Abstract
Objective
To identify potential diagnostic biomarkers distinguishing Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis (EBV-HLH) from infectious mononucleosis (EBV-IM) in pediatric patients using a retrospective case-control design.
Methods
This study enrolled a total of 160 pediatric patients, including 132 with Epstein-Barr virus-associated infectious mononucleosis (EBV-IM) and 28 with EBV-associated hemophagocytic lymphohistiocytosis (EBV-HLH). Serum levels of CD4⁺ T cells, CD8⁺ T cells, and D-dimer were quantified by flow cytometry and immunoturbidimetry, respectively. The CD4⁺/CD8⁺ ratio was calculated from absolute counts. These parameters, along with clinical and laboratory features, were compared between the EBV-IM and EBV-HLH groups. Binary logistic regression was used to analyze the risk factors for the progression of EBV infection to EBV-HLH. The clinical value of CD4⁺/CD8⁺ ratio and D-dimer levels in diagnosing EBV-HLH was assessed using receiver operating characteristic (ROC) curve analysis.
Results
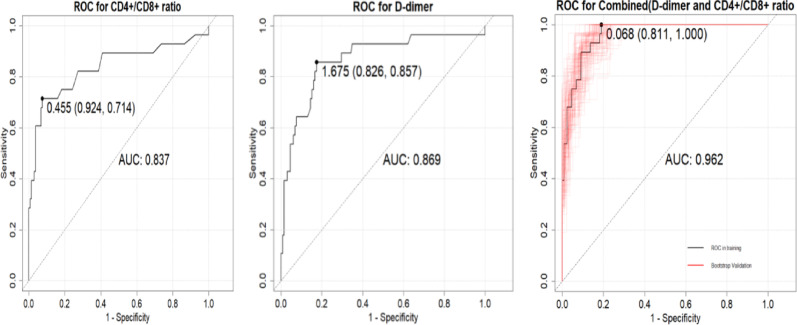
The average age of the EBV-HLH group was significantly lower than that of the IM group (p < 0.05). The EBV-HLH group had significantly higher levels of NLR(Neutrophil to Lymphocyte Ratio), PLR(Platelet to Lymphocyte Ratio), D-dimer, EBV-DNA, disease duration, CD4⁺/CD8⁺ ratio, and AST/ALT compared to the IM group (p < 0.05).Binary logistic regression analysis indicated that a higher CD4⁺/CD8⁺ ratio (OR = 17.60, 95% CI: 1.89–163.64; p < 0.05) and elevated D-dimer levels (OR = 1.31 per 1 mg/L increase, 95% CI: 1.08–1.59; p < 0.05) were significantly associated with EBV-HLH. Building upon the identified associations, we evaluated the diagnostic performance of these biomarkers. ROC curve analysis demonstrated that CD4⁺/CD8⁺ ratio > 0.455 (Youden index = 0.638, sensitivity = 92.4%, specificity = 71.4%) and D-dimer > 1.675 mg/L (Youden index = 0.683, sensitivity = 82.6%, specificity = 85.7%) optimally discriminated EBV-HLH from EBV-IM. The combined model significantly enhanced diagnostic accuracy (Youden index = 0.811), with AUC values of 0.837 (95%CI: 0.76–0.91), 0.869 (95%CI: 0.80–0.94), and 0.962 (95%CI: 0.935–0.989) for CD4⁺/CD8⁺ ratio, D-dimer, and their combination, respectively.
Conclusion
Elevated CD4⁺/CD8⁺ ratio and D-dimer serve as potential diagnostic biomarkers for pediatric EBV-HLH. Their combined detection enhances differentiation from EBV-IM, though validation through prospective studies is warranted.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-025-11440-1.
Keywords: Epstein-Barr virus infections, Lymphohistiocytosis, Hemophagocytic, Infectious mononucleosis, D-dimer, CD4⁺/CD8⁺ ratio, Pediatrics
Introduction
Epstein-Barr virus (EBV) is classified as a γ-subfamily member of the Herpesviridae family and is a double-stranded DNA virus. EBV is primarily transmitted through saliva but can also spread via blood transfusion and allogeneic grafts. In individuals under six years of age, initial infection is usually asymptomatic or presents with mild upper respiratory symptoms, whereas approximately 50% of adolescents develop infectious mononucleosis (IM) [1].
IM is a benign, self-limiting disease caused by EBV infection. Common clinical manifestations include fever, tonsillitis, and cervical lymphadenopathy, often accompanied by hepatosplenomegaly and peripheral blood atypical lymphocytosis [2]. While most cases have a favorable prognosis, a small proportion of patients may develop multisystem complications, such as EBV-associated hemophagocytic syndrome [3].
Hemophagocytic syndrome, also known as hemophagocytic lymphohistiocytosis (HLH), is a life-threatening inflammatory response syndrome caused by excessive immune system activation. Uncontrolled activation of cytotoxic T cells, natural killer cells, and macrophages leads to the excessive secretion and release of cytokines, resulting in a cytokine storm. This, in turn, causes hemophagocytic infiltration in organs, multiple organ failure, and, in severe cases, death [4–6]. Secondary HLH is the most common type of HLH, with infection-associated HLH being the predominant form. It is linked to various infectious agents, including bacteria, fungi, viruses, and protozoa. Among viral infections, herpesvirus infections, particularly EBV-associated HLH (EBV-HLH), constitute a major subset of secondary HLH. EBV-HLH accounts for approximately 70% of infection-associated HLH cases and has a higher prevalence among children and adolescents in Asia [7].In clinical practice, the initial symptoms of EBV-HLH are often atypical and challenging to recognize. Some patients present with symptoms resembling infectious mononucleosis. However, the disease progresses rapidly, leading to high mortality rates in patients who delay medical attention or receive an unclear diagnosis. Therefore, early and accurate identification of EBV-HLH is crucial [8, 9].Previous studies on the immune status of EBV-HLH patients have shown that these patients exhibit immune dysfunction, with abnormal counts in different subsets of T lymphocytes [10]. Additionally, research indicates that EBV-HLH patients experience coagulation dysfunction. The excessive activation of macrophages leads to the overproduction of plasminogen activators, promoting a hyperfibrinolytic state. Elevated D-dimer (D-D) levels suggest a hypercoagulable and fibrinolytic state in the body [11]. However, studies investigating the association between CD4⁺/CD8⁺ ratio and D-dimer in EBV-HLH remain limited.Based on this, our study aims to observe peripheral blood CD4⁺/CD8⁺ ratio and D-dimer levels in EBV-HLH patients and explore their value in predicting EBV-HLH.
Patients and methods
Patients
This retrospective study examined pediatric patients diagnosed with EBV-IM and EBV-HLH at Anhui Provincial Children’s Hospital from May 2018 to December 2024. Clinical data were collected, including sex, age, disease onset time, lymphocyte subsets, EBV-DNA copy number, AST/ALT, ALB, LDH, WBC, NLR, PLR, and D-dimer levels. Blood samples were collected from all patients on the day of admission for laboratory testing.The study protocol was approved by the Institutional Review Board of our hospital (Ethics Approval Number: EYLL-2023-041) and followed the guidelines outlined in the Declaration of Helsinki. Due to its retrospective nature, the committee waived the requirement for informed consent.
Lymphocyte subset analysis by flow cytometry
Peripheral venous blood was collected from pediatric patients. After adding anti-CD3, CD4, and CD8 monoclonal antibodies, the samples were incubated for 30 min under light-protected conditions. Following centrifugation and supernatant removal, cells were washed with phosphate-buffered saline (PBS), resuspended in 1% paraformaldehyde fixative, and filtered through a 70-µm cell strainer. Fixed samples were stored at 4 °C in the dark. Using the negative control tube for gating strategy, absolute counts of CD3⁺, CD4⁺, and CD8⁺ T lymphocytes were quantified by multiparameter flow cytometry. The CD4⁺/CD8⁺ ratio was subsequently calculated.
Statistical methods
Statistical analysis was performed using SPSS 25.0 and R (version 4.3.2). For univariate analysis, normality tests were conducted for continuous variables. Depending on the sample size, either the Kolmogorov-Smirnov (K-S) test (for samples > 50) or the Shapiro-Wilk (S-W) test was used, with P < 0.05 indicating a non-normal distribution.For variables following a normal distribution, an independent sample t-test was used, and results were expressed as mean ± standard deviation (SD). For non-normally distributed variables, the Mann-Whitney test was applied, and results were described as median (Q1-Q3). Categorical variables were analyzed using the chi-square test, with Pearson’s chi-square test, continuity correction, or Fisher’s exact test applied based on expected frequencies. Categorical variables were presented as frequency (percentage).A P value of < 0.05 in univariate analysis was considered statistically significant. Perform multivariable binary logistic regression using the variables found to be significant in univariate analysis, with a p-value < 0.05 considered statistically significant, to identify influencing factors for EBV-HLH.Receiver operating characteristic (ROC) curves were constructed to evaluate the influential capacity of the CD4+/CD8 + ratio and D-dimer level on the development of EBV-associated hemophagocytic lymphohistiocytosis (EBV-HLH) in children with Epstein-Barr virus (EBV) infection.
Diagnostic criteria
Patients meeting the HLH-2004 criteria and exhibiting active EBV infection were classified as having EBV-HLH [12]. The diagnosis of HLH requires at least five of the following eight criteria:1.Fever,2.Splenomegaly,3.Cytopenia affecting two or more blood cell lineages (hemoglobin < 90 g/L, platelets < 100 × 10⁹/L, and/or neutrophils < 1.0 × 10⁹/L),4.Hypertriglyceridemia (≥ 265 mg/dL) and/or hypofibrinogenemia (≤ 150 mg/dL),5.Evidence of hemophagocytosis in bone marrow, spleen, liver, or lymph nodes,6.Decreased or absent natural killer (NK) cell activity,7.Elevated ferritin levels (≥ 500 µg/L),8Increased interleukin-2 receptor levels (soluble CD25).Patients with primary HLH were excluded from the study, as this includes familial HLH and HLH caused by genetic mutations in RAB27A, LYST, AP3B1, SH2D1A, and BIRC4.
The diagnosis of EBV-IM was based on a combination of clinical symptoms and laboratory findings [13]. Key clinical indicators included:1.Fever,2.Pharyngotonsillitis,3.Cervical lymphadenopathy,4.Splenomegaly,5.Hepatomegaly,6.Eyelid edema.Biomarkers for EBV-IM included:1.Positive anti-EBV-VCA-IgM and anti-EBV-VCA-IgG antibodies with negative anti-EBV-NA-IgG antibodies,2.Negative anti-EBV-VCA-IgM antibody but positive anti-EBV-VCA-IgG with low-affinity antibodies,3.A ≥ 4-fold increase in anti-EBV-VCA-IgG levels between two serum samples,4.Positive polymerase chain reaction (PCR) detection of EBV DNA.A diagnosis of EBV-IM required at least three clinical indicators and one non-specific laboratory test result.
Results
Patient characteristics
Among the 160 pediatric patients included in the study, 28 were diagnosed with EBV-HLH, comprising 15 males and 13 females, with an average age of 2.66 years. Among them, 22 cases (78.6%) had hepatomegaly, and 20 cases (71.4%) had splenomegaly. A total of 132 patients were diagnosed with EBV-IM, including 82 males and 50 females, with an average age of 4 years. Among these, 106 cases (80.3%) had hepatomegaly, and 95 cases (72%) had splenomegaly. There were no significant differences between the two groups in terms of sex, hepatomegaly, or splenomegaly (P > 0.05).The average age in the EBV-HLH group was significantly lower than that in the IM group (P < 0.05). Additionally, the EBV-HLH group showed significantly higher levels of NLR, PLR, D-dimer, EBV-DNA, disease onset time, CD4⁺/CD8⁺ ratio, and AST/ALT compared to the IM group (P < 0.05). However, there was no significant difference in PT between the two groups (P > 0.05) (Table 1).
Table 1.
Baseline characteristics and single factor test results between the two groups
| Variable | IM | EBV-HLH | t/Z/X2 | P |
|---|---|---|---|---|
| Age(year) | 4(3–6) | 2.66(1.46–4.25) | 2.441 | 0.014 |
| NLR | 0.27(0.17–0.39) | 0.46(0.28–1.12) | −3.562 | 0.000 |
| PLR | 17.19(10.82–26.3) | 33.07(18.12–69.85) | −4.342 | 0.000 |
| PT | 12.6(11.9-13.43) | 12.55(11.8-13.62) | −0.137 | 0.892 |
| D-dimer(mg/L) | 0.78(0.54–1.45) | 4.8(1.96–10.75) | −6.119 | 0.000 |
| EBV-DNA | 0.13(0.04–0.71) | 2.77(1.25–31.05) | −5.926 | 0.000 |
| Onset time(day) | 4(2–7) | 7(5–10) | −3.084 | 0.002 |
| CD4⁺/CD8⁺ ratio | 0.2(0.14–0.29) | 0.78(0.32–1.56) | −5.601 | 0.000 |
| AST/ALT | 1.12 ± 0.53 | 2.07 ± 1.83 | −2.733 | 0.011 |
| Sex | 0.707 | 0.400 | ||
| Female | 50(37.9%) | 13(46.4%) | ||
| Male | 82(62.1%) | 15(53.6%) | ||
| Hepatomegaly | 0.043 | 0.835 | ||
| No | 26(19.7%) | 6(21.4%) | ||
| Yes | 106(80.3%) | 22(78.6%) | ||
| Splenomegaly | 0.003 | 0.954 | ||
| No | 37(28%) | 8(28.6%) | ||
| Yes | 95(72%) | 20(71.4%) |
NLR neutrophil to lymphocyte ratio, PLR platelet to lymphocyte ratio, PT alanine aminotransferase, AST aspartate aminotransferase, ALT alanine aminotransferase
Analysis of factors influencing EBV-HLH development
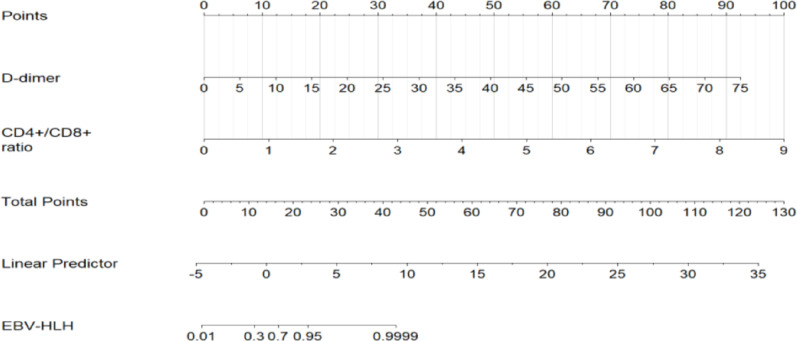
A binary logistic regression model was established, using the occurrence of EBV-HLH as the dependent variable and incorporating the statistically significant indicators from Table 1 as independent variables. The results demonstrated that elevated D-dimer (OR = 1.31 per 1 mg/L increase, 95% CI: 1.08–1.59, P = 0.007) and higher CD4⁺/CD8⁺ ratio (OR = 17.60, 95% CI: 1.89–163.64, P = 0.012) were independently associated with EBV-HLH diagnosis after adjusting for covariates (Table 2). A nomogram (Fig. 1) was constructed to illustrate these predictive variables, with each assigned a point value ranging from 0 to 100. By summing these points and locating them on the total score scale, the corresponding probability of EBV-HLH development could be determined.
Table 2.
Binary logistic regression results
| Variable | β | S.E | Z-value | OR | 95%CI(lower) | 95%CI(upper) | P-value |
|---|---|---|---|---|---|---|---|
| (Intercept) | −5.097 | 1.775 | −2.872 | 0.006 | 0 | 0.198 | 0.004 |
| Age | −0.102 | 0.229 | −0.446 | 0.903 | 0.576 | 1.416 | 0.656 |
| NLR | 0.225 | 0.847 | 0.265 | 1.252 | 0.238 | 6.589 | 0.791 |
| PLR | 0.012 | 0.025 | 0.469 | 1.012 | 0.963 | 1.063 | 0.639 |
| D-dimer | 0.267 | 0.099 | 2.697 | 1.307 | 1.076 | 1.587 | 0.007 |
| EB-DNA | 0.168 | 0.136 | 1.232 | 1.182 | 0.906 | 1.544 | 0.218 |
| Onset time | 0.078 | 0.061 | 1.275 | 1.081 | 0.959 | 1.22 | 0.202 |
| CD4⁺/CD8⁺ ratio | 2.868 | 1.138 | 2.52 | 17.596 | 1.892 | 163.643 | 0.012 |
| AST/ALT | 0.142 | 0.791 | 0.179 | 1.152 | 0.245 | 5.428 | 0.858 |
NLR neutrophil to lymphocyte ratio, PLR platelet to lymphocyte ratio, PT alanine aminotransferase, AST aspartate aminotransferase, ALT alanine aminotransferase
Fig. 1.
The nomogram for prediction of EBV-HLH
Evaluation of the diagnostic performance of CD4⁺/CD8⁺ ratio and D-dimer for EBV-HLH
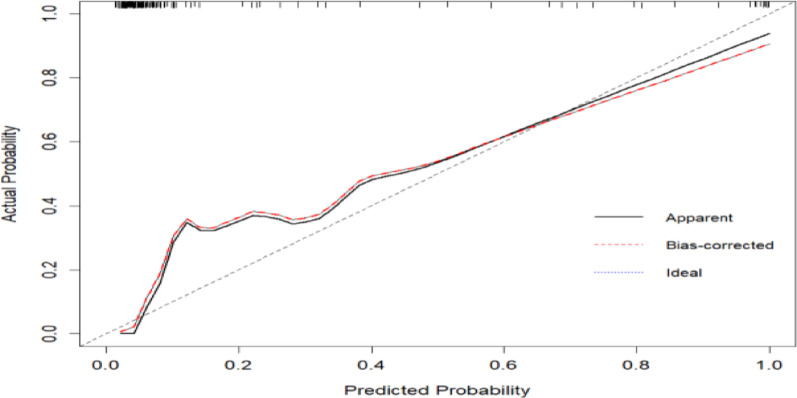
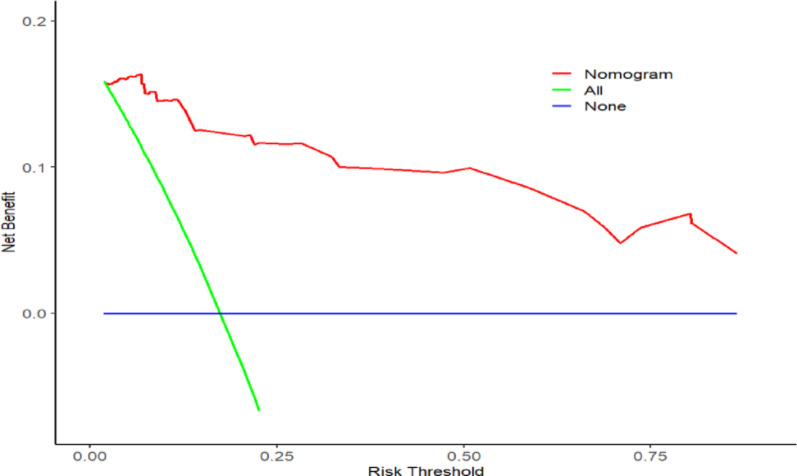
The model was built using the significant factors identified in the logistic regression analysis, and a nomogram was constructed using the rms package. The ROC curve was plotted using the pROC package, with bootstrapped resampling performed 1,000 times. The results showed that the model’s ROC had an AUC of 0.962 (95% CI: 0.9353–0.989), with a specificity of 0.811 and a sensitivity of 1.Through bootstrap resampling (1,000 iterations), the AUC was estimated at 0.962 (95% CI: 0.9311–0.9646), indicating consistent internal validation. A calibration curve was generated using the rms package with 1,000 bootstrap resampling iterations. The results demonstrated that the model curve closely overlapped with the calibration curve and was near the ideal curve, suggesting good agreement between predicted and actual probabilities.Additionally, a Decision Curve Analysis (DCA) was performed using the rmda package. The DCA indicated that the model provided a certain net benefit when the threshold probability was between 0 and 0.2 (Figs. 2, 3, and 4).
Fig. 2.
For CD4⁺/CD8⁺ ratio, D-dimer, and their combination, the optimal cut-off values for diagnosis were 0.455 (AUC 0.837, sensitivity 0.924, specificity 0.714), 1.675 mg/L (AUC 0.869, sensitivity 0.826, specificity 0.857), and 0.068 (AUC 0.962, sensitivity 0.962, specificity 1.000), respectively
Fig. 3.
Calibration curves of the derivation cohort and internal validation cohort
Fig. 4.
Decision curve of the derivation and validation cohorts
Discussion
In typical EBV infections such as IM, B lymphocytes infected by EBV activate a large number of cytotoxic T lymphocytes, leading to the destruction of EBV-infected B lymphocytes and facilitating patient recovery [14, 15].In contrast, for EBV-HLH patients, natural killer (NK) cells and/or T lymphocytes become infected by EBV, resulting in monoclonal proliferation. The disease progresses rapidly, causing multi-organ system dysfunction and posing a severe threat to the patient’s life [16, 17].
The pathogenesis of EBV-HLH is complex and not yet fully understood. However, immune dysfunction is widely recognized as one of its primary causes [18]. In EBV-HLH research, differences in lymphocyte subsets significantly impact patient prognosis. CD4 + T cells, also known as helper T cells, promote the proliferation and differentiation of B cells, T cells, and other immune cells. They can abnormally stimulate the monocyte-macrophage system, leading to its rapid proliferation and activation and the production of large amounts of inflammatory cytokines. The cytokines secreted by CD4 + T cells that induce macrophage proliferation are the initiating factors of HLH. CD8 + T cells, including cytotoxic T lymphocytes (CTLs) and suppressor T cells, primarily function by specifically killing target cells through cytotoxic effects and secreting inhibitory factors to weaken or suppress immune responses. Persistent and abnormal activation of cytotoxic CD8 + T cells, along with the resulting cytokine storm, is the core pathogenic mechanism of HLH. The CD4⁺/CD8⁺ ratio is a sensitive indicator for diagnosing immune dysfunction in humans [10, 19, 20]. When the balance between CD4 + T cells and CD8 + T cells is disrupted, immune dysregulation occurs, leading to excessive cytokine secretion and disease progression.This study found that the EBV-HLH group exhibited a higher CD4⁺/CD8⁺ ratio, which aligns with the findings of Kelkar et al. [21]. This observation contrasts with that reported by Chaturvedi et al. [10]. Their group demonstrated increased expression of PD1, Ki67, and Granzyme B in activated CD8 + T cells (CD8 + CD38high cells) alongside elevated cytokine secretion. We propose that due to persistent activation, CD8 + T cells gradually become hyporesponsive and lose the capacity to secrete effector cytokines following stimulation. Consequently, this impairs their ability to dampen or suppress the immune response, ultimately compromising viral clearance [22].
Coagulation dysfunction is common in HLH, and it is primarily mediated by cytokine storms. Abnormal cytokines can directly or indirectly affect platelets, fibrinogen, and liver function, ultimately disrupting coagulation and fibrinolysis. Studies have shown that in HLH patients, activated macrophages upregulate urokinase plasminogen activator receptor expression on monocytes under the influence of related cytokines. This receptor binds to urokinase-type plasminogen activator, enhancing local fibrinolysis and leading to fibrin degradation [23]. D-dimer is a specific degradation product of cross-linked fibrin by plasmin and serves as an indicator of secondary fibrinolysis. Elevated D-dimer levels suggest increased fibrinolytic activity, reflecting both hypercoagulable and fibrinolytic states, making it a useful biomarker for thromboembolic diseases. Previous studies have indicated that elevated D-dimer is an important independent and persistent risk factor for cardiovascular events and cancer [24]. It is also an early indicator of macrophage activation syndrome (MAS) in febrile patients with active rheumatic disease and a marker for severe infections [25, 26]. A 2021 study suggested that EBV infection in HLH patients can significantly affect coagulation function, with D-dimer demonstrating a high sensitivity (88.90%) in predicting EBV-HLH [21]. In our study, peripheral blood D-dimer levels were significantly higher in EBV-HLH patients compared to IM patients. This finding suggests that elevated peripheral blood D-dimer levels may help distinguish EBV-HLH from IM. The underlying mechanism may involve the excessive production of plasminogen activators by activated macrophages in EBV-HLH patients, leading to the degradation of cross-linked fibrin and subsequent abnormal elevation of D-dimer levels.
In this diagnostic analysis, both elevated CD4⁺/CD8⁺ ratio (Youden index = 0.638) and D-dimer (Youden index = 0.683) showed significant discriminatory capacity for EBV-HLH. Optimal diagnostic thresholds were > 0.455 for CD4⁺/CD8⁺ ratio and > 1.675 mg/L for D-dimer. The combined model achieved markedly higher accuracy (AUC = 0.962, 95%CI: 0.935–0.989) than either marker alone (vs. CD4⁺/CD8⁺ ratio AUC = 0.837, *P*=0.002; vs. D-dimer AUC = 0.869, *P*=0.008).
There are several limitations to this study. First, as a retrospective analysis, we were unable to monitor the progression from fever to the diagnosis of EBV-HLH in patients. Second, due to the single-center nature of this study, the reproducibility of our results needs to be validated through larger, multicenter studies.
Conclusions
CD4⁺/CD8⁺ ratio and D-dimer are potential diagnostic biomarkers for distinguishing EBV-HLH from EBV-IM in pediatric patients. Higher levels of CD4⁺/CD8⁺ ratio and elevated D-dimer were significantly associated with EBV-HLH in our cohort.Their combined use may improve diagnostic accuracy for EBV-HLH, pending validation in prospective studies.
Supplementary Information
Acknowledgements
Not applicable.
Author contributions
Conceptualization, Y.C.,B.C., W.J., W.L.,J.J.and Y.D.; methodology, Y.C. and B.C.; software, Y.C.; validation, B.C.; formal analysis, Y.C.; investigation, Y.C.; data curation, Y.C.; writing—original draft, Y.C.; writing—review and editing, Y.C. and B.C.; visualization, Y.C.; supervision, B.C.W.J., W.L.,and Y.D.; and project administration, Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study received no external funding.
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Anhui Provincial Children’s Hospital (Ethical Approval No.: EYLL-2023-041) and conducted in accordance with the Declaration of Helsinki. Patient data were de-identified prior to analysis and accessible only to the research team. The Ethics Committee formally waived informed consent under national regulations (Article 39, Regulations on Ethical Review of Biomedical Research Involving Humans, China 2016) due to the retrospective design.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Canna SW, Marsh RA. Pediatric hemophagocytic lymphohistiocytosis. Blood. 2020;135(16):1332–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WeiBo G, LiJuan H, XiaoLu M, et al. A predictive model for identifying secondary underlying diseases of hemophagocytic lymphohistiocytosis. Front Immunol. 2023;14:1143181–1143181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang R, Wu D, Wang L, Liu P, Zhu X, Huang L, Chen M, Lv X. A predictive model for Epstein-barr virus-associated hemophagocytic lymphohistiocytosis. Front Immunol. 2024;15:1503118. 10.3389/fimmu.2024.1503118. PMID: 39703509; PMCID: PMC11655318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paul RL, AnnaCarin H, Melissa H, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133(23):2465–77. [DOI] [PubMed] [Google Scholar]
- 5.Salama HA, Jazieh AR, Alhejazi AY, et al. Highlights of the management of adult Histiocytic disorders: Langerhans cell histiocytosis, Erdheim-Chester disease, Rosai-Dorfman disease, and hemophagocytic lymphohistiocytosis. Clin Lymph Myelom Leuk. 2021. 10.1016/j.clml.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrissette K, Bridwell R, Lentz S, et al. Hemophagocytic lymphohistiocytosis in the emergency department: recognizing and evaluating a hidden threat. J Emerg Med. 2021. 10.1016/j.jemermed.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ponnatt TS, Lilley CM, Mirza KM. Hemophagocytic Lymphohistiocytosis. Arch Pathol Laboratory Med. 2021;146(4):507–19. [DOI] [PubMed] [Google Scholar]
- 8.Sepulveda EF, Basile SDG. Hemophagocytic syndrome: primary forms and predisposing conditions[J]. Curr Opinion Immunol. 2017;49:20–6. [DOI] [PubMed] [Google Scholar]
- 9.Xun L, Haipeng Y, Zhenghui X, et al. A Three-Step Screening Procedure for Early Identification of Children at High Risk of Hemophagocytic Lymphohistiocytosis.[J]. J Clin Immunol. 2023;43(5):989–98. [DOI] [PubMed] [Google Scholar]
- 10.Chaturvedi V, Marsh RA, Zoref-Lorenz A, et al. T-cell activation profiles distinguish hemophagocytic lymphohistiocytosis and early sepsis. Blood. 2021. 10.1182/blood.2020009499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gan JP, Xie LL, Lu XL. Clinical early warning and treatment of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. Chin J Pediatr Emerg Med. 2024;31(11):818–24. 10.3760/cma.j.issn.1673-4912.2024.11.004. [Google Scholar]
- 12.Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J, Janka G. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124–31. 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 13.Principle suggestions for diagnosis and treatment of main nontumorous. Epstein-Barr virus-associated diseases in children] [J]. Zhonghua er ke za zhi = Chinese J Pediatr. 2016;54(8):563–8. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe Y, Mashimo S, Ichige H, Nagata H, Kojima M. Scrub typhus mimicking the clinical course of infectious mononucleosis: a case report. J Rural Med. 2021;16(1):62–6. 10.2185/jrm.2020-037. Epub 2021 Jan 5. PMID: 33442438; PMCID: PMC7788302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hicks J, Boswell B, Noble V. Traumatic splenic laceration: a rare complication of infectious mononucleosis in an athlete. Curr Sports Med Rep. 2021;20(5):250–1. 10.1249/JSR.0000000000000840. [DOI] [PubMed] [Google Scholar]
- 16.Garonzi C, Chinello M, Cesaro S. Emapalumab for adult and pediatric patients with hemophagocytic lymphohistiocytosis. Expert Rev Clin Pharmacol. 2021;14(5):527–34. Epub 2021 Mar 23. PMID: 33686916. [DOI] [PubMed] [Google Scholar]
- 17.Cutini I, Gozzini A, Nozzoli C, Boncompagni R, Innocenti C, Fani A, Saccardi R. Toxoplasmosis-associated hemophagocytic lymphohistiocytosis in allogeneic transplantation. J Clin Immunol. 2021;41(4):843–6. 10.1007/s10875-021-00979-8. Epub 2021 Jan 30. PMID: 33515363. [DOI] [PubMed] [Google Scholar]
- 18.Cai L, Xing Y, Xia Y, Zhang Z, Luo Z, Tang Y, Chen Y, Xu X. Comparative study of biomarkers for the early identification of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in infectious mononucleosis. BMC Infect Dis. 2023;23(1):728. 10.1186/s12879-023-08654-6. PMID: 37880605; PMCID: PMC10601177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qi A, Yi W, Shaoyan H, et al. Clinical significance of lymphocyte subset changes in hemophagocytic lymphohistiocytosis of children.[J]. Exp Therapeutic Med. 2016;12(6):3549–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yunyun Z, Chengrong H,,Hao Z, et al. Characteristics of immunological events in Epstein-Barr virus infection in children with infectious mononucleosis. Front Pediatr. 2023;11:1060053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelkar MG, Bargir UA, Malik-Yadav R, Gupta M, Dalvi A, Jodhawat N, Shinde S, Madkaikar MR. CD8 + T cells exhibit an exhausted phenotype in hemophagocytic lymphohistiocytosis. J Clin Immunol. 2021;41(8):1794–803. 10.1007/s10875-021-01109-0. [DOI] [PubMed] [Google Scholar]
- 22.Shi J, Chu C, Yu M, et al. Clinical warning of hemophagocytic syndrome caused by Epstein-Barr virus. Ital J Pediatr. 2021. 10.1186/s13052-020-00949-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heissig B, Salama Y,,Takahashi S, et al. The multifaceted role of plasminogen in inflammation[J]. Cell Signal. 2020;75:109761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simes J, Robledo KP, White HD, et al. D-Dimer predicts Long-Term Cause-Specific mortality, cardiovascular events, and cancer in patients with stable coronary heart disease: LIPID Study. Circulation. 2018;138(7):712–23. [DOI] [PubMed] [Google Scholar]
- 25.Groom AA, AnnaCarin H, Fabrizio BD. Macrophage activation syndrome in the era of biologic therapy. Nat Rev Rheumatol. 2016;12(5):259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodelo RJ, Rosa LDG,,Valencia LM, et al. d -dimer is a significant prognostic factor in patients with suspected infection and sepsis[J]. Am J Emerg Med. 2012;30(9):1991–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.