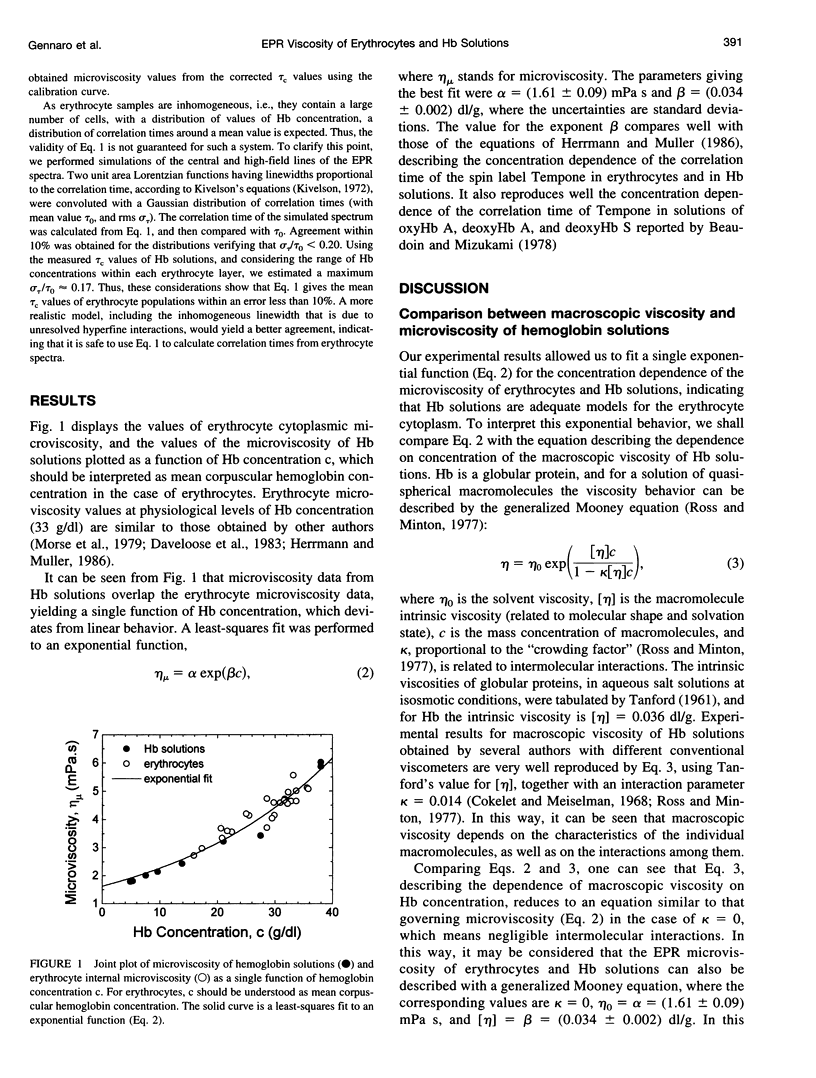
Abstract
The hypothesis that the internal viscosity of erythrocytes is governed by the intracellular hemoglobin (Hb) concentration is examined. Here viscosity is determined by labeling of the cytoplasmic reduced glutathione with the spin label maleimido-Tempo. Erythrocyte populations with different Hb concentrations in isosmotic conditions were obtained through incomplete lysis, followed by cell resealing, and discontinuous density gradient separation. This procedure maintains normal cell shape and volume. Microviscosity of membrane-free Hb solutions was measured by addition of spin labeled glutathione. It was found that microviscosity values are similar for the erythrocyte cytoplasm and for Hb solutions of equivalent concentrations, showing that the erythrocyte membrane does not have any influence on internal microviscosity. The dependence of the microviscosity on the concentration of Hb solutions was compared with results of macroscopic viscosity obtained by other authors. It is concluded that microviscosity is sensitive to individual properties of the Hb molecule (intrinsic viscosity), but that it is not sensitive to intermolecular interactions. As the microviscosity behavior as a function of Hb concentration is the same in Hb solutions as in the erythrocyte cytoplasm, the inferences regarding macroscopic viscosity in Hb solutions could be translated to the rheological properties of the erythrocyte cytoplasm. Thus, these properties could be predicted from the values of the mean corpuscular Hb concentration.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaudoin A. G., Mizukami H. ESR correlation times of 2,2,6,6-tetramethyl piperidone-N-oxyl (Tempone) in solutions of hemoglobin A and hemoglobin S. Biochim Biophys Acta. 1978 Jan 25;532(1):41–47. doi: 10.1016/0005-2795(78)90445-2. [DOI] [PubMed] [Google Scholar]
- Cokelet G. R., Meiselman H. J. Rheological comparison of hemoglobin solutions and erythrocyte suspensions. Science. 1968 Oct 11;162(3850):275–277. doi: 10.1126/science.162.3850.275. [DOI] [PubMed] [Google Scholar]
- Daveloose D., Fabre G., Berleur F., Testylier G., Leterrier F. A new spin label method for the measurement of erythrocyte internal microviscosity. Biochim Biophys Acta. 1983 Aug 17;763(1):41–49. doi: 10.1016/0167-4889(83)90023-x. [DOI] [PubMed] [Google Scholar]
- Endre Z. H., Kuchel P. W. Viscosity of concentrated solutions and of human erythrocyte cytoplasm determined from NMR measurement of molecular correlation times. The dependence of viscosity on cell volume. Biophys Chem. 1986 Aug;24(3):337–356. doi: 10.1016/0301-4622(86)85039-6. [DOI] [PubMed] [Google Scholar]
- Eriksson L. E., Beving H. Calcium- and lead-activated morphological changes in human erythrocytes: a spin label study of the cytoplasm. Arch Biochem Biophys. 1993 Jun;303(2):296–301. doi: 10.1006/abbi.1993.1286. [DOI] [PubMed] [Google Scholar]
- Herrmann A., Müller P. Correlation of the internal microviscosity of human erythrocytes to the cell volume and the viscosity of hemoglobin solutions. Biochim Biophys Acta. 1986 Jan 23;885(1):80–87. doi: 10.1016/0167-4889(86)90041-8. [DOI] [PubMed] [Google Scholar]
- Mach O., Lacko L. Density gradient in a dextran medium. Anal Biochem. 1968 Mar;22(3):393–397. doi: 10.1016/0003-2697(68)90281-9. [DOI] [PubMed] [Google Scholar]
- Morse P. D., 2nd, Lusczakoski D. M., Simpson D. A. Internal microviscosity of red blood cells and hemoglobin-free resealed ghosts: a spin-label study. Biochemistry. 1979 Oct 30;18(22):5021–5029. doi: 10.1021/bi00589a033. [DOI] [PubMed] [Google Scholar]
- Morse P. D., 2nd, Ruhlig M., Snipes W., Keith A. D. A spin-label study of the viscosity profile of sarcoplasmic reticular vesicles. Arch Biochem Biophys. 1975 May;168(1):40–56. doi: 10.1016/0003-9861(75)90226-x. [DOI] [PubMed] [Google Scholar]
- Morse P. D., 2nd, Warth J. A. Direct measurement of the internal viscosity of sickle erythrocytes as a function of cell density. Biochim Biophys Acta. 1990 Jun 12;1053(1):49–55. doi: 10.1016/0167-4889(90)90025-9. [DOI] [PubMed] [Google Scholar]
- Nash G. B., Meiselman H. J. Red cell and ghost viscoelasticity. Effects of hemoglobin concentration and in vivo aging. Biophys J. 1983 Jul;43(1):63–73. doi: 10.1016/S0006-3495(83)84324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison J. K., Lyons J. M., Mehlhorn R. J., Keith A. D. Temperature-induced phase changes in mitochondrial membranes detected by spin labeling. J Biol Chem. 1971 Jun 25;246(12):4036–4040. [PubMed] [Google Scholar]
- Ross P. D., Minton A. P. Hard quasispherical model for the viscosity of hemoglobin solutions. Biochem Biophys Res Commun. 1977 Jun 20;76(4):971–976. doi: 10.1016/0006-291x(77)90950-0. [DOI] [PubMed] [Google Scholar]
- Schwoch G., Passow H. Preparation and properties of human erythrocyte ghosts. Mol Cell Biochem. 1973 Dec 15;2(2):197–218. doi: 10.1007/BF01795474. [DOI] [PubMed] [Google Scholar]
- Sutera S. P., Gardner R. A., Boylan C. W., Carroll G. L., Chang K. C., Marvel J. S., Kilo C., Gonen B., Williamson J. R. Age-related changes in deformability of human erythrocytes. Blood. 1985 Feb;65(2):275–282. [PubMed] [Google Scholar]


