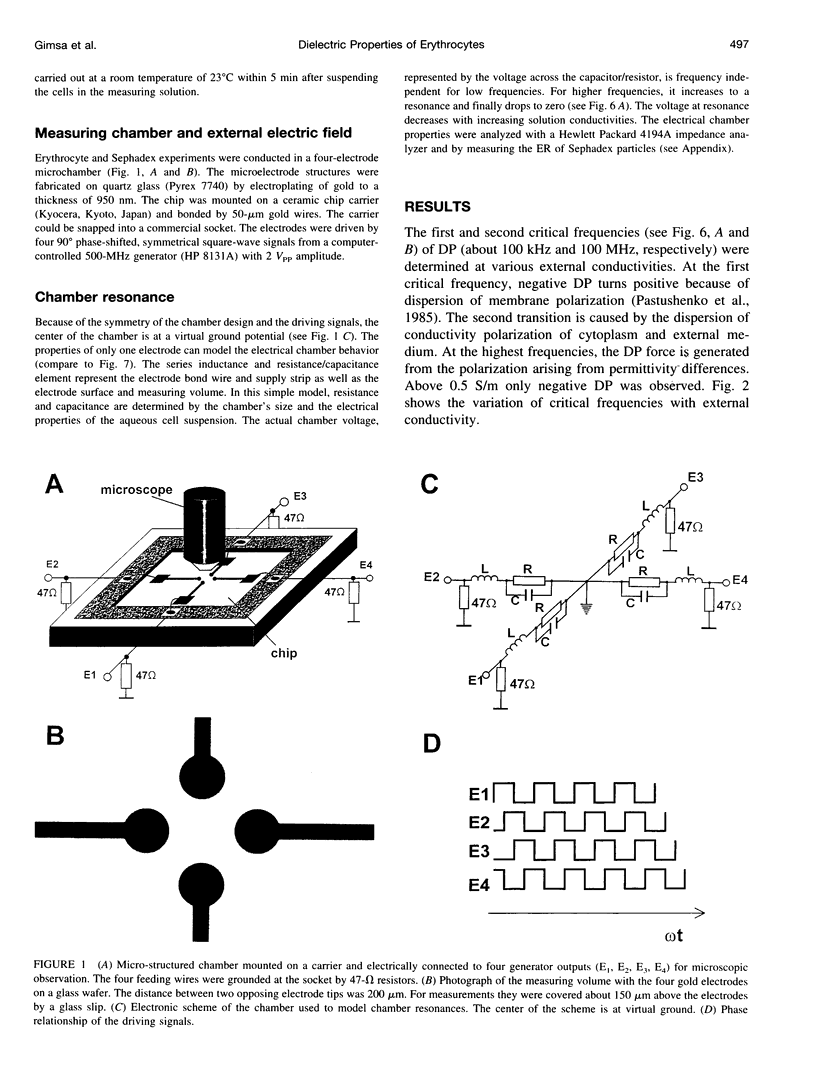
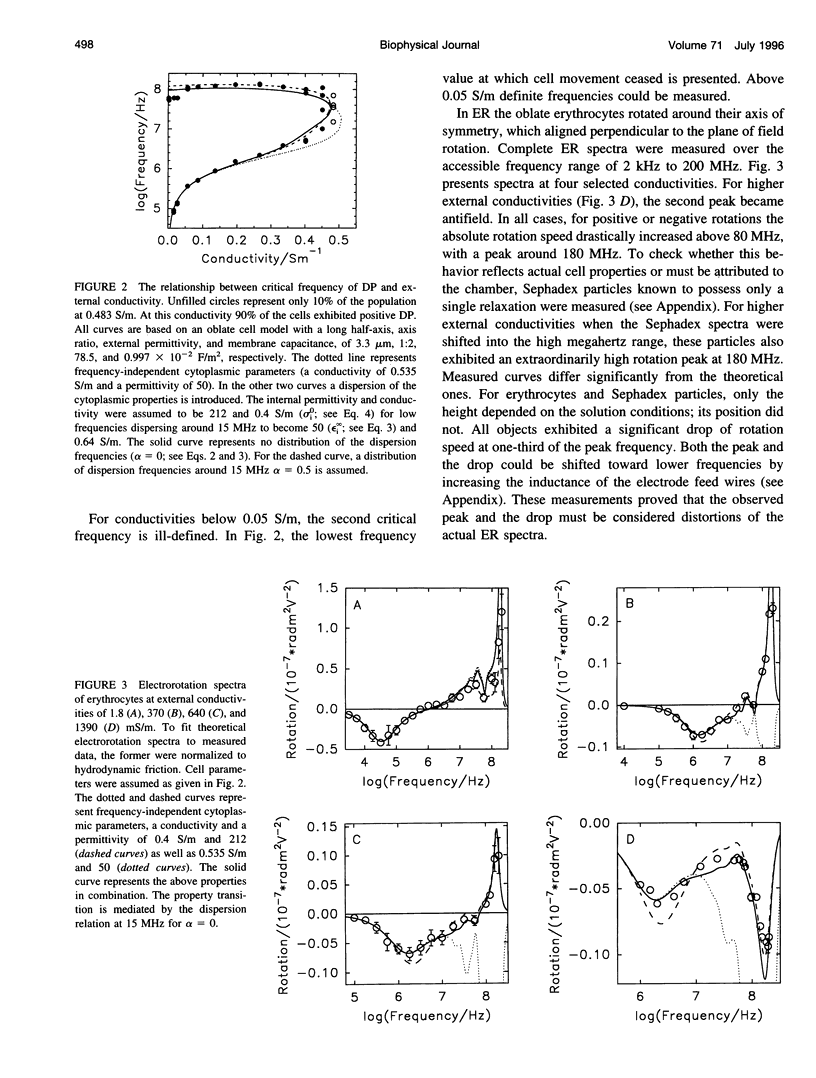
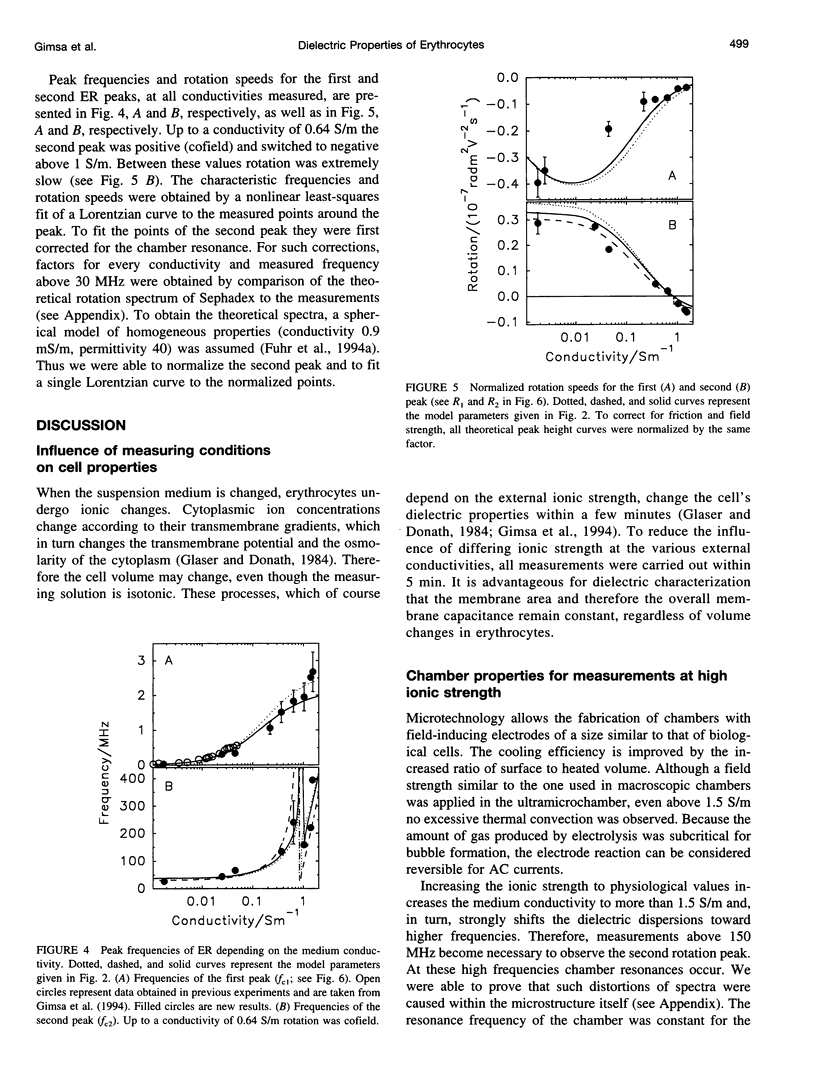
Abstract
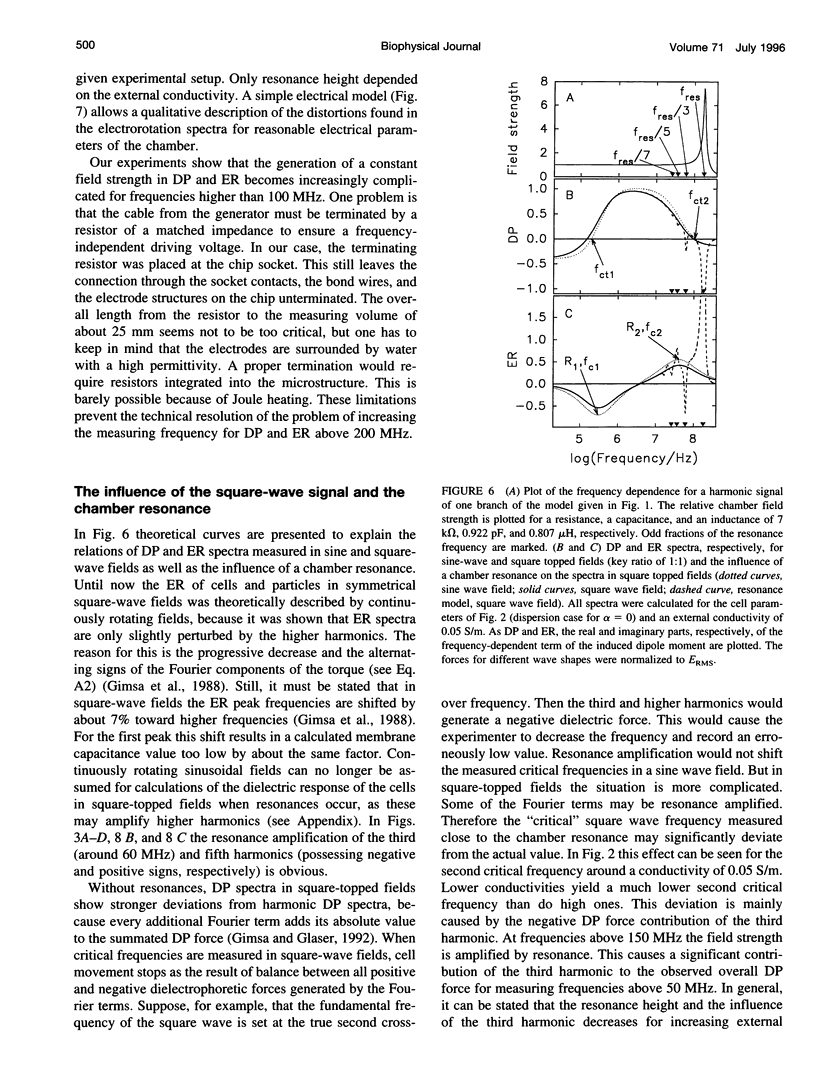
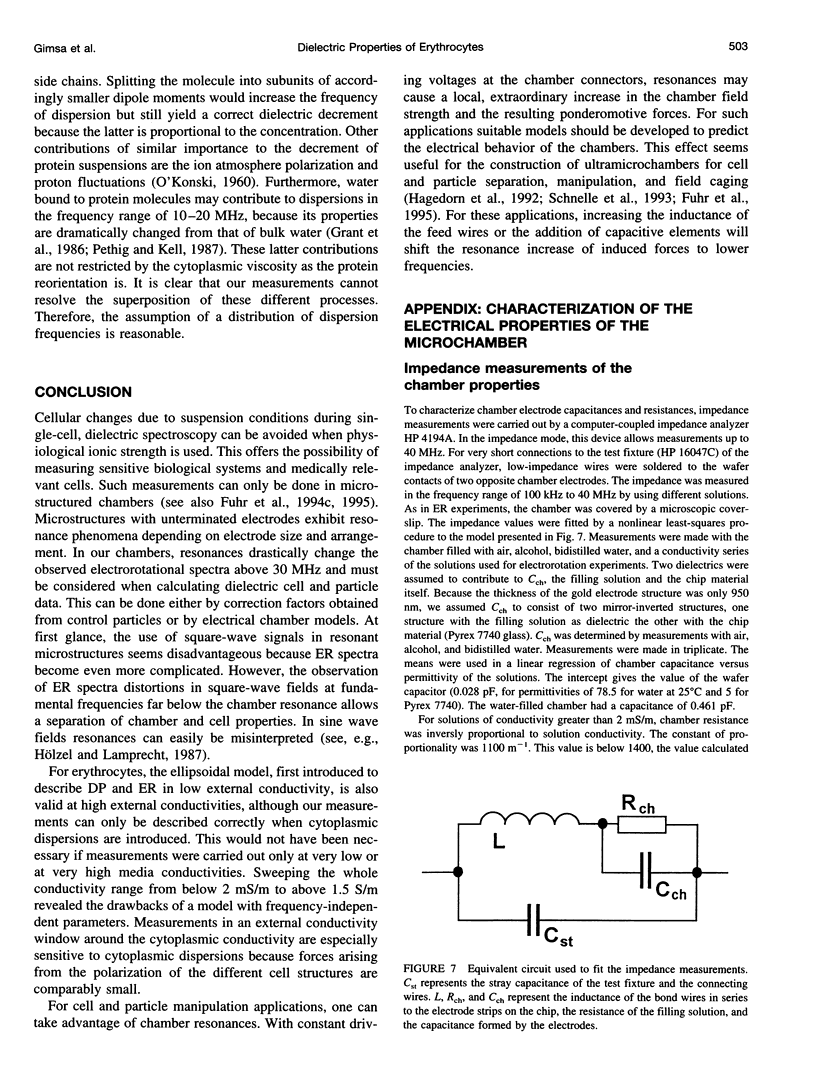
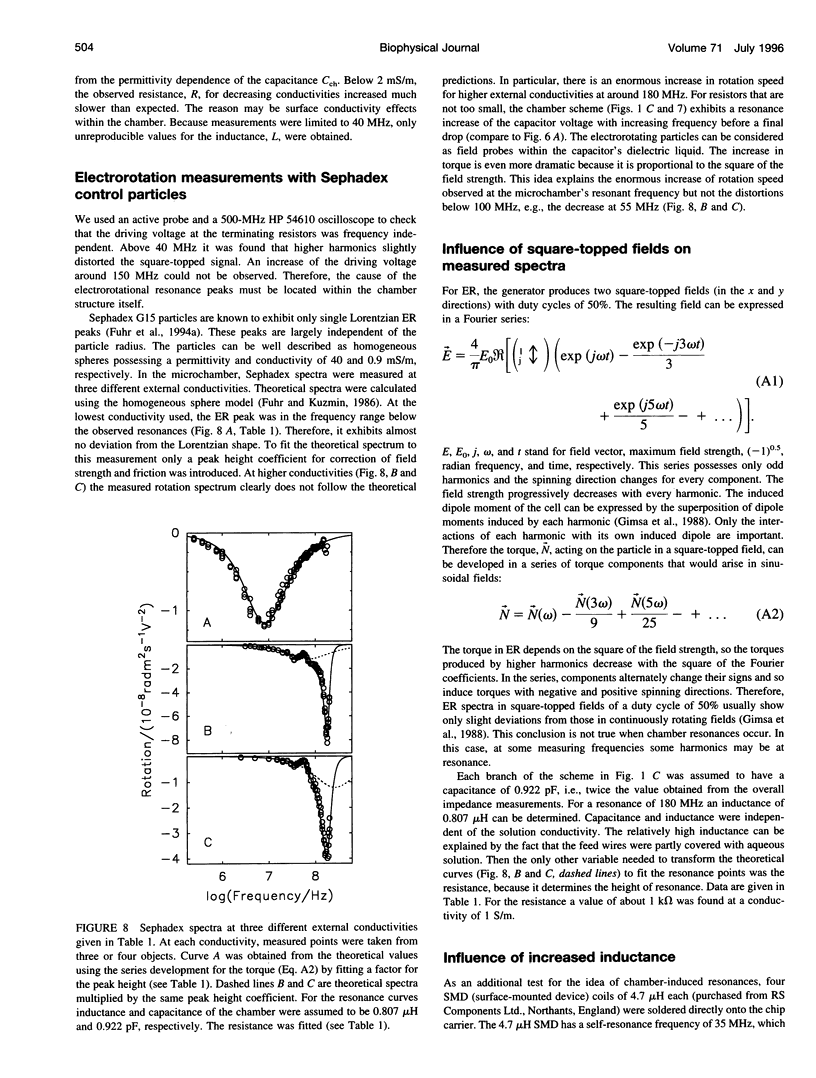
Usually dielectrophoretic and electrorotation measurements are carried out at low ionic strength to reduce electrolysis and heat production. Such problems are minimized in microelectrode chambers. In a planar ultramicroelectrode chamber fabricated by semiconductor technology, we were able to measure the dielectric properties of human red blood cells in the frequency range from 2 kHz to 200 MHz up to physiological ion concentrations. At low ionic strength, red cells exhibit a typical electrorotation spectrum with an antifield rotation peak at low frequencies and a cofield rotation peak at higher ones. With increasing medium conductivity, both electrorotational peaks shift toward higher frequencies. The cofield peak becomes antifield for conductivities higher than 0.5 S/m. Because the polarizability of the external medium at these ionic strengths becomes similar to that of the cytoplasm, properties can be measured more sensitively. The critical dielectrophoretic frequencies were also determined. From our measurements, in the wide conductivity range from 2 mS/m to 1.5 S/m we propose a single-shell erythrocyte model. This pictures the cell as an oblate spheroid with a long semiaxis of 3.3 microns and an axial ratio of 1:2. Its membrane exhibits a capacitance of 0.997 x 10(-2) F/m2 and a specific conductance of 480 S/m2. The cytoplasmic parameters, a conductivity of 0.4 S/m at a dielectric constant of 212, disperse around 15 MHz to become 0.535 S/m and 50, respectively. We attribute this cytoplasmic dispersion to hemoglobin and cytoplasmic ion properties. In electrorotation measurements at about 60 MHz, an unexpectedly low rotation speed was observed. Around 180 MHz, the speed increased dramatically. By analysis of the electric chamber circuit properties, we were able to show that these effects are not due to cell polarization but are instead caused by a dramatic increase in the chamber field strength around 180 MHz. Although the chamber exhibits a resonance around 180 MHz, the harmonic content of the square-topped driving signals generates distortions of electrorotational spectra at far lower frequencies. Possible technological applications of chamber resonances are mentioned.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asami K., Takahashi Y., Takashima S. Dielectric properties of mouse lymphocytes and erythrocytes. Biochim Biophys Acta. 1989 Jan 17;1010(1):49–55. doi: 10.1016/0167-4889(89)90183-3. [DOI] [PubMed] [Google Scholar]
- Bao J. Z., Davis C. C., Schmukler R. E. Frequency domain impedance measurements of erythrocytes. Constant phase angle impedance characteristics and a phase transition. Biophys J. 1992 May;61(5):1427–1434. doi: 10.1016/S0006-3495(92)81948-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J. Z., Davis C. C., Schmukler R. E. Impedance spectroscopy of human erythrocytes: system calibration and nonlinear modeling. IEEE Trans Biomed Eng. 1993 Apr;40(4):364–378. doi: 10.1109/10.222329. [DOI] [PubMed] [Google Scholar]
- Bao J. Z., Davis C. C., Swicord M. L. Microwave dielectric measurements of erythrocyte suspensions. Biophys J. 1994 Jun;66(6):2173–2180. doi: 10.1016/S0006-3495(94)81013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow D. J., Thornton J. M. The distribution of charged groups in proteins. Biopolymers. 1986 Sep;25(9):1717–1733. doi: 10.1002/bip.360250913. [DOI] [PubMed] [Google Scholar]
- Becker F. F., Wang X. B., Huang Y., Pethig R., Vykoukal J., Gascoyne P. R. Separation of human breast cancer cells from blood by differential dielectric affinity. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):860–864. doi: 10.1073/pnas.92.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beving H., Eriksson L. E., Davey C. L., Kell D. B. Dielectric properties of human blood and erythrocytes at radio frequencies (0.2-10 MHz); dependence on cell volume fraction and medium composition. Eur Biophys J. 1994;23(3):207–215. doi: 10.1007/BF01007612. [DOI] [PubMed] [Google Scholar]
- Egger M., Donath E. Electrorotation measurements of diamide-induced platelet activation changes. Biophys J. 1995 Jan;68(1):364–372. doi: 10.1016/S0006-3495(95)80197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhr G., Glaser R., Hagedorn R. Rotation of dielectrics in a rotating electric high-frequency field. Model experiments and theoretical explanation of the rotation effect of living cells. Biophys J. 1986 Feb;49(2):395–402. doi: 10.1016/S0006-3495(86)83649-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhr G., Glasser H., Müller T., Schnelle T. Cell manipulation and cultivation under a.c. electric field influence in highly conductive culture media. Biochim Biophys Acta. 1994 Dec 15;1201(3):353–360. doi: 10.1016/0304-4165(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Fuhr G., Kuzmin P. I. Behavior of cells in rotating electric fields with account to surface charges and cell structures. Biophys J. 1986 Nov;50(5):789–795. doi: 10.1016/S0006-3495(86)83519-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhr G., Müller T., Schnelle T., Hagedorn R., Voigt A., Fiedler S., Arnold W. M., Zimmermann U., Wagner B., Heuberger A. Radio-frequency microtools for particle and liver cell manipulation. Naturwissenschaften. 1994 Dec;81(12):528–535. doi: 10.1007/BF01139998. [DOI] [PubMed] [Google Scholar]
- Gascoyne P. R., Pethig R., Burt J. P., Becker F. F. Membrane changes accompanying the induced differentiation of Friend murine erythroleukemia cells studied by dielectrophoresis. Biochim Biophys Acta. 1993 Jun 18;1149(1):119–126. doi: 10.1016/0005-2736(93)90032-u. [DOI] [PubMed] [Google Scholar]
- Gimsa J., Schnelle T., Zechel G., Glaser R. Dielectric spectroscopy of human erythrocytes: investigations under the influence of nystatin. Biophys J. 1994 Apr;66(4):1244–1253. doi: 10.1016/S0006-3495(94)80908-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant E. H., McClean V. E., Nightingale N. R., Sheppard R. J., Chapman M. J. Dielectric behavior of water in biological solutions: studies on myoglobin, human low-density lipoprotein, and polyvinylpyrrolidone. Bioelectromagnetics. 1986;7(2):151–162. doi: 10.1002/bem.2250070206. [DOI] [PubMed] [Google Scholar]
- Hagedorn R., Fuhr G., Müller T., Gimsa J. Traveling-wave dielectrophoresis of microparticles. Electrophoresis. 1992 Jan-Feb;13(1-2):49–54. doi: 10.1002/elps.1150130110. [DOI] [PubMed] [Google Scholar]
- Herrmann A., Müller P. Correlation of the internal microviscosity of human erythrocytes to the cell volume and the viscosity of hemoglobin solutions. Biochim Biophys Acta. 1986 Jan 23;885(1):80–87. doi: 10.1016/0167-4889(86)90041-8. [DOI] [PubMed] [Google Scholar]
- Huang Y., Hölzel R., Pethig R., Wang X. B. Differences in the AC electrodynamics of viable and non-viable yeast cells determined through combined dielectrophoresis and electrorotation studies. Phys Med Biol. 1992 Jul;37(7):1499–1517. doi: 10.1088/0031-9155/37/7/003. [DOI] [PubMed] [Google Scholar]
- Hölzel R., Lamprecht I. Cellular spin resonance of yeast in a frequency range up to 140 MHz. Z Naturforsch C. 1987 Nov-Dec;42(11-12):1367–1369. doi: 10.1515/znc-1987-11-1242. [DOI] [PubMed] [Google Scholar]
- Kaler K. V., Xie J. P., Jones T. B., Paul R. Dual-frequency dielectrophoretic levitation of Canola protoplasts. Biophys J. 1992 Jul;63(1):58–69. doi: 10.1016/S0006-3495(92)81586-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek P., Zielinsky J. J., Fikus M., Tsong T. Y. Determination of electric parameters of cell membranes by a dielectrophoresis method. Biophys J. 1991 May;59(5):982–987. doi: 10.1016/S0006-3495(91)82312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORTTUNG W. H. EVIDENCE FOR A PERMANENT DIPOLE MOMENT IN HEMOGLOBIN FROM KERR EFFECT OPTICAL DISPERSION. J Am Chem Soc. 1965 Feb 20;87:924–926. doi: 10.1021/ja01082a049. [DOI] [PubMed] [Google Scholar]
- Orttung W. H. Anisotropy of proton fluctuations and the Kerr effect of protein solutions. Theoretical considerations. J Phys Chem. 1968 Nov;72(12):4058–4066. doi: 10.1021/j100858a020. [DOI] [PubMed] [Google Scholar]
- Orttung W. H. Anisotropy of proton fluctuations in proteins. Calculations for simple models. J Phys Chem. 1968 Nov;72(12):4066–4071. doi: 10.1021/j100858a021. [DOI] [PubMed] [Google Scholar]
- Paul R., Otwinowski M. The theory of the frequency response of ellipsoidal biological cells in rotating electrical fields. J Theor Biol. 1991 Feb 21;148(4):495–519. doi: 10.1016/s0022-5193(05)80233-4. [DOI] [PubMed] [Google Scholar]
- Pauly H., Schwan H. P. Dielectric properties and ion mobility in erythrocytes. Biophys J. 1966 Sep;6(5):621–639. doi: 10.1016/S0006-3495(66)86682-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pethig R., Kell D. B. The passive electrical properties of biological systems: their significance in physiology, biophysics and biotechnology. Phys Med Biol. 1987 Aug;32(8):933–970. doi: 10.1088/0031-9155/32/8/001. [DOI] [PubMed] [Google Scholar]
- Pilwat G., Zimmermann U. Determination of intracellular conductivity from electrical breakdown measurements. Biochim Biophys Acta. 1985 Nov 7;820(2):305–314. doi: 10.1016/0005-2736(85)90125-7. [DOI] [PubMed] [Google Scholar]
- SCHWAN H. P. Electrical properties of tissue and cell suspensions. Adv Biol Med Phys. 1957;5:147–209. doi: 10.1016/b978-1-4832-3111-2.50008-0. [DOI] [PubMed] [Google Scholar]
- Schnelle T., Hagedorn R., Fuhr G., Fiedler S., Müller T. Three-dimensional electric field traps for manipulation of cells--calculation and experimental verification. Biochim Biophys Acta. 1993 Jun 11;1157(2):127–140. doi: 10.1016/0304-4165(93)90056-e. [DOI] [PubMed] [Google Scholar]
- Sukhorukov V. L., Arnold W. M., Zimmermann U. Hypotonically induced changes in the plasma membrane of cultured mammalian cells. J Membr Biol. 1993 Feb;132(1):27–40. doi: 10.1007/BF00233049. [DOI] [PubMed] [Google Scholar]
- Takashima S., Asami K. Calculation and measurement of the dipole moment of small proteins: use of protein data base. Biopolymers. 1993 Jan;33(1):59–68. doi: 10.1002/bip.360330107. [DOI] [PubMed] [Google Scholar]
- Takashima S., Asami K., Takahashi Y. Frequency domain studies of impedance characteristics of biological cells using micropipet technique. I. Erythrocyte. Biophys J. 1988 Dec;54(6):995–1000. doi: 10.1016/S0006-3495(88)83037-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima S., Asami K., Takahashi Y. Frequency domain studies of impedance characteristics of biological cells using micropipet technique. I. Erythrocyte. Biophys J. 1988 Dec;54(6):995–1000. doi: 10.1016/S0006-3495(88)83037-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T. X. Contributions of suspending medium to electrical impedance of blood. Biochim Biophys Acta. 1994 Nov 11;1201(2):179–185. [PubMed] [Google Scholar]