Abstract
Background
Curing is an essential process for transforming tobacco leaves into an economic product. Optimizing curing parameters to regulate the orderly senescence and apoptosis of tobacco cells serves as a direct approach to enhancing the softness of leaves and a critical guarantee of quality. Therefore, understanding the intrinsic mechanisms underlying leaf softening during curing is vital for curing conditions and improving tobacco usability.
Results
To elucidate the degradation patterns of key cell wall components and dynamic changes in related enzyme activities during the softening of tobacco leaves during curing stage, we systematically analyzed the softening progression, cell wall structure, and dynamic transformation of key metabolites in the flue-cured variety Yunyan 87. The results demonstrated that leaf softness peaked during the yellowing stage (minimal softness value: 11.83mN). The cell wall structure progressively disintegrated throughout curing. Pectin methylesterase (PME), polygalacturonase (PG), and pectin lyase (PL) exhibited higher activities in fresh leaves and the yellowing stage, with corresponding values of 8.33 and 20.56 U/g for PME, 1.20 and 1.33 U/g for PG, and 2.39 and 4.93 U/g for PL. The cell wall matrix content decreased significantly during curing, reaching 412.84 mg/g in the drying stage.LC-MS/MS analysis identified 1,220 metabolites with significant alterations during curing, 54 of which were closely associated with cell wall metabolism. We further delineated metabolic pathways for cellulose, pectin, and lignin.
Conclusions
This study comprehensively investigated the metabolic basis of tobacco leaf softening during curing, identifying the fresh leaves and yellowing stage as critical regulatory nodes. These findings provide valuable references for optimizing tobacco curing parameters.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-025-07111-7.
Keywords: Flue-cured tobacco, Softness mechanisms, Cell wall components, Metabolic pattern, Curing stage
Introduction
Softening is an important physiological change caused by the degradation of cell wall material during the postharvest processing of fruits and leaves, which plays a very important role in both plant processing quality and storage and has a remarkable impact on agricultural production and food processing [1]. The plant cell wall is a complex network of structures consisting mainly of cellulose, hemicellulose, pectin, and lignin, which provide structural support and protection for the cell [2–5]. Numerous studies have shown that postharvest fruit firmness is closely related to changes in cell wall composition, such as the degradation of pectin and cellulose and lignin accumulation [6–11]. Studies by Li [12] have shown that softness is closely associated with key quality factors, such as total sugar, reducing sugar, and potassium contents. Improving softness helps to enhance processing quality. In summary, their findings further indicate a strong relationship between leaf softness and tobacco quality, with softer leaves typically exhibiting better quality, whereas harder leaves are associated with lower quality.Postharvest leaf softening is closely related to cell wall metabolism [12, 13]. Tobacco, a model crop commonly used in research, gradually changes colour from green to yellow under suitable temperature and humidity conditions after harvest. Key cell wall components such as cellulose, hemicellulose, and lignin gradually decompose during the curing process. These components break down into small molecular compounds that enter other cellular metabolic pathways, supporting energy metabolism and respiration, participating in the leaf’s antioxidant systems [14–17]. Some decomposition products also serve as integral components of tobacco aroma and quality. As cell wall materials degrade, the cellular structure progressively disintegrates, initiating the senescence and apoptosis program in cells [18, 19]. The softening and transformation of substances accompanying this process have important impacts on the processing performance and usability of the tobacco leaves [20]. To effectively increase cell wall degradation efficiency, external factors can be applied to regulate the process. For example, mechanical stress-induced defects in the cellulose structure can create sites conducive to enzymatic degradation, thereby facilitating the breakdown of cellulose [21]. Additionally, high temperatures accelerate cell wall relaxation, facilitating the degradation of cellulose and pectin [22]. Furthermore, the degradation and reorganization of lignin can be influenced by exogenous additives or the modulation of lignin synthesis, thereby regulating both cell wall plasticity and stability [23, 24]. Postharvest fruits and leaves are strongly affected by the ambient temperature and humidity. During tobacco processing, significant changes occur in leaf color, sugar content, amino acids, and aroma compounds with variations in temperature and moisture content.The degradation of the cell walls of postharvest plant tissues is influenced mainly by the activity of cell wall-degrading enzymes. Increasing the activity of degrading enzymes can effectively improve the degradation efficiency of cell wall components [25]. Short-term high-temperature treatment enhances the activity of cellulase (Cx) and PG promoting cellulose decomposition and accelerating the formation of water-soluble pectin. However, sustained high temperatures (> 45°C) inactivatePME and PG, leading to protopectin accumulation. Concurrently, elevated temperatures stimulate respiration rates (increasing by 2–3 fold per 10°C rise), accelerating the consumption of cell wall polysaccharide breakdown products and inducing tissue softening [26–29].
However, when the cell wall is damaged or exposed to environmental stress, the cell wall integrity monitoring system detects changes in the wall through receptor-like kinases (RLKs) and signalling pathways. In response, the system activates appropriate repair mechanisms, prompting the cell to remodel its wall by regulating the synthesis and assembly of its structural components, thereby adapting to external stress [30–32]. Degradation products of cell wall components can also undergo re-cross-linking, which enhances the stability of the cell wall and reduces its softness [15, 16, 33–35]. These mechanisms work together to prevent the complete degradation of cell wall components to some extent.
Although numerous studies have investigated the mechanisms involved in plant tissue softening, studies on the mechanisms of leaf softening in postharvest plants under continuous processing and warming conditions are lacking. In particular, there is a gap in the understanding of how key metabolites regulate cell wall degradation and drive changes in softness, which has not been systematically analysed. Therefore, this study aimed to investigate the mechanism of tobacco leaf softening using tobacco as a model organism. Our approach integrated morphological observations, softness measurements, ultrastructural analysis, determination of cell wall components and related enzyme activities, and metabolomics technology to identify key metabolites. This comprehensive approach will extend the metabolic network analysis and provide a more complete understanding of the dynamic regulation of cell wall degradation during softening. Ultimately, this research provides a theoretical basis for the application of temperature control technologies in related fields, thereby improving the quality and efficiency of food processing.
Materials and methods
Plant growth and sampling
Nicotiana tabacum L. var Yunyan 87 was cultivated at the research farm of the Guizhou Academy of Tobacco Science in Fuquan city, Guizhou Province, China (26°74′N, 107°50′E), which is located at an altitude of 1200 m and has a subtropical monsoon climate. The soil composition consisted of 25320 mg/kg organic matter, 138.73 mg/kg available nitrogen (N), 36.32 mg/kg phosphorus (P), and 218.69 mg/kg potassium (K), and the pH value was 6.2. The experiment was conducted with a randomized block design, with each plot covering an area of 121 m2 and a planting density of 1.1 m × 0.55 m, which was replicated three times. Seeds were sown on January 20th, followed by transplantation on April 25th. The average temperatures ranged from 16.13°C to 23.17°C in May, 19.23°C to 24.93°C in June, 22.87°C to 29.83°C in July, and 21.30°C to 30.37°C in August. The base fertilizer regime included 525 kg/ha of compound fertilizer (with an N: P:K ratio of 10:10:25), 450 kg/ha of fermented oilseed meal and 375 kg/ha of calcium-magnesium-phosphate fertilizer. On the day of transplantation, each plant was treated with 150–200 mL of a water-soluble fertilizer solution containing 1% compound fertilizer and 0.28% cyhalothrin emulsifiable concentrate. Ten days after transplantation, an additional 100–150 mL of water-soluble fertilizer, formulated with 4% compound fertilizer, was applied per plant. This application was repeated 30 days posttransplantation, and the same volume and concentration of fertilizer were maintained. All cultivation and management measures were uniformly maintained for consistency [36].
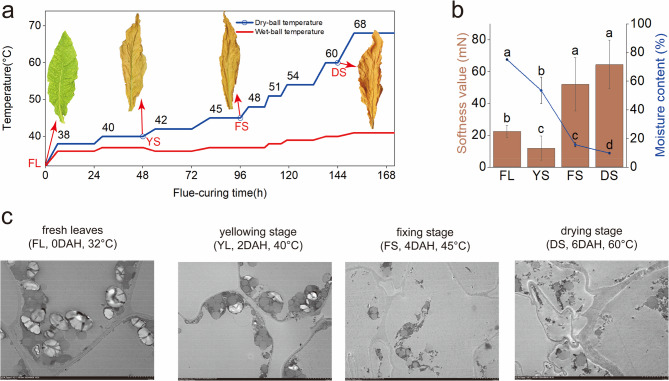
Fresh leaf (FL, 0 DAH, 32°C, 105 days after transplanting) samples were collected from the labelled plants in the field. The remaining marked leaves were transferred to a standard tobacco curing room and subjected to curing following the defined protocol. Detailed information on the curing process is provided in Fig. 1a. Curing leaves at the end of the yellowing stage (YS, 2 DAH, 40°C), fixing stage (FS, 4 DAH, 45°C), and drying stage (DS, 6 DAH, 60°C) were collected (Abbreviations: DAH = Day After Harvest). For each stage, 20 leaves (FL, YS, FS, DS) were selected and assigned to five groups (biological replicates, n = 5 per group). Using the half-leaf method, each leaf sample was divided into two parts: one portion was immediately frozen in liquid nitrogen for subsequent cell wall composition analysis, cell wall-degrading enzyme activity assays, and metabolomic sequencing (LC-MS), while the other portion was processed immediately to determine moisture content and softness values.
Fig. 1.
Multiscale evolution of tobacco leaf properties during curing. a Curing process and morphological features: Fresh leaves (FL), Yellowing stage (YS), Fixing stage (FS), Drying stage (DS). The blue line indicates the dry-bulb temperature in the curing chamber, and the red line represents the wet-bulb temperature. Red arrows point to representative leaf morphology at corresponding stages. b Bar graph shows softness values (left Y-axis; higher values indicate poorer flexibility). Line graph displays relative moisture content (right Y-axis, %). Different lowercase letters denote significant differences in softness and moisture content across stages (Duncan’s test, p < 0.05). c Ultrastructural Observations: Transmission electron microscopy (TEM) images (Scale bar = 5 μm, consistent across all panels). From left to right: FL, YS, FS, and DS
Moisture content
The moisture content of the tobacco leaves was determined by strict adherence to the method outlined in “Tobacco and tobacco products-Preparation of test sample and determination of water content-Oven method” (YC/T 31-1996). The specific procedure is as follows: first weigh the dry weighing dish and record the mass as m₀; add the sample to be tested and weigh, recording the mass as m₁; after drying to constant weight, weigh again and record as m₂. The moisture content (%) is calculated using the following formula: Moisture % = [(m₁ - m₂)/(m₁ - m₀)] × 100.
Softness analysis
The softness value of the tobacco leaves was determined as described by Wu [37]. Tobacco leaf softness was measured with an RH-R1000 instrument, in which the rectangular section of the leaf was placed into the measuring slot. The instrument recorded the total bending resistance of the leaf vane and the friction on both sides of the measuring slot, which were used to quantify the softness of the tobacco leaf. Softness is expressed in millinewtons (mN). From each replicated tobacco leaf sample, 5 × 2 cm rectangular sections were cut from the tip (veins 3–4), middle (veins 6–7), and base (veins 11–12). The leaf softness value was measured at an ambient temperature of 22 ± 1°C and a relative humidity of 60 ± 2%.
Ultrastructural observation
Ultrastructural observation of tobacco leaves was performed as described by Wu [19]. Sample sections of 1 mm² (2 cm from the midrib) were cut from the middle portion of the labelled leaves. Ultrastructural changes were studied by observing ultrathin sections of the leaf palisade tissue at 0, 48, 72 and 144 h with a Hitachi H-600 electron microscope (Kyoto, Japan).
Cell wall-related substance content
The contents of cellulose, hemicellulose, lignin, protopectin, and soluble pectin were determined with the assay kits:
Cellulose Content Assay Kit (Solarbio, China; Cat. No.BC4285): Samples were homogenized in acidic extraction buffer, water-bathed, and centrifuged to obtain crude cell walls. After starch removal with alkaline solution and drying, hydrolysis was performed with concentrated sulfuric acid in an ice bath. The supernatant was centrifuged, and absorbance at 620 nm was measured for quantification via a standard curve [38].
Hemicellulose Content Assay Kit (Solarbio, China; Cat. No.BC4445): Dried samples were reacted with buffer and centrifuged. The pellet was treated with alkaline extraction buffer; the supernatant was mixed with anthrone-sulfuric acid reagent, developed at 90°C, cooled, and measured at 540 nm for standard curve quantification [38].
Lignin Content Assay Kit (Solarbio, China; Cat. No.BC4200): Dried samples were treated with acetyl bromide solution, centrifuged, washed, mixed with glacial acetic acid, hydrolyzed at 80°C, and centrifuged. The supernatant absorbance at 280 nm was quantified using a standard curve [39].
Protopectin Content Assay Kit (Solarbio, China; Cat. No.BC3685): Samples were homogenized in ammonium oxalate buffer, water-bathed, and centrifuged. Starch was removed with acid-alcohol solution, followed by hot acid extraction of the supernatant. Absorbance at 530 nm was measured for standard curve quantification [40].
and Soluble Pectin (WSP) Content Assay Kit (Solarbio, China; Cat. No.BC4125): Samples were water-extracted, homogenized, and centrifuged. The supernatant was reacted with concentrated sulfuric acid and m-hydroxydiphenyl for color development. Absorbance at 530 nm was measured for standard curve quantification [40].
Measurements of enzyme activity
The activities of PG, PL, PME, endo-β−1,4-glucanase, exo-β−1,4-glucanase, and β-GAL were sequentially measured with the following assay kits:
Polygalacturonase(PG) Activity Assay Kit (Solarbio, China; Cat. No.2665): Samples were homogenized in an ice bath, centrifuged at high speed at 4°C, and the supernatant was reacted with polygalacturonic acid substrate at 40°C for 2 h. The reaction was terminated by boiling water bath, and activity was determined spectrophotometrically at 540 nm via a standard curve [41].
Pectin Lyase(PL) Activity Assay Kit (Solarbio, China; Cat. No.BC2640): Samples were homogenized and centrifuged to collect supernatant, which was reacted with pectin substrate at 40°C for 30 min. Enzyme activity was calculated based on real-time absorbance increments at 235 nm [42].
Pectin Esterase(PE) Activity Assay Kit (Solarbio, China; Cat. No.BC2700): Supernatant from homogenized/centrifuged samples was added to a thermostatic reaction system (37°C) containing pectin substrate at pH 7.8. Activity was quantified by NaOH consumption required to maintain constant pH [43].
Cellulase(CL) Activity Assay Kit (Solarbio, China; Cat. No.BC2545): Samples were homogenized/sonicated and centrifuged. The supernatant was incubated with carboxymethyl cellulose sodium substrate at 40°C for 30 min. After termination by boiling, activity was measured spectrophotometrically at 540 nm using a standard curve [44].
1,4-β-D-Glucan Cellobiohydrolase(C1) Activity Assay Kit (Solarbio, China; Cat. No.BC4300): Supernatant from homogenized/centrifuged samples was reacted with p-nitrophenyl cellobioside at 37°C for 1 h. Activity was determined by direct absorbance measurement at 400 nm via standard curve [45].
Lactase/β-Galactosidase(β-GAL) Activity Assay Kit (Solarbio, China; Cat. No.BC2585): Supernatant from homogenized/centrifuged samples was incubated with p-nitrophenyl-β-D-galactopyranoside at 37°C for 30 min. Enzyme activity was calculated based on absorbance increment rate at 400 nm [46].
LC-MS analysis
Tobacco samples were lyophilized using a freeze-dryer (Pilot FD8-15.6 V, SIM, America), pulverized through an 80-mesh sieve after drying, A 50 µg sample of freeze-dried powde was accurately weighed using an analytical balance (XPR2U, Mettler-Toledo, Switzerland) and transferred into a 1.5 mL Microcentrifuge tube(Eppendorf, Germany), followed by the addition of 800 µL of precooled methanol: water extraction solution (7:3, v/v) and 20 µL of internal standard 1 (d3-Leucine, 13C9-Phenylalanine, d5-Tryptophan, 13C3-Progesterone). The mixture was homogenized for 10 min at 50 Hz using a tissue homogenizer (JXFSTPRP, Shanghai Jingxin, China) and then subjected to 30 min of ultrasonic treatment in a 4°C water bath. Prior to homogenization, the sample was briefly vortexed using a vortex mixer (QL-901, Qilinbeier Instrument, China) to ensure adequate mixing. After standing at −20°C for 1 h, the samples were centrifuged at 14000 rpm for 15 min at 4°C using a high-speed refrigerated centrifuge (Centrifuge 5430, Eppendorf, Germany). The supernatant (600 µL) was filtered through a 0.22 μm membrane, and 20 µL of the filtered solution from each sample was pooled to create a QC sample for assessing reproducibility and stability in LC‒MS analysis. Both the filtered and QC samples were subsequently transferred to 1.5 mL sample vials for instrumental analysis. Metabolite separation and detection were performed with a Waters 2777 C UPLC system (Waters, USA) coupled with a Q Exactive HF high-resolution mass spectrometer (Thermo Fisher Scientific, USA). Chromatographic separation was performed with a Hypersil GOLD aQ column (1.9 μm, 2.1 × 100 mm; Thermo Fisher Scientific, USA). The mobile phases consisted of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). The gradient elution conditions were as follows: 0–2 min, 5% B; 2–22 min, 5–95% B; 22–27 min, 95% B; 27–27.1 min, 95% B to 5% B; and 27.1–30 min, 5% B, with a flow rate of 0.3 mL/min and a column temperature of 40°C [36].
The mass spectrometer was operated in both positive and negative ion modes. The mass scan range for precursor ions was 125–1500 m/z (positive) and 100–1500 m/z (negative), with a resolution of 120,000 at m/z 200. The automatic gain control (AGC) target for positive and negative ions was set to 1e6 and 3e6, respectively, with a maximum injection time (IT) of 100 ms. The top 3 precursor ions were selected for fragmentation, and secondary data were collected at a resolution of 30,000. The AGC for positive and negative ions in the second stage was set to 2e5 and 1e5, respectively, with an IT of 50 ms. The stepped NCE was set to 20, 40, and 60 eV. The ion source parameters were as follows: sheath gas flow rate, 40 l/min; auxiliary gas flow rate, 12 l/min; spray voltages, 3.80 kV (positive mode) and 3.20 kV (negative mode); ion transfer tube temperature, 320°C; and auxiliary gas heater temperature, 350°C.
The mass spectrometry data were imported into Compound Discoverer 3.3 software (Thermo Fisher Scientific, USA) and analysed with the BGI Metabolomics Database (BMDB), mzCloud, and ChemSpider online databases to obtain metabolite peak areas and identification results. Subsequent bioinformatics analysis involved comprehensive data preprocessing, quality control, global analysis, and differential screening between groups with the BGI online platform (https://biosys.bgi.com/). Differentially abundant metabolites (DAMs) were identified on the basis of VIP ≥ 1, fold change ≥ 1.20 or ≤ 0.80, and p value ≤ 0.05. Hierarchical cluster analysis (HCA) was performed on the samples and metabolites, and the results are presented as heatmaps and dendrograms. Pearson correlation coefficients (PCCs) between samples were calculated with the cor function in R. HCA was performed with the pheatmap R package. Metabolites were annotated with the KEGG compound database (http://www.kegg.jp/kegg/compound/) and mapped to corresponding pathways in the KEGG pathway database (http://www.kegg.jp/kegg/pathway.html).
Data analysis
The data are presented as the means ± standard deviations from repeated experiments. Statistical significance was determined with one-way ANOVA in SPSS version 25.0, with the significance level set at p < 0.05. All the photographs and figures were processed and analysed with Adobe Illustrator 2020 or OriginPro 2021 software.
Results
Assessments of physical properties and ultrastructure
As shown in Fig. 1, with increasing postharvest processing time, the processing temperature gradually increased, and the colour of the tobacco leaf blade transitioned from green to yellow during the curing process. At the FL, the leaf blade, main veins, and branch veins were predominantly green. During the YS, the leaf blade turned yellow, whereas the main and branch veins remained green. In the FS, the branch veins turned completely white, whereas the main veins remained mostly unchanged. During the drying stage, the main veins of the tobacco leaf had turned either white or yellow (Fig. 1a). The softness value of the tobacco leaves initially decreased but then increased with increasing postharvest processing time. During leaf softening and wilting, the softness value of tobacco leaves at the YS(11.83 mN) was significantly lower than that of FL(22.43 mN). However, with increasing temperature and prolonged curing duration, the softness values at the FS(52.00 mN) and DS (62.41 mN) became significantly higher than those observed in both FL and the YS. Moreover, the moisture content of the tobacco leaves decreased significantly throughout the process, from 75.11% in the FL to 9.67% in the DS. The change in softness value during the curing process was closely associated with the moisture content (Fig. 1b). Ultrastructural analysis of the tobacco leaf cell wall (Fig. 1c) revealed gradual destabilization of the cell structure and function, with increasing damage leading to complete deconstruction under conditions of extended processing and elevated temperature. At the FL, the leaf cell wall was intact, and the starch granules were structurally full. During the YS, the starch granules in the chloroplasts progressively decomposed, while the number of lipid droplets increased significantly, and the mitochondria showed signs of partial swelling. As processing continued through the FS and the drying stage, the leaf cell wall tension decreased, chloroplasts and mitochondria were severely damaged, lipid droplets fused abnormally, starch granules were nearly completely decomposed, and the cellular energy supply was impaired. The observed alterations in leaf ultrastructure during the curing process align with findings reported by Wu et al. [17, 19]. As the cell wall lost its structural function, the cell structure collapsed, and the degree of leaf softness value continued to increase.
Assessments of cell wall substance content and enzyme activity
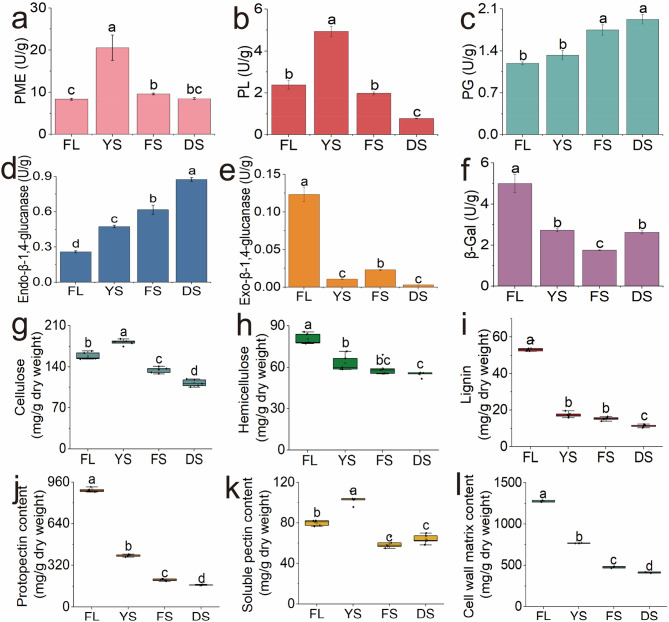
The changes in the softness of tobacco leaves during the curing process were closely associated with alterations in the cell wall structure and the composition of cell wall substances. Additionally, the changes in the contents of these substances were linked to the activities of key enzymes. Figure 2 illustrated the dynamic changes in the activities of key cell wall enzymes and their associated metabolites during the curing process of tobacco leaves. As postharvest processing continued and the temperature increased, Changes in cell wall-degrading enzyme activities are shown in Fig. 2a-f. Specifically, PME and PL exhibited a parabolic trend, with activities increasing rapidly from the FL to the YS, reaching 21.73 U/g and 4.93 U/g, respectively, which were twice as high as the activity values of the fresh tobacco leaf stage, and subsequently declined significantly as curing progressed. The activities of PG and endo-β−1,4-glucanase significantly increased throughout the curing process. In contrast, the activities of β-gal and exo-β−1,4-glucanase significantly decreased during the curingcuring process, especially that of exo-β−1,4-glucanase, whose activity was significantly lower than that of fresh tobacco after the yellowing stage (2 DAH, 40°C).
Fig. 2.
Dynamic changes in cell wall components and key enzymatic activities of tobacco leaves during curing stage. Enzyme activities of PME a, PL b, PG c, endo-β−1,4-glucanase d, exo-β−1,4-glucanase e and β-gal f; The contents of cellulose g, hemicellulose h, lignin i, protopectin j, soluble pectin k, and cell wall matrix content l; The horizontal axes of all subplots consistently represent the curing stages from left to right as: Fresh leaf (FL), yellowing stage (YS), fixing stage (FS), and drying stage (DS)
Cellulose, hemicellulose, protopectin, and lignin are key components of the cell wall, and changes in their contents play crucial roles in maintaining the structural stability of the cell wall [47]. Figure 2(g-h) illustrates the transformation and metabolism of these key substances during the curing process of tobacco leaves. In fresh tobacco leaves, the contents of cellulose, hemicellulose, lignin, and protopectin were 158.59 mg/g, 79.61 mg/g, 53.10 mg/g, and 904.60 mg/g, respectively. By the DS, these values declined significantly to 110.43 mg/g, 55.62 mg/g, 11.68 mg/g, and 168.69 mg/g. The contents of these cell wall components, including cellulose, hemicellulose, protopectin, and lignin, decreased throughout the curing process, reflecting the natural maturation and senescence of tobacco cells [48]. In contrast, The soluble pectin content exhibited stage-specific dynamics: 79.63 mg/g in FL, 106.21 mg/g at YS, 59.80 mg/g during FS representing the peak level,, and 66.42 mg/g at DS.Crucially, the significant increase at YS demonstrated enzymatic conversion of protopectin to soluble pectin. Cell wall matrix content plummeted during the curing process, measuring 1,275.83 mg/g in fresh tobacco leaves, 767.31 mg/g at theYS, 477.60 mg/g at the FS, and 412.84 mg/g at the DS.
Screening and clustering analysis of cell Wall-Related metabolites
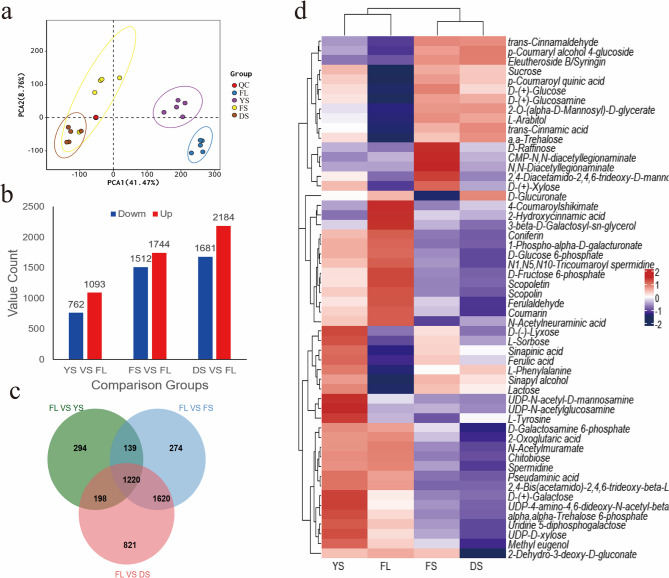
The base peak chromatograms (BPC) of analyzed samples are presented in Supplementary Figure S1, showing results in both positive ionization mode (Pos) and negative ionization mode (Neg). PCA revealed distinct patterns of metabolite changes during the curing process, as shown in Fig. 3a. PCA1 and PCA2 explained 50.23% of the variance in the broad-targeted metabolomics analysis of tobacco leaves across different stages. Specifically, the metabolites of the FL and the YS were significantly separated from the main components of the FS and the DS. Notably, the metabolite distribution in the DS was clustered around that of the FS. These results suggested that processing time and temperature had a substantial effect on the distribution and transformation patterns of metabolites. The differentially abundant metabolite contents across different curing stages are depicted in Fig. 3b. The number of differentially abundant metabolites gradually increased with increasing postharvest processing time and elevated temperature, with upregulated metabolites consistently outnumbering downregulated metabolites. The Venn diagrams of the differentially abundant metabolites in tobacco leaves at different stages are shown in Fig. 3c. The 1220 common differentially abundant metabolites changed throughout the curing process, Among the 1,220 differentially abundant metabolites identified throughout the curing process, 307 were successfully characterized (Supplementary Table S1). These annotated metabolites were classified into 15 major categories, prominently featuring 48 terpenoids, 40 heterocyclic compounds and lipids, 31 phenylpropanoids, and 12 flavonoids, among others (Figure S3).
Fig. 3.
Integrated metabolomic profiling of tobacco leaves during curing stage. a Principal component analysis (PCA) plot of metabolomic data showing separation of sample groups across curing stages. b Bar graph displaying the count of metabolites upregulated and downregulated in the yellowing stage (YS), fixing stage (FS), and drying stage (DS) relative to the fresh leaves(FL). c Venn diagram illustrating the overlap of differentially abundant metabolite profiles identified in the YS, FS, and DS stages compared to FL. d Heatmap showing relative abundance of clustered metabolites associated with cell wall components, selected from the global metabolome via pathway screening (Table 1), color scale represents z-scores of log₂-transformed relative abundance with red indicating above-average abundance, blue indicating below-average abundance, and saturation corresponding to the absolute value of the z-score.
On the basis of the statistical analysis of metabolic pathways associated with key cell wall substances (Table 1), a clustering analysis was performed on the broad-targeted metabolomics data (Fig. 3d). The key cell wall substances, such as cellulose, lignin and pectin metabolism-related substances, all presented clear categorical clustering in the analysis, as did relatively more consistent dynamic patterns of change. Lignin metabolism-related metabolites, including sinapinic acid, ferulic acid, and sinapyl alcohol, exhibited a distinct decreasing trend during the yellowing stage. Key metabolites related to cellulose and pectin metabolism, such as D-(+)-glucosamine, UDP-4-amino-4,6-dideoxy-N-acetyl-beta-L-altrosamine, 2,4-bis(acetamido)−2,4,6-trideoxy-beta-L-altropyranose, and D-(+)-galactose, gradually decreased during the curing process. Similarly, the content of possible lignin decomposition products such as coumarin and scopolin tended to decrease significantly during the curing process.
Table 1.
Key cell wall components involved in KEGG metabolic pathways and associated references
| Cell wall components | Associated metabolic pathways | References |
|---|---|---|
| Pectin |
Fructose and mannose metabolism (map00051) Galactose metabolism (map00052) |
Mohnen, D [49]. Scheller, H. V [50]. |
| Cellulose |
Sucrose metabolism (map00500) Nucleotide sugars metabolism (map00520) |
Somerville, C [2]. Endler, A [51]. Seifert, G. J [52]. Tao, J [53]. |
| Hemicellulose | Pentose and glucuronate interconversions (map00040) |
Scheller, H. V [54]. Rennie, E. A [55]. |
| Lignin | Phenylpropanoid biosynthesis (map00940) |
Boerjan, W [56]. Bonawitz, N. D [57]. |
Metabolic pathway analysis of key cell wall metabolites
Analysis of cellulose and pectin pathway
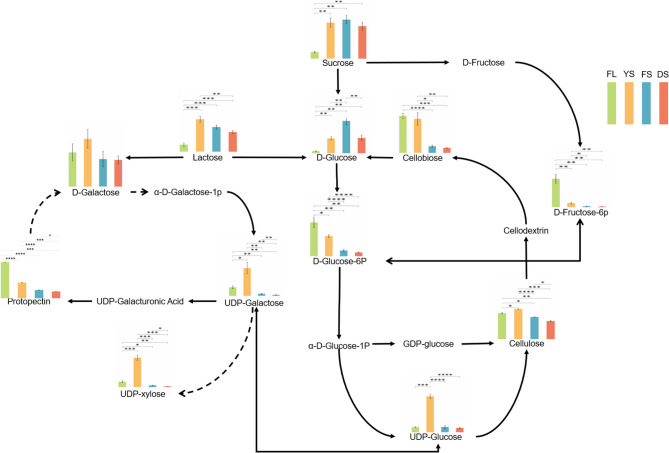
The cellulose metabolic pathway involves the degradation and metabolism of cellulose and is one of the important carbon and nitrogen compound metabolic pathways in tobacco cells. The content and availability of carbon source substances, such as UDP-glucose, D-glucose-6P, and sucrose, directly or indirectly influence cellulose conversion and metabolism (Fig. 4). During tobacco curing, lactose exhibited its highest relative abundance at the YS, followed by significant declines during FS and DS. Sucrose showed the lowest abundance in FL, increased significantly at YS, and remained stable from FS to DS. D-Glucose-6P displayed the highest abundance in FL, decreased markedly at YS, declined further during FS, and showed no significant change at DS.This shift in cellulose content synergized with the aforementioned sugar dynamics: cellulose levels increased significantly from the FL to the YS, followed by a pronounced decline from YS to the DS., whereas the content of cellobiose decreased significantly throughout the curing process. Pectin, which is primarily composed of galacturonic acid units linked by α−1,4 glycosidic bonds, also underwent metabolic changes during curing. Pectin content exhibited a continuous decreasing trend throughout curing, with the most significant decline occurring from FL to the YS. This reduction aligns with the enzymatic activity dynamics of PME, PG and β-GAL). As shown in Fig. 4, sucrose accumulated substantially from FL to YS, directly linked to starch degradation—ultrastructural evidence confirmed prominent starch granule breakdown during this stage. Concurrently, UDP-D-galactose and UDP-glucose (precursors for pectin synthesis) showed highly significant increases. UDP-xylose, generated from UDP-D-galactose via epimerization, exhibited fully synchronized changes, serving as a critical substrate for hemicellulose biosynthesis. The availability of UDP-xylose directly governs xylan synthesis efficiency [56]; for example, UXS gene knockout mutants display reduced xylan content, decreased molecular weight, and diminished cell wall thickness [58].
Fig. 4.
Metabolic pathways of cellulose and pectin in tobacco leaves during the curing stages. The bars arranged left to right showing relative abundances of key metabolites in the fresh leaf (FL, green), yellowing stage (YS, yellow), fixing stage (FS, blue), and drying stage (DS, red). *, **, ***, and **** indicate significant differences at p < 0.05, p < 0.01, p < 0.001 and p < 0.0001, respectively (Student’s t test)
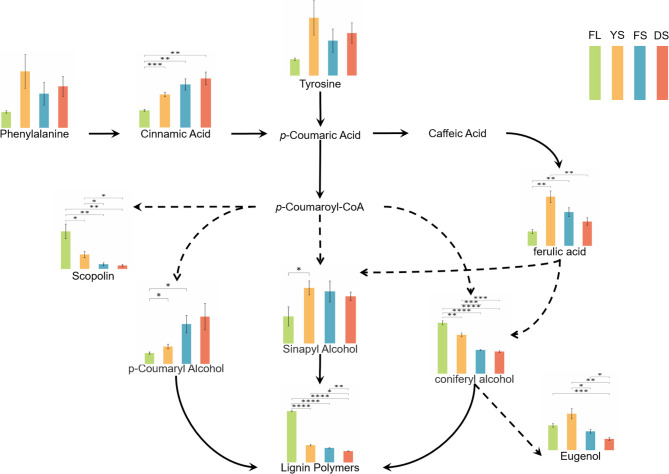
Analysis of lignin pathway
Lignin metabolism involves a series of reactions starting from phenylalanine, ultimately leading to the formation of lignin monomers, which then polymerize to form lignin. Figure 5 illustrates the dynamic changes in key lignin metabolites during the curing process of tobacco leaves. Tobacco curing is intrinsically a progressive stress process. Adversity stress induces alterations in enzymatic activities such as phenylalanine ammonia-lyase (PAL) and cinnamyl alcohol dehydrogenase (CAD), which promote lignin biosynthesis [59]. As essential precursors for lignin synthesis, the contents of phenylalanine and tyrosine peaked during YS and subsequently declined in FS and DS. Phenylalanine catalysed by enzymes to form cinnamic acid, whose content significantly increased throughout the curing process. Further examination of downstream metabolic pathways revealed that ferulic acid content increased significantly from the fresh leaf (FL) to YS, followed by a sharp decline. Scopolin—another metabolite derived from p-coumaroyl-CoA—exhibited a continuous and significant decrease throughout curing. Despite the decline in most precursors and intermediate metabolites, sinapyl alcohol and p-coumaroyl alcohol content in tobacco leaves showed marked increases during later stages. This phenomenon may stem from hindered lignin polymerization or, more plausibly, retrograde depolymerization of lignin into its monomeric units during advanced curing. Concurrently, the declining trend in coniferyl alcohol suggests its metabolic flux was redirected toward alternative pathways under impaired lignin polymerization. Notably, eugenol—a potential downstream product—demonstrated significant accumulation from FL to YS.
Fig. 5.
Metabolic pathways of lignin in tobacco leaves during the curing stages. The bars arranged left to right showing relative abundances of key metabolites in the fresh leaf (FL, green), yellowing stage (YS, yellow), fixing stage (FS, blue), and drying stage (DS, red). *, **, ***, and **** indicate significant differences at p < 0.05, p < 0.01, p < 0.001 and p < 0.0001, respectively (Student’s t test)
Discussion
Together, changes in cell structure and function, the degradation of cell wall components, the increased activity of degradative enzymes, and alterations in metabolites form a highly coordinated dynamic regulatory network. Studies have shown that the cell wall is primarily composed of polysaccharides such as cellulose, hemicellulose, and pectin, and the stability of its network structure plays a crucial role in determining the stiffness of plant tissues [60, 61]. Cell wall degradation and the activity of degrading enzymes are key factors in plant softening. By regulating these processes, the hardness and morphology of plant tissues can be effectively controlled, offering potential for manipulating tissue properties during postharvest processing [61, 62]. The postharvest processing of tobacco plays a crucial role in the transformation of tobacco compounds and the development of quality. An effective processing process can promote tobacco senescence and programmed cell apoptosis, improve the softness of tobacco leaves, and enhance both their quality and overall usability [63, 64]. This study revealed that the softness values of tobacco leaves continued to decrease before the yellowing stage, reaching a minimum of 11.83 mN at 48 h of curing.Subsequently, Wu’s experimental results revealed that the softness values of tobacco leaves continuously decreased during the first 72 h of curing—consistent with our findings, as this period corresponds to the yellowing stage characterized by moderate temperatures and higher moisture retention. However, upon entering the FS, elevated curing temperatures and extensive dehydration triggered substantial degradation of cell wall components and structural disruption. This phenomenon is further corroborated by the ultrastructural observations in our study. the integrity of the cell wall gradually deteriorated, starch granules progressively decomposed, chloroplasts and mitochondria were increasingly damaged, lipid droplets underwent abnormal fusion, and the tension in the cell wall decreased and relaxed, ultimately leading to complete disintegration of the cell wall (Fig. 1c). Cosgrove et al. [22] proposed that the expansive growth of cell walls relies on water-driven component slippage. During tobacco curing, cell wall dehydration may facilitate the reformation of hydrogen bonds between cellulose microfibrils, reducing slippage space and causing densification of microfibril alignment, thereby increasing rigidity. Bashline et al. [47] further emphasized that the arrangement patterns of cell wall components directly govern mechanical properties, suggesting that dehydration-induced realignment contributes to tissue hardening.This was due to the sharp decrease in tobacco leaf moisture in the later stages of curing in this study, leading to an increase in softness value, indicating that the moisture content in the cells was an important factor affecting the softness value of the tobacco leaves [65, 66]. These findings were essentially consistent with Wu’s conclusion that the thylakoids in chloroplasts remained relatively intact during the fresh tobacco leaf period. When the curing time reached 48 h, the chloroplast membranes and thylakoid structures were severely damaged. When the curing time reached 72 h, very few chloroplasts, basal vesicles, and plastid remnants remained, with most having been degraded. These findings indicated that changes in the arrangement and size of cells, as well as their interstitial spaces, are key factors in determining the mechanical properties of plant tissues, such as hardness and softness. During postharvest processing, the interstitial spaces of tobacco plants increased, with some cell walls slightly invaginated, the cellular structure remaining relatively intact, and the leaves beginning to experience initial relaxation and improved softness. This stage of change was similar to the ripening process of fruit, where the cell size increased, the hardness decreased, and the softness improved [45].
Numerous studies have confirmed that the degradation of cell walls in postharvest plant tissues is influenced primarily by the activity of cell wall-degrading enzymes [67]. Increasing the activity of these enzymes can effectively increase the degradation efficiency of cell wall components. Furthermore, the content of cell wall materials is positively correlated with the softness value of tobacco leaves [37]. In this study, the activities of PME and PL in tobacco followed a parabolic trend during the curing process, with a rapid increase from the fresh tobacco stage to the yellowing stage, followed by a notable decrease in the FS and DS. Short-time high-temperature treatment can enhance the activity of cellulase and PG promoting cellulose breakdown and accelerating the formation of water-soluble pectin [26, 27, 29]. However, sustained high temperatures cause the disulfide bonds inPME to break, reducing or even inactivating its enzyme activity [68]. In general, this pattern facilitated the conversion of pro-pectin into soluble pectin in tobacco leaves, which was consistent with the observed decrease in the pro-pectin content and increase in the soluble pectin content (Fig. 2). Therefore, it can be concluded that during the yellowing stage of tobacco curing, the curing temperature remains relatively mild, and the duration of treatment is not yet prolonged enough to significantly affect the activity of key enzymes involved in cell wall degradation. Instead, it may even enhance the activity of certain enzymes, such as PME and PL. However, as curing progresses, the continued rise in temperature, prolonged duration, and reduction of moisture content in tobacco cells impose significant constraints on the activity of cell wall-degrading enzymes.An analysis of the pectin metabolic pathway revealed that the contents of the main precursor carbon source substances for pectin synthesis, such as lactose, D-Gal, and UDP-D-galactose, tended to increase and then decrease, which was overall favourable for the synthesis of pectin; however, the pectin content decreased instead of increasing, indicating that PME and PL had greater abilities to promote the catabolism of pectin. Furthermore, the activities of enzymes such as endo-β−1,4-glucanase, β-gal, and exo-β−1,4-glucanase remained high throughout the curing process, effectively catalysing the decomposition of cellulose and hemicellulose. This finding also reflected the observed decrease in the contents of cellulose and hemicellulose during curing. The findings of this study align with those of Wu [17, 37]. With the increased demethylation of pectin, the degradation of cellulose and hemicellulose significantly accelerated, leading to the gradual degradation of the main components of the cell wall. This resulted in a loosening of the cell wall structure and a reduction in its toughness. Consistent with the results of metabolic pathway analysis, the activities of cellulose endonuclease and exonuclease were found to be enhanced, especially the rebound in cellulose exonuclease activity, which promoted the further degradation of cellulose breakdown products. As the activities of PME and PL gradually decreased, the demethylation of pectin was limited, and the conversion rate slowed. Moreover, although the activity of PG gradually increased, its role as a terminal enzyme for pectin degradation was constrained by a decrease in substrate availability, thereby limiting its ability to promote pectin degradation and ultimately slowing the overall degradation process. The synthesis pathways for cellulose and pectin significantly increased the D-glucose content and decreased the UDP-glucose content, suggesting that the conversion from D-glucose to UDP-glucose may have been impeded. This was supported by metabolite changes, where the precursors and intermediates for cell wall synthesis rapidly increased from the fresh tobacco leaf stage to the yellowing stage, accompanied by significant activation of the anabolic pathway. This phenomenon may be linked to the protective mechanisms of plant tissues, which respond to unfavourable conditions by employing various defence mechanisms, such as cell wall defect signalling and the activation of repair mechanisms when the cell wall is damaged [31, 32, 69].
In the lignin synthesis pathway, The levels of key lignin precursors undergo significant changes. From the FL (Fresh Leaf) to YS (Yellowing Stage) stage, the relative content of coniferyl alcohol is higher while that of sinapyl alcohol is lower, favoring the formation of G-rich lignin which is more readily degraded by laccase. In later stages, the content of these two precursors reverses, favoring the formation of S-rich lignin. S-rich lignin readily crosslinks with ferulic acid to form complexes that are more recalcitrant to degradation [4, 56]. This aligns with the observed changes in lignin content in this study (Fig. 5). Ferulic acid content increases significantly from FL to YS. This is likely released during the enzymatic breakdown of protopectin into soluble pectin by PME/PG/PL [15, 49]. From YS to DS, its content decreases significantly (Fig. 5). High temperatures may decompose ferulic acid, leading to the dissociation of lignin-carbohydrate complexes (LCCs) and facilitating lignin degradation [34, 70].Ferulic acid released from the cell wall may be converted to sinapic acid, enhancing cellular membrane stability [71]. The reason for this might be that the lignin synthesis pathway was blocked, and the excessive accumulation of intermediate products also increased the possibility of re-cross-linking, thus increasing the mechanical strength and stability of the cell wall.This cross-linking process not only enhances cellular tissue resistance to ROS bursts—delaying the disruption of the antioxidant enzyme system—but also reinforces the mechanical strength and stability of cell walls, thereby supporting the overall structural integrity of plant tissues [22, 72].
This study utilized tobacco as a model plant to investigate the dynamic changes in major cell wall components and key enzyme activities during the curing process under sustained high temperatures. It also conducted a systematic analysis of metabolic pathways related to cellulose, pectin, and lignin. However, the research did not comprehensively analyze the inhibitory effects of moisture status variations in tobacco leaves on plant enzyme activity during different curing stages, nor did it explore the synergistic influences of other identified metabolites (such as alkaloids, flavonoids, and terpenoids) on the cell wall deconstruction process. Additionally, potential impacts of sustained high-temperature treatment on phyllosphere microbiota and their role in cell wall senescence and apoptosis remain unexamined. To gain a more holistic and systematic understanding of how continuous high-temperature curing affects tobacco cell walls and critical organelles, further studies employing cell wall in situ localization techniques and multi-omics analyses are still required.
Conclusions
In this study, we systematically investigated the dynamics of tobacco leaf softness during the curing process, including changes in cellular ultrastructure, cell wall components, and key enzyme activities. Our results demonstrate that leaf softness is closely associated with moisture content. Key enzymes such asPME, PG and PL exhibit higher activity in fresh leaves and during the yellowing stage, facilitating the degradation of cell wall constituents including cellulose, hemicellulose, and pectin. Using LC‒MS/MS, we screened and analyzed the dynamic patterns of 54 cell wall-related metabolites. The findings reveal that during the fresh leaf and yellowing stages, tobacco leaves maintain relatively high moisture content and undergo moderately curing temperatures. This phase represents a critical control point for enhancing leaf quality and usability by optimizing curing parameters to regulate the physiological and biochemical processes of cell wall transformation.
Supplementary Information
Acknowledgements
We extend our gratitude to Shenzhen BGI Co., Ltd. for providing technical support in metabolite detection and to Beijing Zhongke Baice Testing Technology Co., Ltd. for their assistance in ul-trastructural observation.
Authors’ contributions
Author Contributions: Conceptualization: G.L., C.Z., K.W.; Investigation: G.L., C.Z., Z.Z., Y.L.; Resources: K.W.; Writing—Original Draft Preparation: G.L.; Writing—Review and Editing: J.T., X.Z., B.X., K.W.; Supervision: S.W.; K.W.; Funding Acquisition: K.W.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (32160648), Key Research and Development Program (110202202016, 2022XM17), Science and Technology Program of Science and Technology Department of Guizhou Province (QKHJC-ZK [2022] YB288).
Data availability
The raw data supporting the conclusions of this article will be made available by the authors on request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guanhui Li and Cheng Zhang contributed equally to this work.
Contributor Information
Benbo Xu, Email: benboxu@yangtzeu.edu.cn.
Kesu Wei, Email: weiks8816@163.com.
References
- 1.Zhang BC, Gao Y, Zhang LJ, Zhou YH. The plant cell wall: biosynthesis, construction, and functions. J Integr Plant Biol. 2021;63:251–72. 10.1111/jipb.13055. [DOI] [PubMed] [Google Scholar]
- 2.Somerville C, Bauer S, Brininstool G, Facette M, Hamann T, Milne J, Osborne E, Paredez A, Persson S, Raab T, Vorwerk S, Youngs H. Toward a systems approach to understanding plant cell walls. Science. 2004;306:2206–11. 10.1126/science.1102765. [DOI] [PubMed] [Google Scholar]
- 3.Mercado JA, Pliego-Alfaro F, Quesada MA. Fruit shelf life and potential for its genetic improvement. Breed Fruit Qual. 2011;81–104. 10.1002/9780470959350.ch4.
- 4.Zhang Q, Wang L, Wang Z, Zhang R, Liu P, Liu M, Liu Z, Zhao Z, Wang L, Chen X, Xu H. The regulation of cell wall lignification and lignin biosynthesis during pigmentation of winter jujube. Hortic Res. 2021;8:238. 10.1038/s41438-021-00670-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang L, Cong P, He J, Bu H, Qin S, Lyu D. Differential pulp cell wall structures lead to diverse fruit textures in Apple (Malus domestica). Protoplasma. 2022;259:905–21. 10.1007/s00709-021-01727-w. [DOI] [PubMed] [Google Scholar]
- 6.Paniagua C, Pose S, Morris VJ, Kirby AR, Quesada MA, Mercado JA. Fruit softening and pectin disassembly: an overview of nanostructural pectin modifications assessed by atomic force microscopy. Ann Botany. 2014;114:1375–89. 10.1093/aob/mcu149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li M, Wei KS, Xue Y, Wu SJ, Liu YJ, Chen DM, Yan XF, Kang C. Microscopic Spatiotemporal changes in cell wall cellulose and pectin during Nicotiana tabacum L. leaf growth and senescence based on label-free Raman microspectroscopic imaging combined with multivariate curve resolution. Industrial Crops Prod. 2024;222:119865. 10.1016/j.indcrop.2024.119865. [Google Scholar]
- 8.Fasoli M, Dell’Anna R, Amato A, Balestrini R, Dal Santo S, Monti F, Zenoni S. Active rearrangements in the cell wall follow polymer concentration during postharvest withering in the berry skin of vitis vinifera cv. Corvina. Plant Physiol Biochem. 2019;135:411–22. 10.1016/j.plaphy.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 9.Gao Y, Guo Y, Su Z, Yu Y, Zhu Z, Gao P, Wang X. Transcriptome analysis of genes related to fruit texture in watermelon. Sci Hort. 2020;262:109075. 10.1016/j.scienta.2019.109075. [Google Scholar]
- 10.Jiang Y, Yin H, Wang D, Zhong Y, Deng Y. Exploring the mechanism of Akebia trifoliata fruit cracking based on cell-wall metabolism. Food Res Int. 2022;157:111219. 10.1016/j.foodres.2022.111219. [DOI] [PubMed] [Google Scholar]
- 11.Castro RI, Muñoz-Vera M, Morales-Quintana L. Evaluation of cell wall modification in two strawberry cultivars with contrasted softness. Agronomy. 2021;11:1100. 10.3390/agronomy11061100. [Google Scholar]
- 12.Li G, Wei B, Chen L, Zheng H, Wu S, Li D, et al. Correlation analysis between tobacco leaf structure and quality indicators. J Mountain Agric Biology. 2025;44(2):63–6892. 10.15958/j.cnki.sdnyswxb.2025.02.009. [Google Scholar]
- 13.Wu SJ, Song ZP, Xu ZC, Wang SF, Li FQ, Cheng CZ, Gong CR. Cell wall physiological changes of tobacco leaves during curing process. Chin Tob Sci. 2010;31:73–7. 10.3969/j.issn.1007-5119.2010.03.017. [Google Scholar]
- 14.Pauly M, Keegstra K. Cell-wall carbohydrates and their modification as a resource for biofuels. Plant J. 2008;54(4):559–68. 10.1111/j.1365-313X.2008.03463.x. [DOI] [PubMed] [Google Scholar]
- 15.Willats WGT, McCartney L, Mackie W, Knox JP, Pectin. Cell biology and prospects for functional analysis. Plant Mol Biol. 2001;47:9–27. 10.1023/A:1010662911148. [PubMed] [Google Scholar]
- 16.Buanafina MM, d. O, Morris P. The impact of cell wall feruloylation on plant growth, responses to environmental stress, plant pathogens and cell wall degradability. Agronomy. 2022;12:1847. 10.3390/agronomy12081847. [Google Scholar]
- 17.Wu S, Song C, He F, Sun J, Gong C. Changes of cell ultrastructure and some physiological indexes and physical properties of tobacco leaves during bulk flue-curing. China Agric Sci. 2011;44:125–32. [Google Scholar]
- 18.Wang D, Seymour GB. Molecular and biochemical basis of softening in tomato. Mol Hortic. 2022;2:5. 10.1186/s43897-022-00026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu S, Guo Y, Adil MF, Sehar S, Cai B, Xiang Z, Tu Y, Zhao D, Shamsi IH. Comparative proteomic analysis by iTRAQ reveals that plastid pigment metabolism contributes to leaf color changes in tobacco (Nicotiana tabacum) during curing. Int J Mol Sci. 2020;21:2394. 10.3390/ijms21072394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou L, Chen L, Xu X, Wei X, Wang X, Zhou W, Xia J, Chen G. Correlation analysis between softness and quality of neutral flavor type flue-cured tobacco leaf. Hunan Agric Sci. 2016;11:67–70. 10.16498/j.cnki.hnnykx.2016.011.021. [Google Scholar]
- 21.Ciesielski PN, Wagner R, Bharadwaj VS, Killgore J, Mittal A, Beckham GT, Decker SR, Himmel ME, Crowley MF. Nanomechanics of cellulose deformation reveal molecular defects that facilitate natural Deconstruction. Proc Natl Acad Sci United States Am (PNAS). 2019;116:9825–30. 10.1073/pnas.1900161116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cosgrove DJ. Diffuse growth of plant cell walls. Plant Physiol. 2018;176:16–27. 10.1104/pp.17.01160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weng S, Deng M, Chen S, Yang R, Li J, Zhao X, Ji S, Wu L, Ni L, Zhang E, Wang C, Qi L, Liao K, Chen Y, Zhang W. Application of pectin hydrolyzing bacteria in tobacco to improve flue-cured tobacco quality. Front Bioeng Biotechnol. 2024;12:1340160. 10.3389/fbioe.2024.1340160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mottiar Y, Vanholme R, Boerjan W, Ralph J, Mansfield SD. Designer lignins: harnessing the plasticity of lignification. Curr Opin Biotechnol. 2016;37:190–200. 10.1016/j.copbio.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Qian L, Yang S, Li Y, Liu Y, Li X, Sun H. Research progress on softening mechanism of postharvest fruit. Food Ind Sci Technol. 2024;4:371–8. 10.13386/j.issn1002-0306.2023040058. [Google Scholar]
- 26.Vicente AR, Costa ML, Martínez GA, Chaves AR, Civello PM. Effect of heat treatments on cell wall degradation and softening in strawberry fruit. Postharvest Biol Technol. 2005;38:213–22. 10.1016/j.postharvbio.2005.06.005. [Google Scholar]
- 27.Luo Z, Xi Y, Fu G, Lü C. Effect of heat treatment on cell wall components in relation to cell wallHydrolase of excised bamboo shoots. Acta Horticulturae Sinica. 2002;43–6. https://www.ahs.ac.cn/CN/Y2002/V29/I1/43.
- 28.Sharma S, Shree B, Sharma D, Kumar S, Kumar V, Sharma R, et al. Vegetable microgreens: the gleam of next generation super foods, their genetic enhancement, health benefits and processing approaches. Food Res Int. 2022;155:111038. 10.1016/j.foodres.2022.111038. [DOI] [PubMed] [Google Scholar]
- 29.Lohani S, Trivedi PK, Nath P. Changes in activities of cell wall hydrolases during ethylene-induced ripening in banana: effect of 1-MCP, ABA and IAA. Postharvest Biol Technol. 2004;31(2):119–26. 10.1016/j.postharvbio.2003.08.001. [Google Scholar]
- 30.Hamann T. The plant cell wall integrity maintenance mechanism—concepts for organization and mode of action. Plant Cell Physiol. 2015;56:215–23. 10.1093/pcp/pcu164. [DOI] [PubMed] [Google Scholar]
- 31.Anderson CT, Kieber JJ. Dynamic construction, perception, and remodeling of plant cell walls. Annu Rev Plant Biol. 2020;71:39–69. 10.1146/annurev-arplant-081519-035846. [DOI] [PubMed] [Google Scholar]
- 32.Wolf S. Plant cell wall signalling and receptor-like kinases. Biochem J. 2017;474:471–92. 10.1042/BCJ20160238. [DOI] [PubMed] [Google Scholar]
- 33.Alvira P, Tomás-Pejó E, Ballesteros M, Negro MJ. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis. Bioresour Technol. 2010;101:4851–61. 10.1016/j.biortech.2009.11.093. [DOI] [PubMed] [Google Scholar]
- 34.Selig MJ, Viamajala S, Decker SR, Tucker MP, Himmel ME, Vinzant TB. Deposition of lignin droplets produced during dilute acid pretreatment of maize stems retards enzymatic hydrolysis of cellulose. Biotechnol Prog. 2007;23:1333–9. 10.1021/bp0702018. [DOI] [PubMed] [Google Scholar]
- 35.Kang X, Kirui A, Dickwella Widanage MC, Mentink-Vigier F, Cosgrove DJ, Wang T. Lignin-polysaccharide interactions in plant secondary cell walls revealed by solid-state NMR. Nat Commun. 2019;10:347. 10.1038/s41467-018-08252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei K, Chen X, Cheng Z, Wang H, Wang F, Yang L, Wu S, Yang Y, Tu Y, Wang Y, Liang C. LC-MS/MS-based metabolic profiling unraveling the impact of varying degrees of curing on metabolite transformations in tobacco. Front Plant Sci. 2024;15:1473527. 10.3389/fpls.2024.1473527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu S, Cao G, Adil MF, Tu Y, Wang W, Cai B, Zhao D, Shamsi IH. Changes in water loss and cell wall metabolism during postharvest withering of tobacco (Nicotiana tabacum L.) leaves using tandem mass tag-based quantitative proteomics approach. Plant Physiol Biochem. 2020;150:121–32. 10.1016/j.plaphy.2020.02.040. [DOI] [PubMed] [Google Scholar]
- 38.Hu Y, Liu T, Chen N, Feng C, Lu W, Guo H. Simultaneous bio-reduction of nitrate and Cr(VI) by mechanical milling activated corn straw. J Hazard Mater. 2022;429:128258. 10.1016/j.jhazmat.2022.128258. [DOI] [PubMed] [Google Scholar]
- 39.Wang H, Wang Z-X, Tian H-Y, Zeng Y-L, Xue H, Mao W-T, et al. The miR172a–SNB module orchestrates both induced and adult-plant resistance to multiple diseases via MYB30-mediated lignin accumulation in rice. Mol Plant. 2025;18(1):59–75. 10.1016/j.molp.2024.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Wang YY, Hu YP, Ren HF, Zhao XQ, Yuan ZH. Integrated transcriptomic, metabolomic, and functional analyses unravel the mechanism of bagging delaying fruit cracking of pomegranate (Punica granatum L.). Food Chem. 2024;451139384. 10.1016/j.foodchem.2024.139384. [DOI] [PubMed]
- 41.Wu Y, Li X, Li Y, Ma H, Chi H, Ma Y, et al. Degradation of de-esterified pctin/homogalacturonan by the polygalacturonase GhNSP is necessary for pollen exine formation and male fertility in cotton. Plant Biotechnol J. 2022;20(6):1054–68. 10.1111/pbi.13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jia LL, Li Y, Liu GS, He JG. UV-C delays senescence in ‘lingwu long’ jujube fruit by regulating ROS and henylpropanoid metabolism. Plant Physiol Biochem. 2023;194:383–93. 10.1016/j.plaphy.2022.11.030. [DOI] [PubMed] [Google Scholar]
- 43.Zhang DZ, Liu JJ, Zhang YB, Wang HR, Wei SW, Zhang X et al. Morphophysiological, proteomic and metabolomic analyses reveal cadmium tolerance mechanism in common wheat (Triticum aestivum L.). J Hazardous Mat. 2023;445130499. 10.1016/j.jhazmat.2022.130499. [DOI] [PubMed]
- 44.Huang WJ, Shi HY, Weng Q, Ding S, Lou LP. Disparities and mechanisms of carbon and nitrogen conversion during food waste composting with different bulking agents. J Environ Manag. 2024;351119629. 10.1016/j.jenvman.2023.119629. [DOI] [PubMed]
- 45.Huang Y, Qin S, He J, Lyu D. Integration of cell wall fraction, organic matter content, and membrane to understand crispness changes in apples. Sci Hort. 2023;321:112309. 10.1016/j.scienta.2023.112309. [Google Scholar]
- 46.Han F, Tu ZD, Zhu Z, Liu DC, Meng QC, Yu QF, et al. Targeting endogenous reactive oxygen species removal and regulating regenerative microenvironment at annulus fibrosus defects promote tissue repair. ACS Nano. 2023;(8):7645–61. 10.1021/acsnano.3c00093. [DOI] [PubMed] [Google Scholar]
- 47.Bashline L, Lei L, Li SD, Gu Y. Cell wall, cytoskeleton, and cell expansion in higher plants. Mol Plant. 2014;7(4):586–600. 10.1093/mp/ssu018. [DOI] [PubMed] [Google Scholar]
- 48.Li M, Zhang K, Long R, Sun Y, Kang J, Zhang T, et al. iTRAQ-based comparative proteomic analysis reveals tissue-specific and novel early-stage molecular mechanisms of salt stress response in carex rigescens. Environ Exp Bot. 2017;143:99–114. 10.1016/j.envexpbot.2017.08.010. [Google Scholar]
- 49.Mohnen D. Pectin structure and biosynthesis. Curr Opin Plant Biol. 2008;11:266–77. 10.1016/j.pbi.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Scheller HV, Jensen JK, Sørensen SO, Harholt J, Geshi N. Biosynthesis of pectin. Physiol Plant. 2007;129:283–95. 10.1111/j.1399-3054.2006.00834.x. [Google Scholar]
- 51.Endler A, Persson S. Cellulose synthases and synthesis in Arabidopsis. Mol Plant. 2011;4:199–211. 10.1093/mp/ssq079. [DOI] [PubMed] [Google Scholar]
- 52.Seifert GJ. Nucleotide sugar interconversions and cell wall biosynthesis. Curr Opin Plant Biol. 2004;7:277–84. 10.1016/j.pbi.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Tao J, Chen Q, Chen S, Lu P, Chen Y, Jin J, Li J, Xu Y, He W, Long T, Deng X, Yin H, Li Z, Fan J, Cao P. Metagenomic insight into the microbial degradation of organic compounds in fermented plant leaves. Environ Res. 2022;214:113902. 10.1016/j.envres.2022.113902. [DOI] [PubMed] [Google Scholar]
- 54.Scheller HV, Ulvskov P, Hemicelluloses. Annu Rev Plant Biol. 2010;61:263–89. 10.1146/annurev-arplant-042809-112315. [DOI] [PubMed] [Google Scholar]
- 55.Rennie EA, Scheller HV. Xylan biosynthesis. Curr Opin Biotechnol. 2014;26:100–7. 10.1016/j.copbio.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 56.Boerjan W, Ralph J, Baucher M. Lignin biosynth annual rev plant biology. 2003;519–46. 10.1146/annurev.arplant.54.031902.134938. [DOI] [PubMed]
- 57.Bonawitz ND, Chapple C. The genetics of lignin biosynthesis: connecting genotype to phenotype. Annu Rev Genet. 2010;44:337–63. 10.1146/annurev-genet-102209-163508. [DOI] [PubMed] [Google Scholar]
- 58.Zhao XH, Liu N, Shang N, Zeng W, Ebert B, Rautengarten C, et al. Three UDP-xylose transporters participate in Xylan biosynthesis by conveying cytosolic UDP-xylose into the golgi lumen in Arabidopsis. J Exp Bot. 2018;69(5):1125–34. 10.1093/jxb/erx448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou P, Li Q, Liu G, Xu N, Yang Y, Zeng W, Wang S. Integrated analysis of transcriptomic and metabolomic data reveals critical metabolic pathways involved in polyphenol biosynthesis in Nicotiana tabacum under chilling stress. Funct Plant Biol. 2018;46(1):30–43. 10.1071/FP18099. [DOI] [PubMed] [Google Scholar]
- 60.Yilmaz N, Kodama Y, Numata K. Revealing the architecture of the cell wall in living plant cells by bioimaging and enzymatic degradation. Biomacromolecules. 2020;21:95–103. 10.1021/acs.biomac.9b00979. [DOI] [PubMed] [Google Scholar]
- 61.Fan X, Jiang W, Gong H, Yang Y, Zhang A, Liu H, Cao J. Cell wall polysaccharides degradation and ultrastructure modification of apricot during storage at a near freezing temperature. Food Chem. 2019;300:125194. 10.1016/j.foodchem.2019.125194. [DOI] [PubMed] [Google Scholar]
- 62.Goulao L, Oliveira C. Cell wall modifications during fruit ripening: when a fruit is not the fruit. Trends Food Sci Technol. 2008;19:4–25. 10.1016/j.tifs.2007.07.002. [Google Scholar]
- 63.Wu S, Guo Y, Issaka Joan H, Tu Y, Faheem M. iTRAQ-based comparative proteomic analysis reveals high temperature accelerated leaf senescence of tobacco (Nicotiana tabacum L.) during flue-curing. Genomics. 2020;112:3075–88. 10.1016/j.ygeno.2020.05.019. [DOI] [PubMed] [Google Scholar]
- 64.Xu XL, Song WW, Zhou WH, Wang XY, Guan J, Zhou LX. Determination of softness and cytological observation of flue-cured tobacco leaves. Hunan Agricultural Sci. 2019;3:76–9. 10.16498/j.cnki.hnnykx.2019.003.021. [Google Scholar]
- 65.Chang A, Zhan J, He K, Liang S, Lyu G, Yu J. Relationship between softness and physical properties in flue-cured tobacco leaves. J Hunan Agricultural Univ (Natural Sciences). 2016;42:365–9. 10.13331/j.cnki.jhau.2016.04.004. [Google Scholar]
- 66.Zhan J, Shen X, He K, Liang S, Li L, Jiao S, Yu J. Study on relationship between softness and physical characteristics of flue-cured tobacco and the suitable softness range in Jiangxi Province. J Agricultural Sci Technol. 2017;19:131–7. 10.13304/j.nykjdb.2016.254. [Google Scholar]
- 67.Fu QJ, Sun TT, Dou YQ, Cheng S, Cai XJ. Softness of flue-cured tobacco leaves and its relationship with main physicochemical indexes. Tob Sci Technol. 2021;54:77–81. 10.16135/j.issn1002-0861.2020.0294. [Google Scholar]
- 68.Lu C, Li W, Feng X, Chen J, Hu S, Tan Y, et al. The dynamic remodeling of plant cell wall in response to heat stress. Genes. 2025. 10.3390/genes16060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lei L, Singh A, Bashline L, Li S, Yingling YG, Gu Y. Cellulose synthase interactive1 is required for fast recycling of cellulose synthase complexes to the plasma membrane in Arabidopsis. Plant Cell. 2015;27:2926–40. 10.1105/tpc.15.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ralph J, Lundquist K, Brunow G, Lu F, Kim H, Schatz PF, et al. Lignins: natural polymers from oxidative coupling of 4-hydroxyphenyl- propanoids. Phytochem Rev. 2004;3(1):29–60. 10.1023/B:PHYT.0000047809.65444.a4. [Google Scholar]
- 71.Vanholme R, De Meester B, Ralph J, Boerjan W. Lignin biosynthesis and its integration into metabolism. Curr Opin Biotechnol. 2019;56:230–9. 10.1016/j.copbio.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 72.Grantham NJ, Wurman-Rodrich J, Terrett OM, Lyczakowski JJ, Stott K, Iuga D, et al. An even pattern of Xylan substitution is critical for interaction with cellulose in plant cell walls. Nat Plants. 2017;3(11):859–65. 10.1038/s41477-017-0030-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.