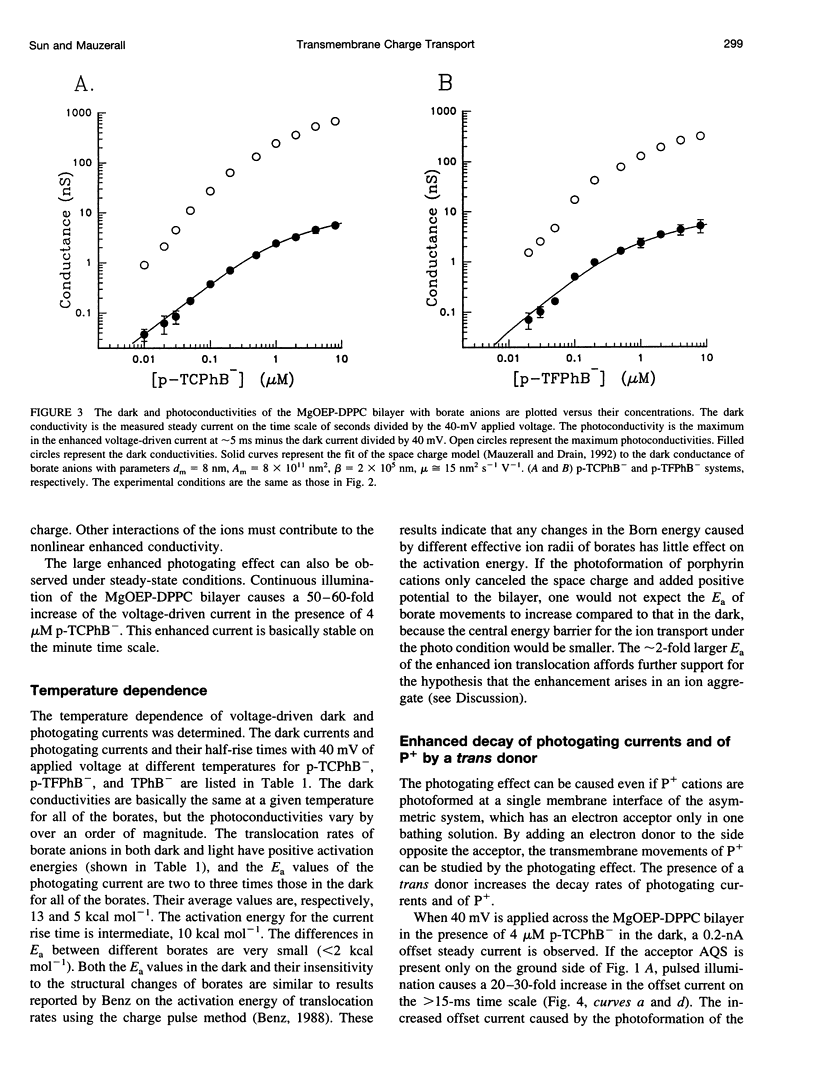
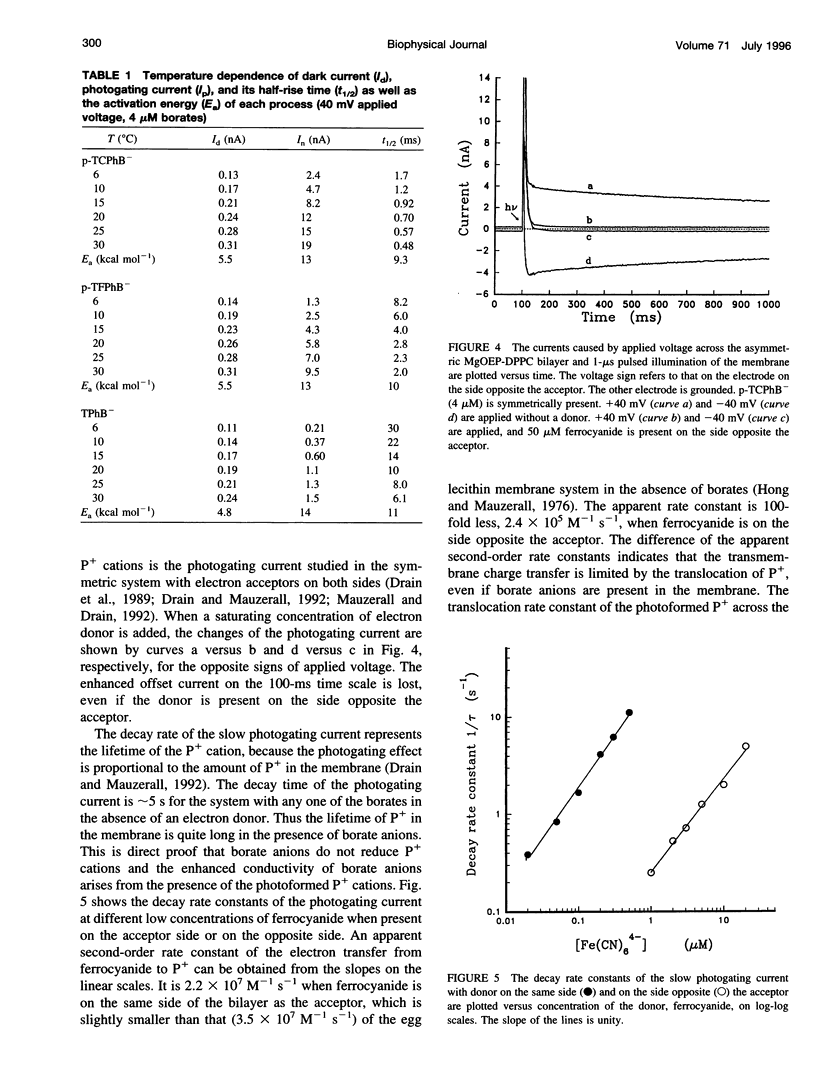


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen O. S., Feldberg S., Nakadomari H., Levy S., McLaughlin S. Electrostatic interactions among hydrophobic ions in lipid bilayer membranes. Biophys J. 1978 Jan;21(1):35–70. doi: 10.1016/S0006-3495(78)85507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O. S., Finkelstein A., Katz I., Cass A. Effect of phloretin on the permeability of thin lipid membranes. J Gen Physiol. 1976 Jun;67(6):749–771. doi: 10.1085/jgp.67.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O. S., Fuchs M. Potential energy barriers to ion transport within lipid bilayers. Studies with tetraphenylborate. Biophys J. 1975 Aug;15(8):795–830. doi: 10.1016/S0006-3495(75)85856-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Läuger P., Janko K. Transport kinetics of hydrophobic ions in lipid bilayer membranes. Charge-pulse relaxation studies. Biochim Biophys Acta. 1976 Dec 14;455(3):701–720. doi: 10.1016/0005-2736(76)90042-0. [DOI] [PubMed] [Google Scholar]
- Benz R. Structural requirement for the rapid movement of charged molecules across membranes. Experiments with tetraphenylborate analogues. Biophys J. 1988 Jul;54(1):25–33. doi: 10.1016/S0006-3495(88)82927-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafiso D. S., Hubbell W. L. EPR determination of membrane potentials. Annu Rev Biophys Bioeng. 1981;10:217–244. doi: 10.1146/annurev.bb.10.060181.001245. [DOI] [PubMed] [Google Scholar]
- Cevc G. Membrane electrostatics. Biochim Biophys Acta. 1990 Oct 8;1031(3):311–382. doi: 10.1016/0304-4157(90)90015-5. [DOI] [PubMed] [Google Scholar]
- Drain C. M., Christensen B., Mauzerall D. Photogating of ionic currents across a lipid bilayer. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6959–6962. doi: 10.1073/pnas.86.18.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drain C. M., Mauzerall D. C. Photogating of ionic currents across lipid bilayers. Hydrophobic ion conductance by an ion chain mechanism. Biophys J. 1992 Dec;63(6):1556–1563. doi: 10.1016/S0006-3495(92)81739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin J. C., Cafiso D. S. Internal electrostatic potentials in bilayers: measuring and controlling dipole potentials in lipid vesicles. Biophys J. 1993 Jul;65(1):289–299. doi: 10.1016/S0006-3495(93)81051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig B. H., Hubbell W. L., Flewelling R. F. Electrostatic interactions in membranes and proteins. Annu Rev Biophys Biophys Chem. 1986;15:163–193. doi: 10.1146/annurev.bb.15.060186.001115. [DOI] [PubMed] [Google Scholar]
- Ilani A., Mauzerall D. The potential span of photoredox reactions of porphyrins and chlorophyll at the lipid bilayer-water interface. Biophys J. 1981 Jul;35(1):79–92. doi: 10.1016/S0006-3495(81)84775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läuger P., Benz R., Stark G., Bamberg E., Jordan P. C., Fahr A., Brock W. Relaxation studies of ion transport systems in lipid bilayer membranes. Q Rev Biophys. 1981 Nov;14(4):513–598. doi: 10.1017/s003358350000247x. [DOI] [PubMed] [Google Scholar]
- Mauzerall D. C., Drain C. M. Photogating of ionic currents across lipid bilayers. Electrostatics of ions and dipoles inside the membrane. Biophys J. 1992 Dec;63(6):1544–1555. doi: 10.1016/S0006-3495(92)81738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. The electrostatic properties of membranes. Annu Rev Biophys Biophys Chem. 1989;18:113–136. doi: 10.1146/annurev.bb.18.060189.000553. [DOI] [PubMed] [Google Scholar]
- Redwood W. R., Pfeiffer F. R., Weisbach J. A., Thompson T. E. Physical properties of bilayer membranes formed from a synthetic saturated phospholipid in n-decane. Biochim Biophys Acta. 1971 Mar 9;233(1):1–6. doi: 10.1016/0005-2736(71)90351-8. [DOI] [PubMed] [Google Scholar]
- Woodle M. C., Mauzerall D. Photoinitiated ion movements in bilayer membranes containing magnesium octaethylporphyrin. Biophys J. 1986 Sep;50(3):431–439. doi: 10.1016/S0006-3495(86)83479-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


