Abstract
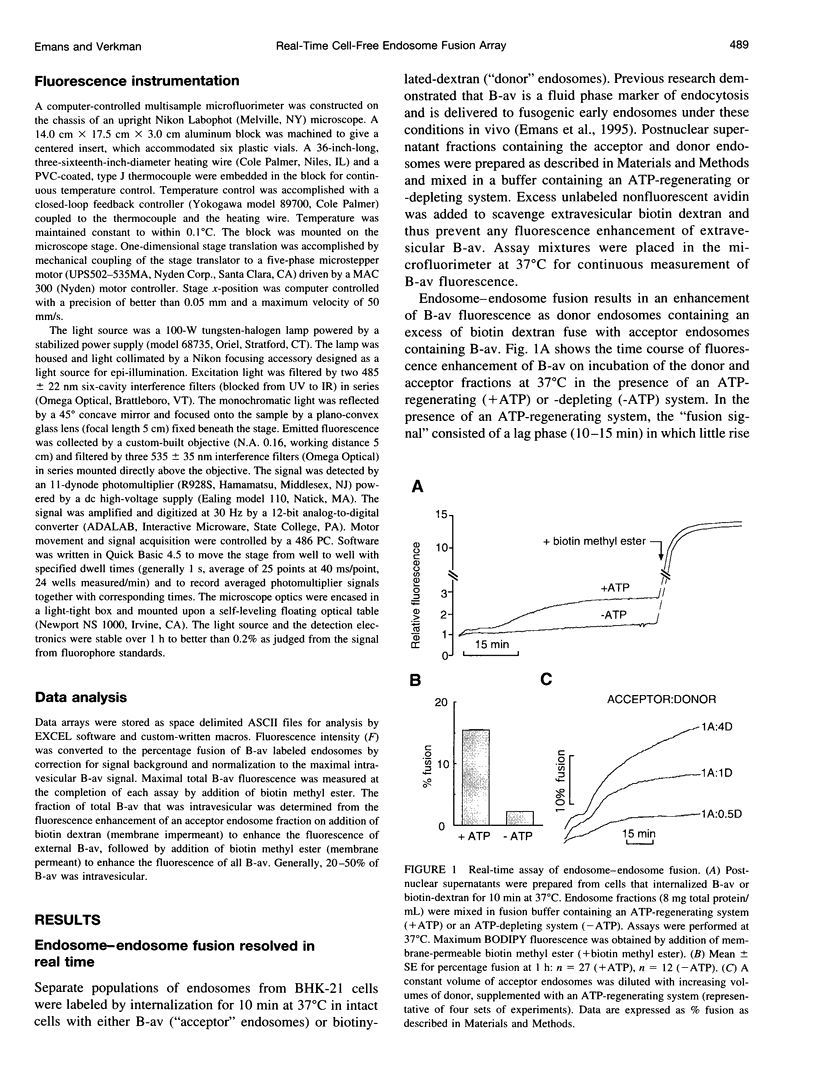
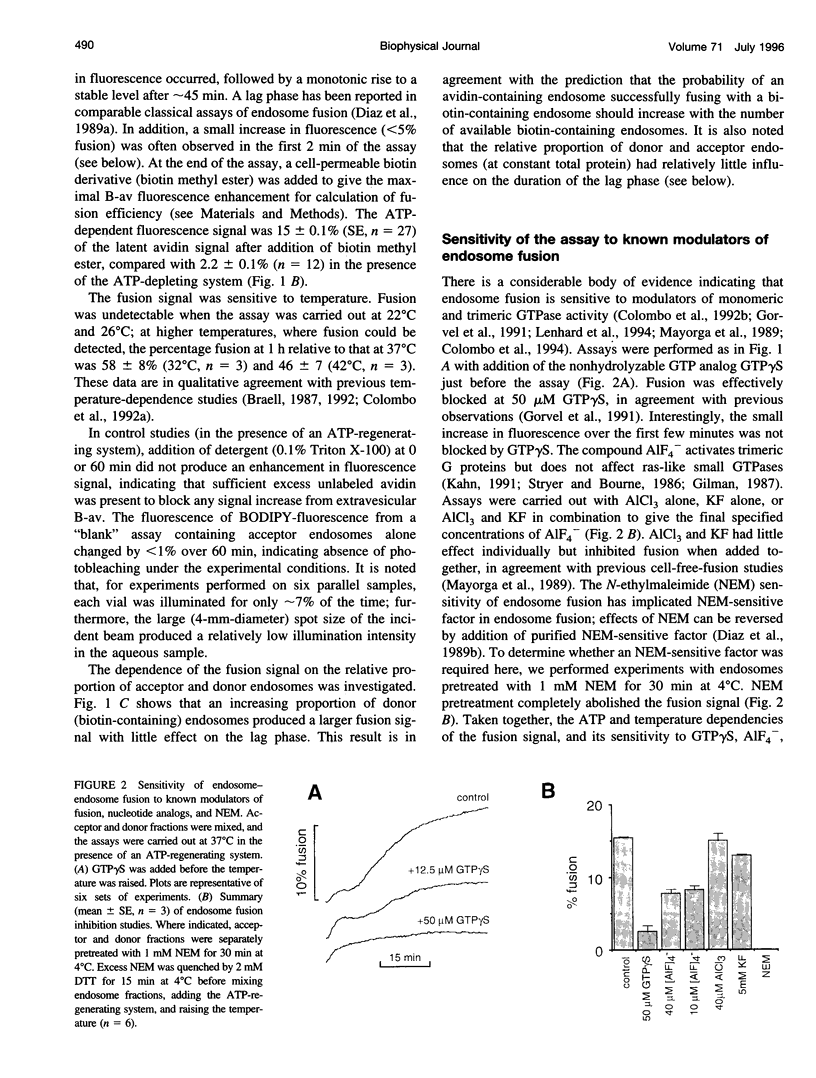
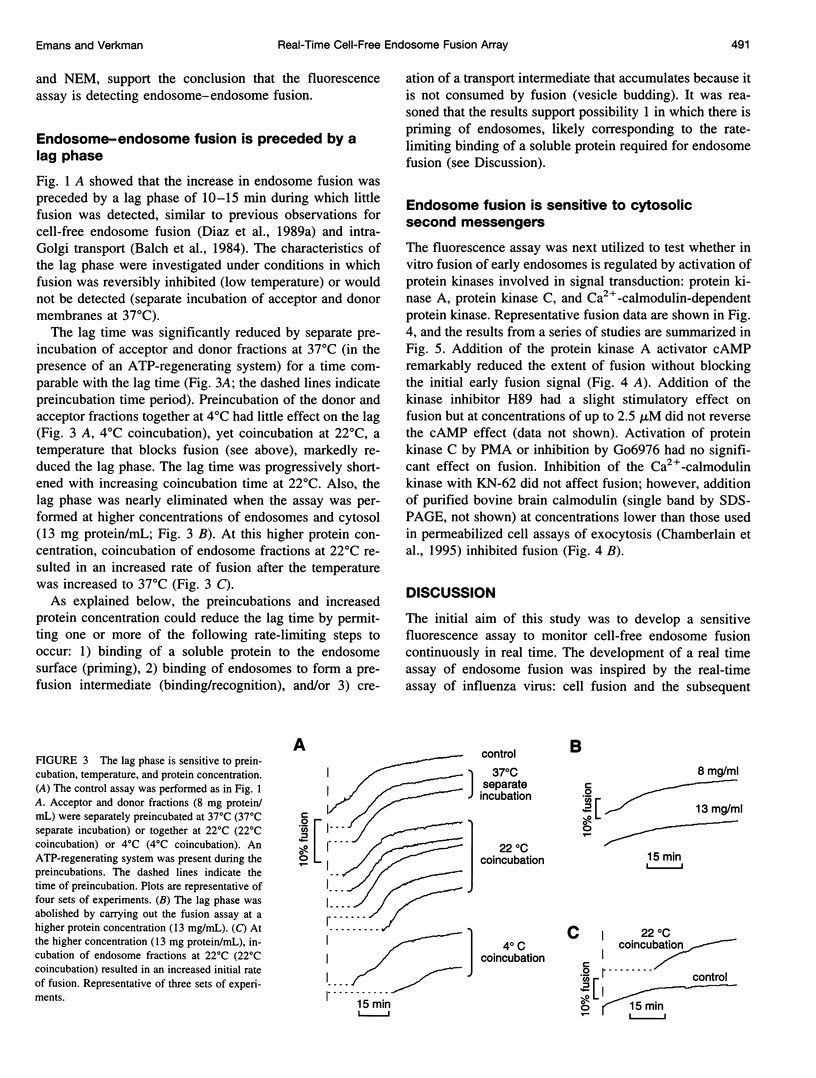
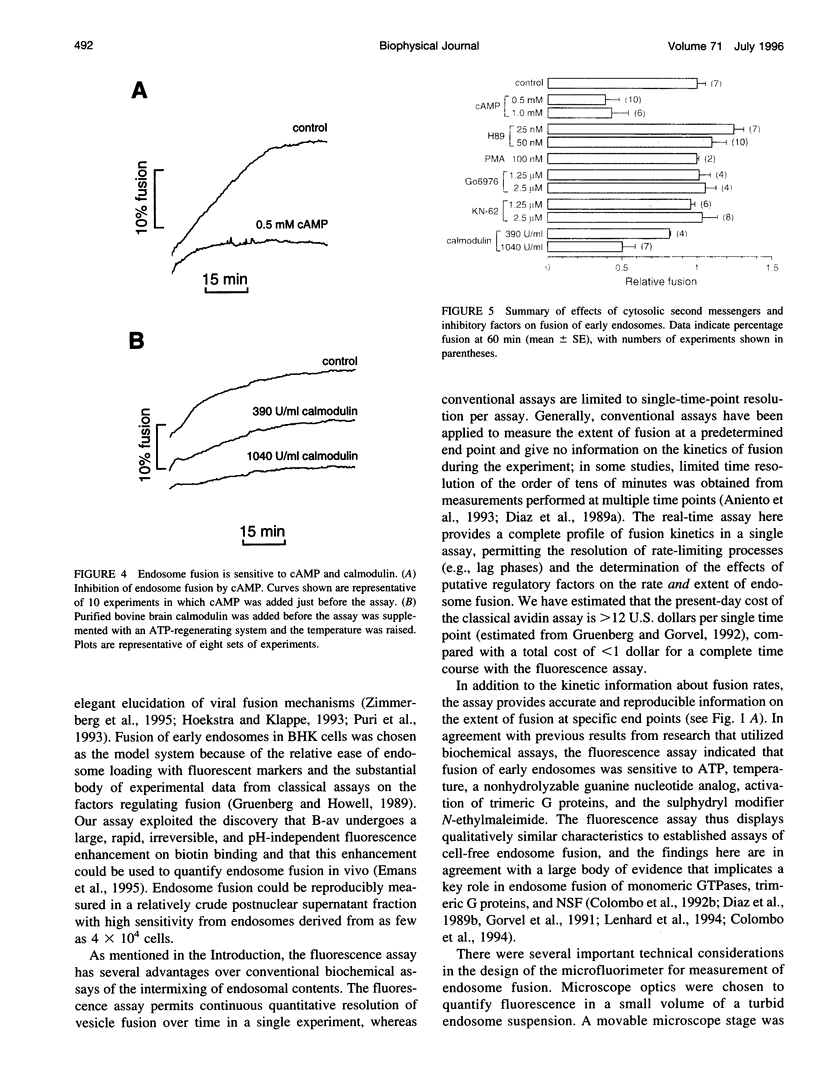
A quantitative real-time assay of cell-free endosomal vesicle fusion was developed and applied to study fusion mechanisms in endosomes from baby hamster kidney (BHK-21) cells. The assay is based on an irreversible approximately 10-fold increase in BODIPY-avidin fluorescence on binding of biotinylated conjugates. BODIPY-avidin and biotin-dextran were internalized for 10 min at 37 degrees C into separate populations of BHK-21 cells, and endosome fractions were prepared. Postnuclear supernatant fractions underwent ATP- and temperature-dependent fusion, as measured in a sensitive custom-built microfluorimeter by the continuous increase in BODIPY-avidin fluorescence. Fusion processes of efficiency > 2.5% could be detected with 200-ms time resolution in sample volumes of 50 microL containing endosomes derived from approximately 4 x 10(4) cells. The fusion time course consisted of a distinct lag phase (up to 10 min) in which little fusion occurred, followed by an approximately exponential rise (t 1/2 10-30 min; fusion efficiency approximately 15%). The lag phase was reduced by preincubation of separate endosome fractions with ATP at 37 degrees C and by coincubation of endosomes at 22 degrees C before the assay, suggesting a rate-limiting step involving binding of a soluble protein to the endosome membrane. Endosome fusion was strongly inhibited by GTP gamma S, N-ethylmaleimide, and AIF4-. Endosome fusion was not affected by phorbol myristate acetate but was significantly inhibited by cAMP and bovine brain calmodulin. The results establish a sensitive real-time fluorescence assay to quantify the kinetics and extent of endosome fusion in a cell-free system and demonstrate regulation of early endosome fusion by cytosolic second messengers.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aniento F., Emans N., Griffiths G., Gruenberg J. Cytoplasmic dynein-dependent vesicular transport from early to late endosomes. J Cell Biol. 1993 Dec;123(6 Pt 1):1373–1387. doi: 10.1083/jcb.123.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Glick B. S., Rothman J. E. Sequential intermediates in the pathway of intercompartmental transport in a cell-free system. Cell. 1984 Dec;39(3 Pt 2):525–536. doi: 10.1016/0092-8674(84)90459-8. [DOI] [PubMed] [Google Scholar]
- Bradbury N. A., Bridges R. J. Endocytosis is regulated by protein kinase A, but not protein kinase C in a secretory epithelial cell line. Biochem Biophys Res Commun. 1992 May 15;184(3):1173–1180. doi: 10.1016/s0006-291x(05)80006-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Braell W. A., Balch W. E., Dobbertin D. C., Rothman J. E. The glycoprotein that is transported between successive compartments of the Golgi in a cell-free system resides in stacks of cisternae. Cell. 1984 Dec;39(3 Pt 2):511–524. doi: 10.1016/0092-8674(84)90458-6. [DOI] [PubMed] [Google Scholar]
- Braell W. A. Detection of endocytic vesicle fusion in vitro, using assay based on avidin-biotin association reaction. Methods Enzymol. 1992;219:12–21. doi: 10.1016/0076-6879(92)19005-q. [DOI] [PubMed] [Google Scholar]
- Braell W. A. Fusion between endocytic vesicles in a cell-free system. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1137–1141. doi: 10.1073/pnas.84.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain L. H., Roth D., Morgan A., Burgoyne R. D. Distinct effects of alpha-SNAP, 14-3-3 proteins, and calmodulin on priming and triggering of regulated exocytosis. J Cell Biol. 1995 Sep;130(5):1063–1070. doi: 10.1083/jcb.130.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clague M. J., Urbé S., Aniento F., Gruenberg J. Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J Biol Chem. 1994 Jan 7;269(1):21–24. [PubMed] [Google Scholar]
- Colombo M. I., Lenhard J. M., Mayorga L. S., Stahl P. D. Reconstitution of endosome fusion: identification of factors necessary for fusion competency. Methods Enzymol. 1992;219:32–44. doi: 10.1016/0076-6879(92)19007-s. [DOI] [PubMed] [Google Scholar]
- Colombo M. I., Mayorga L. S., Casey P. J., Stahl P. D. Evidence of a role for heterotrimeric GTP-binding proteins in endosome fusion. Science. 1992 Mar 27;255(5052):1695–1697. doi: 10.1126/science.1348148. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey C., Li G., Colombo M. I., Stahl P. D. A regulatory role for ARF6 in receptor-mediated endocytosis. Science. 1995 Feb 24;267(5201):1175–1178. doi: 10.1126/science.7855600. [DOI] [PubMed] [Google Scholar]
- Davey J., Hurtley S. M., Warren G. Reconstitution of an endocytic fusion event in a cell-free system. Cell. 1985 Dec;43(3 Pt 2):643–652. doi: 10.1016/0092-8674(85)90236-3. [DOI] [PubMed] [Google Scholar]
- Diaz R., Mayorga L. S., Weidman P. J., Rothman J. E., Stahl P. D. Vesicle fusion following receptor-mediated endocytosis requires a protein active in Golgi transport. Nature. 1989 Jun 1;339(6223):398–400. doi: 10.1038/339398a0. [DOI] [PubMed] [Google Scholar]
- Diaz R., Mayorga L., Stahl P. In vitro fusion of endosomes following receptor-mediated endocytosis. J Biol Chem. 1988 May 5;263(13):6093–6100. [PubMed] [Google Scholar]
- Emans N., Biwersi J., Verkman A. S. Imaging of endosome fusion in BHK fibroblasts based on a novel fluorimetric avidin-biotin binding assay. Biophys J. 1995 Aug;69(2):716–728. doi: 10.1016/S0006-3495(95)79947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emans N., Gorvel J. P., Walter C., Gerke V., Kellner R., Griffiths G., Gruenberg J. Annexin II is a major component of fusogenic endosomal vesicles. J Cell Biol. 1993 Mar;120(6):1357–1369. doi: 10.1083/jcb.120.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Goda Y., Pfeffer S. R. Cell-free systems to study vesicular transport along the secretory and endocytic pathways. FASEB J. 1989 Nov;3(13):2488–2495. doi: 10.1096/fasebj.3.13.2680705. [DOI] [PubMed] [Google Scholar]
- Goda Y., Pfeffer S. R. Identification of a novel, N-ethylmaleimide-sensitive cytosolic factor required for vesicular transport from endosomes to the trans-Golgi network in vitro. J Cell Biol. 1991 Mar;112(5):823–831. doi: 10.1083/jcb.112.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda Y., Pfeffer S. R. Selective recycling of the mannose 6-phosphate/IGF-II receptor to the trans Golgi network in vitro. Cell. 1988 Oct 21;55(2):309–320. doi: 10.1016/0092-8674(88)90054-2. [DOI] [PubMed] [Google Scholar]
- Gorvel J. P., Chavrier P., Zerial M., Gruenberg J. rab5 controls early endosome fusion in vitro. Cell. 1991 Mar 8;64(5):915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- Gruenberg J., Griffiths G., Howell K. E. Characterization of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle fusion in vitro. J Cell Biol. 1989 Apr;108(4):1301–1316. doi: 10.1083/jcb.108.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J., Howell K. E. Membrane traffic in endocytosis: insights from cell-free assays. Annu Rev Cell Biol. 1989;5:453–481. doi: 10.1146/annurev.cb.05.110189.002321. [DOI] [PubMed] [Google Scholar]
- Hoekstra D., Klappe K. Fluorescence assays to monitor fusion of enveloped viruses. Methods Enzymol. 1993;220:261–276. doi: 10.1016/0076-6879(93)20088-k. [DOI] [PubMed] [Google Scholar]
- Kornfeld S., Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Lenhard J. M., Colombo M. I., Stahl P. D. Heterotrimeric GTP-binding proteins (G proteins) and ADP-ribosylation factor (ARF) regulate priming of endosomal membranes for fusion. Arch Biochem Biophys. 1994 Aug 1;312(2):474–479. doi: 10.1006/abbi.1994.1334. [DOI] [PubMed] [Google Scholar]
- Mayorga L. S., Diaz R., Stahl P. D. Regulatory role for GTP-binding proteins in endocytosis. Science. 1989 Jun 23;244(4911):1475–1477. doi: 10.1126/science.2499930. [DOI] [PubMed] [Google Scholar]
- Peters P. J., Hsu V. W., Ooi C. E., Finazzi D., Teal S. B., Oorschot V., Donaldson J. G., Klausner R. D. Overexpression of wild-type and mutant ARF1 and ARF6: distinct perturbations of nonoverlapping membrane compartments. J Cell Biol. 1995 Mar;128(6):1003–1017. doi: 10.1083/jcb.128.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri A., Clague M. J., Schoch C., Blumenthal R. Kinetics of fusion of enveloped viruses with cells. Methods Enzymol. 1993;220:277–287. doi: 10.1016/0076-6879(93)20089-l. [DOI] [PubMed] [Google Scholar]
- Robinson J. S., Graham T. R., Emr S. D. A putative zinc finger protein, Saccharomyces cerevisiae Vps18p, affects late Golgi functions required for vacuolar protein sorting and efficient alpha-factor prohormone maturation. Mol Cell Biol. 1991 Dec;11(12):5813–5824. doi: 10.1128/mcb.11.12.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L., Bourne H. R. G proteins: a family of signal transducers. Annu Rev Cell Biol. 1986;2:391–419. doi: 10.1146/annurev.cb.02.110186.002135. [DOI] [PubMed] [Google Scholar]
- Söllner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993 Mar 25;362(6418):318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Tooze S. A., Huttner W. B. Cell-free protein sorting to the regulated and constitutive secretory pathways. Cell. 1990 Mar 9;60(5):837–847. doi: 10.1016/0092-8674(90)90097-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub L. M., Ostrom J. A., Kornfeld S. Biochemical dissection of AP-1 recruitment onto Golgi membranes. J Cell Biol. 1993 Nov;123(3):561–573. doi: 10.1083/jcb.123.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman L. S., Wickner W. Molecular characterization of VAC1, a gene required for vacuole inheritance and vacuole protein sorting. J Biol Chem. 1992 Jan 5;267(1):618–623. [PubMed] [Google Scholar]
- Woodman P. G., Mundy D. I., Cohen P., Warren G. Cell-free fusion of endocytic vesicles is regulated by phosphorylation. J Cell Biol. 1992 Jan;116(2):331–338. doi: 10.1083/jcb.116.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zen K., Biwersi J., Periasamy N., Verkman A. S. Second messengers regulate endosomal acidification in Swiss 3T3 fibroblasts. J Cell Biol. 1992 Oct;119(1):99–110. doi: 10.1083/jcb.119.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg J., Blumenthal R., Sarkar D. P., Curran M., Morris S. J. Restricted movement of lipid and aqueous dyes through pores formed by influenza hemagglutinin during cell fusion. J Cell Biol. 1994 Dec;127(6 Pt 2):1885–1894. doi: 10.1083/jcb.127.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]