Abstract
Background and aims
Common variable immunodeficiency (CVID) represents the most frequently diagnosed symptomatic primary immunodeficiency (PID), marked by a heterogeneous presentation involving infectious and non-infectious symptoms. This study investigated the association between serum copeptin levels and right ventricular functions (RVF) and pulmonary complications in patients diagnosed with CVID.
Methods
The study analyzed data from 60 individuals with a confirmed diagnosis of CVID and 30 age- and sex-matched healthy volunteers (HVs). Clinical and biochemical parameters were sourced from existing hospital records.CVID patients were categorized into two subgroups: those with and without pulmonary complications. Comparisons of serum copeptin levels were made between these groups and between the overall CVID cohort and healthy controls. RVF was evaluated using tricuspid annular plane systolic excursion (TAPSE) and supplementary echocardiographic indicators.
Results
The CVID group had a median age of 40 years (interquartile range [IQR]: 30–55), with 51.7% being male, while the HVs group had a median age of 37 years (IQR: 28–47.5), with 60% male. No significant differences in age (p = 0.226) or sex distribution (p = 0.45) were observed between the groups. CVID with pulmonary complications (CVID-P) exhibited significantly elevated copeptin levels compared to those without such complications (p < 0.001). According to ROC analysis, a copeptin cut-off value of 11 pmol/L significantly differentiated patients with CVID-P from those without pulmonary complications (p < 0.001). Moreover, overall copeptin levels were significantly higher in the CVID group than in HVs (p < 0.001). A copeptin cut-off value of 21 pmol/L effectively distinguished CVID patients with low TAPSE from those with normal TAPSE values (p < 0.001). Pulmonary complications and low TAPSE were independently associated with increased copeptin levels (p = 0.006 and p = 0.004, respectively).
Conclusion
The development of pulmonary complications and RV dysfunction were associated with elevated serum copeptin levels in CVID. Measuring serum copeptin concentration may be a useful biomarker in diagnosing and prognosis pulmonary diseases and RV dysfunction in CVID.
Keywords: Common variable immunodeficiency, Copeptin, Right ventricular functions, Pulmonary complications
Background
Common variable immunodeficiency (CVID) is the most common symptomatic primary immunodeficiency (PID) [1]. CVID is characterized by hypogammaglobulinemia and reduced or absent antibody responses to antigens [2]. The incidence is approximately 1:50.000–1:25.000 [3]. CVID is a heterogeneous immunodeficiency group with a wide clinical spectrum. Clinical presentations include recurrent bacterial infections, autoimmunity, lymphoproliferative disorders, enteropathy, allergic diseases, and malignancy [4]. Cardiovascular disorders are rare among clinical manifestations of CVID [5]. Cardiovascular diseases are a major health problem worldwide, and heart failure (HF) is among the leading causes of cardiovascular mortality [6]. Since the left ventricular is usually considered in cardiological evaluation, the right ventricular (RV) has remained in the shadow of the left ventricular, but in recent years, it has been understood that the RV is a determining factor for survival, especially in pathologies such as HF and pulmonary hypertension, and its evaluation is necessary [7]. Right HF patients with preserved ejection fraction (EF) constitute approximately 50% of the HF population and are associated with increased mortality [8]. Approximately 1/3 of patients with CVID have chronic lung disease at the time of diagnosis, which frequently includes bronchiectasis, obstructive and restrictive lung diseases, and nonsarcoid granulomatous diseases [9]. In CVID, chronic pulmonary complications such as bronchiectasis due to recurrent respiratory tract infections may lead to RV strain and dysfunction [10, 11]. Copeptin is a 39-amino acid neuropeptide derived from the C-terminus of vasopressin prohormone released from the hypothalamus [12]. Many pathological and physiological stimuli, including pain, hypoxemia, hypoglycemia, infection, stroke, and shock, trigger copeptin release. Elevated serum copeptin levels are associated with poor prognosis in various diseases, including myocardial infarction, respiratory diseases, HF, stroke, and diabetes mellitus [13]. Serum copeptin level is not routinely measured in the diagnosis and follow-up of CVID. In two previous studies, it was emphasized that serum copeptin levels were increased in acute exacerbation of chronic obstructive pulmonary disease (AECOPD) and patients with RV dysfunction, and serum copeptin may be an interesting marker in these patient groups [14, 15].
This study investigated the potential association between copeptin levels and right ventricular functions (RVF) and pulmonary complications in patients with CVID. Identifying such a relationship may contribute to early diagnosis, individualized treatment, and improved clinical management in this patient population.
In this way, a specific parameter for the development of RV dysfunction and pulmonary complications in the follow-up of patients with CVID may guide clinical practice.
Materials and methods
Study design
This prospective cohort investigation was conducted at Necmettin Erbakan University Faculty of Medicine Hospital in Konya, Türkiye. Ethical clearance was obtained from the institution’s ethics review board (approval number: 2020/2962). Between December 2020 and January 2022, clinical data were collected from hospital records for patients and healthy volunteers (HVs) who met the study’s eligibility criteria. Inclusion criteria for the patient group required individuals to be 18 years or older and have a confirmed diagnosis of CVID. Exclusion criteria encompassed a previous history of myocardial infarction or cerebrovascular events that might influence serum copeptin concentrations, a left ventricular EF below 50%, the presence of moderate to severe aortic or mitral valve disease, and a diagnosis of pulmonary arterial hypertension (PAH). The HVs of inclusion criteria were ≥ 18 years and had no known chronic medical conditions. All HVs were fully informed about the nature and purpose of the study, and written informed consent was obtained from each participant before inclusion (Fig. 1).
Fig. 1.
Flow chart of patient inclusion in this study. CVID common variable immunodeficiency
Data collecting
Demographic characteristics (including age and sex), body mass index (BMI), comorbid conditions (hypertension, diabetes mellitus, cardiovascular diseases, hypercholesterolemia, smoker, cardiac family history etc.), past medical history of pulmonary and cardiac manifestations (bronchiectasis, fibrosis, arrhythmia etc.), laboratory datas (creatinine, immunoglobulin levels and flow cytometric values) and echocardiographic characteristics were extracted from the hospital’s electronic medical records.
Immunoglobulin measurements
Serum immunoglobulin levels were determined using nephelometric methods (Siemens BNII System, Erlangen, Germany). IgE results were expressed in international units (IU)/mL. IgG, IgM, and IgA measurements were expressed in mg/dL; IgG (mg/dl): 700–1600, IgM (mg/dl): 46–304, IgA (mg/dl): 70–400. An estimate of the glomerular filtration rate (eGFR) was derived using the Modification of Diet in Renal Disease (MDRD) method to assess kidney function [16].
Flow cytometric analysis
At the time of initial diagnosis, peripheral venous blood samples (2 mL) were obtained from a cohort of 60 individuals with confirmed CVID. The samples were anticoagulated with EDTA and processed within six hours of collection. Quantification of B and T lymphocyte subsets was performed using multicolor flow cytometry. Cellular analyses were conducted on a BD FACS Canto II Flow Cytometry System (BD Biosciences). The distribution of lymphocyte subpopulations was reported as percentages within reference intervals: CD3⁺ T cells (57–85%), CD3⁺CD4⁺ T cells (30–61%), CD3⁺CD8⁺ T cells (12–42%), CD19⁺ B cells (6–29%), and CD3⁻CD16⁺56⁺ natural killer (NK) cells (4–25%).
Echocardiography
Transthoracic echocardiographic evaluation was conducted using a Philips Epiq 7 C ultrasound system (Bothell, WA, USA) with a 5–1 MHz transducer. Two experienced cardiologists obtained all echocardiographic measurements using the standard protocols and imaging recommendations of the American Society of Echocardiography (ASE) [17]. Measurements were obtained with the patient in the left lateral decubitus position.
Left ventricular EF was calculated using Simpson’s biplane method. Standard imaging views were acquired, including parasternal long-axis, parasternal short-axis, apical four-chamber, and apical long-axis. All images were captured at a frame rate greater than 60 frames per second and recorded over a minimum of five cardiac cycles. Mitral inflow velocities, including early diastolic (E) and late diastolic (A) waves, were measured by pulsed-wave (PW) Doppler from the apical four-chamber view, positioning the sample volume approximately 1 cm below the mitral valve leaflet tips. Measurements were averaged over 5 to 10 cardiac cycles. Tissue Doppler-derived E/E′ ratios were obtained separately for both the septal and lateral mitral annular walls to assess left ventricular diastolic function.RV systolic and diastolic functions were evaluated. Diastolic RV parameters included systolic myocardial velocity (RV SM), early diastolic myocardial velocity (RV EM), late diastolic myocardial velocity (RV AM), and systolic RV parametre included tricuspid annular plane systolic excursion (TAPSE). Pulmonary artery systolic pressure (PAPs) was estimated from tricuspid regurgitation velocity. Left ventricular systolic and diastolic functions were comprehensively assessed. Ejection fraction was measured to evaluate left ventricular systolic function. The Ratio of mitral inflow (E)/ velocity to tissue Doppler (E’) (mitral E/E’ ratio ) was measured to evaluate left ventricular diastolic function.
Blood sampling
This study was conducted in a group of clinically stable outpatients with CVID who did not have conditions such as infection or renal failure. A 5 mL venous blood sample was collected from the antecubital vein of outpatients without the use of any medication that could affect serum copeptin levels, intravenous fluid infusion, or diagnostic imaging procedures. The samples were transferred to heparin-containing tubes and immediately stored on ice at 4 °C. The plasma was then separated by centrifugation at 4,000 rpm for five minutes and stored at -40 °C until analysis. All samples were brought to room temperature and thawed prior to measurement.
Serum copeptin measurement
Serum copeptin concentrations were quantified using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Catalog No: E-EL-H0851 96T; Elabscience, Texas, USA), which operates on the principle of competitive ELISA. The microplate included in the kit was pre-coated with human copeptin peptide (CPP). During the assay, biotinylated anti-human CPP antibodies in the sample or standard compete with a fixed amount of coated antigen for binding sites. After the competitive binding step, unbound substances were removed through washing. Horseradish peroxidase (HRP)-conjugated avidin was added to each well and incubated, followed by tetramethylbenzidine (TMB) substrate. A stop solution terminated the enzymatic reaction, and the resulting colorimetric change was measured spectrophotometrically at 450 ± 2 nm. Copeptin concentrations in the samples were determined by comparing the optical density (OD) values to a standard calibration curve. The copeptin assay’s lower detection limit was 2.44 pmol/L, and its functional sensitivity—defined by a coefficient of variation (CV) below 10%—was above 1.5 pmol/L. Among HVs, the mean copeptin level was 6.8 pmol/L with a standard deviation (SD) of 3.
Statistical analysis
All statistical procedures were executed using SPSS software (IBM, version 22.0; Armonk, NY, USA). The normality of distribution for continuous variables was assessed using the Kolmogorov-Smirnov and Shapiro-Wilk tests. For variables with a normal distribution, results were reported as the mean ± SD; non-normally distributed variables were described using the median and interquartile range (IQR). Categorical variables were absolute counts (n) and corresponding percentages (%). Relationships among categorical variables were evaluated using the Chi-square (χ²) test. For comparisons of continuous variables, the independent samples Student’s t-test was employed when data were normally distributed. In cases of non-normal distribution, the Mann-Whitney U test was utilized. The relationships between continuous variables were examined through Pearson or Spearman correlation, selected according to the normality of the data. The point-biserial correlation assessed the relationships between continuous and binary categorical variables. Logistic regression models were applied to determine the prognostic significance of serum copeptin in terms of pulmonary complications and RV dysfunction. Multivariate regression analysis was used to evaluate the relationship between immunological parameters and copeptin. The most appropriate cut-off points were assessed based on calculated sensitivity and specificity values, which were performed by receiver operating characteristic (ROC) curve analysis. A p-value of less than 0.05 was considered indicative of statistical significance.
Results
Study population
A total of 60 patients with CVID and 30 HVs were included in the study. The median (IQR) age of patients with CVID was 40 ( 30–55) years, and 31 (51.7%) were male.
Arterial hypertension (11.7%) and hypercholesterolemia (10%) were the most common cardiovascular risk factors. Arrhythmia (5%) was the most common cardiac manifestation (Table 1).
Table 1.
Demographic and clinical characteristics of patients with CVID
| Characteristic | Patients (n = 60) |
|---|---|
| Sex, n (%) | |
| Female | 29 (48.3) |
| Male | 31 (51.7) |
| Age, median (IQR), years | 40 (30–55) |
| Cardiovascular risk factors, n (%) | 15 (%25) |
| Arterial hypertension | 7 (11.7) |
| Hypercholesterolemia | 6 (10) |
| Diabetes mellitus | 5 (8.3) |
| Smoker | 5(8.3) |
| Cardiac family history | 3 (5) |
| Obesity | 5 (8.3) |
| Cardiac manifestations, n (%) | 6 (10) |
| Arrhythmia | 3 (5) |
| Pericardial diseases | 2 (3.3) |
| Congenital heart diseases | 1 (1.6) |
| Pulmonary complications, n (%) | |
| Bronchiectasis | 20 (33.3) |
| Fibrosis | 10 (16.7) |
| GLILD | 2 (3.3) |
| Obstructive lung diseases | 16 (26.7) |
| Restrictive lung diseases | 6 (10) |
| Laboratory values | |
| Creatinine, median (IQR), mg/dL | 0.8 (0.7–0.9) |
| GFR, median (IQR), mL/min/1.73 m2 | 107 (86–120) |
| Basal IgG, median (IQR), mg/dL | 3.95 (1.7–5.3) |
| Basal IgM, median (IQR), mg/dL | 0.25 (0.18–0.5) |
| Basal IgA, median (IQR), mg/dL | 0.3 (0.23–0.97) |
| Basal IgE, median (IQR), IU/mL | 18 (15.4–19) |
| CD3+ T cell, median, (IQR), % | 77.5 (70.2–85) |
| CD19+ B cell, median, (IQR), % | 6 (2–11) |
| CD3+CD4+ T cell, median, (IQR), % | 34.5 (27.5–45) |
| CD3+ CD8+ T cell, median, (IQR), % | 39.5 (32–50) |
| CD3− CD16+ CD56+ NK cells, median, (IQR), % | 7 (5-9.7) |
| Copeptin, median (IQR), (pmol/L) | 14 (10–21) |
CVID common variable immunodeficiency, IQR interquartile range, GLILD granulomatous lymphocytic interstitial lung disease, GFR glomerular filtration rate, IgG, immunoglobulin G, IgM, immunoglobulin M, IgA immunoglobulin A, IgE immunoglobulin E, NK natural killer
Demographic, clinical and echocardiographic characteristics of patients with CVID and HVs
The median (IQR) age of HVs was 37 (28–47.5) years, and 18 (60%) were male. There was no significant difference between patients with CVID and HVs in terms of age (p = 0.226) or sex (p = 0.45). Median GFR and creatinine levels were similar in both groups, and there were no statistically significant differences (respectively, p = 0.13, p = 0.40).
In RV diastolic functions of patients with CVID, the median (IQR) RVSM was 13 (12-14.1) cm/sn, the median (IQR) RVEM was 14.8 (14.5–15.1) cm/sn, the median (IQR) RVAM was 13.5 (11.3–16.1) cm/sn and the median TAPSE was 2.2 (2-2.3) cm/sn.
RVSM, RVEM, RVAM, and TAPSE levels were higher in the patients with CVID than in HVs, but there was no statistically significant difference. In other echocardiographic parameters, there was no statistically significant difference between these groups. Serum copeptin levels were significantly higher in the patients with CVID than in HVs (p < 0.001) (Table 2).
Table 2.
Demographic, clinical and echocardiographic characteristics of patients with CVID and HVs
| Variables | CVID patients n = 60 |
Healthy volunteers n = 30 |
P |
|---|---|---|---|
|
Sex, n (%) Female |
29 (48.3) | 12 (40) | 0.45 |
| Male | 31 (51.7) | 18 (60) | |
| Age, median (IQR), years | 40 (30–55) | 37 (28-47.5) | 0.226 |
| Creatinin, median (IQR), mg/dL | 0.8 (0.7–0.9) | 0.76 (0.7–0.81) | 0.13 |
| GFR, median (IQR), mL/min/1.73 m2 | 107 (86–120) | 114 (94.7–126) | 0.40 |
| RVSM, median (IQR), (cm/sn) | 13 (12-14.1) | 11.8 (11.5–12) | 0.08 |
| RVEM, median (IQR), (cm/sn) | 14.8 (14.5–15.1) | 13 (11.3–14) | 0.075 |
| RVAM, median (IQR), (cm/sn) | 13.5 (11.3–16.1) | 8.77 (8.4–9.2) | 0.077 |
| TAPSE, median (IQR), (mm) | 2.2 (2-2.3) | 2.1 (1.9–2.5) | 0.87 |
| PAP, median (IQR), (mmHg) | 28 (25–30) | 23.5 (22–25) | 0.25 |
| EF, median (IQR) | 60 (50–60) | 63 (60–65) | 0.065 |
| E/E’ Septal, median (IQR) | 7.3 (6.5–8.2) | 5.75 (5.6–5.9) | 0.07 |
| E/E’ Lateral, median (IQR) | 6.5 (6-7.1) | 5.67 (5.5–5.8) | 0.076 |
| LVESD, median (IQR), (cm) | 2.6 (2.3–2.97) | 2.82 (2.5–3.1) | 0.16 |
| LVEDD, median (IQR), (cm) | 4.4 (4.1–4.7) | 4.6 (4.33–4.86) | 0.17 |
|
Aortic diameter, median, (IQR), (cm) |
2.4 (2.2–2.7) | 3 (2.83–3.4) | 0.09 |
| Copeptin, median (IQR), (pmol/L) | 14 (10-21.7) | 5.8 (4.6–9.6) | < 0.001 |
Bold p< 0.05 value indicate statistically significant differences
CVID common variable immunodeficiency, HVs healthy volunteers, IQR interquartile range, GFR glomerular filtration rate, RVSM systolic myocardial velocity, RVEM early diastolic myocardial velocity, RVAM late diastolic myocardial velocity, TAPSE tricuspid annular plane systolic excursion, PAP pulmonary artery pressure, EF ejection fraction, E/E’ ratio of mitral inflow (E), velocity to tissue Doppler (E’), LVESD, left ventricular end systolic diameter, LVEDD left ventricular end diastolic diameter
*Intergroup comparisons were conducted using the chi-square, and independent samples T tests, as appropriate
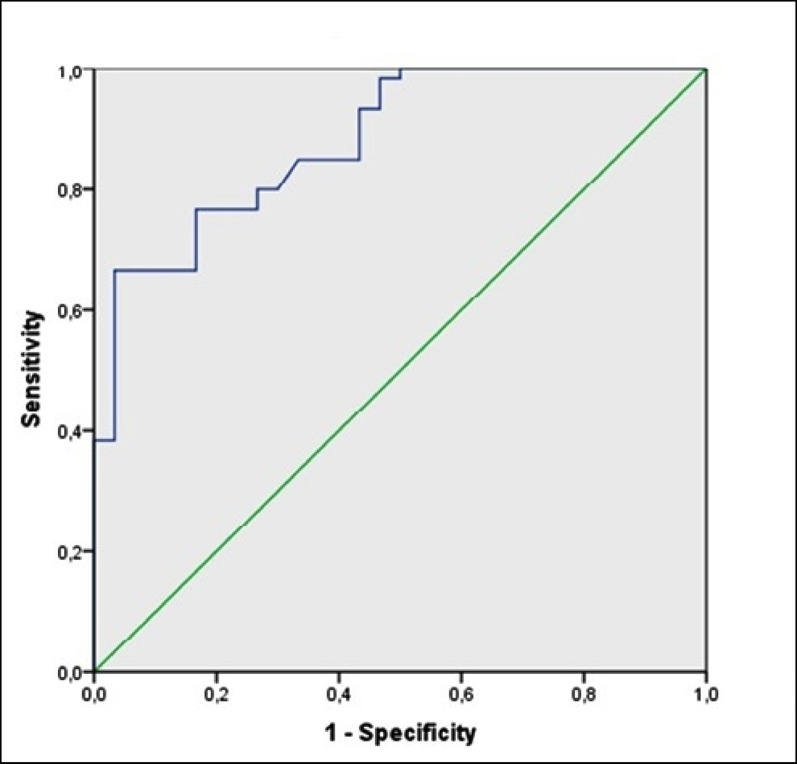
TAPSE is easily obtainable and is a measure of RV longitudinal function, and TAPSE < 16 mm indicates RV systolic dysfunction [18]. Patients with CVID were divided into 2 groups: 10 (16.6%) with low TAPSE (< 16 mm) and 50 (84.4%) without low TAPSE. Median (IQR) copeptin levels were higher in the group with low TAPSE, and this difference was statistically significant (respectively, 22.5 pmol/L vs. 12.5 pmol/L, IQR (21.5–27 vs. 8.8–18 ), p = 0.007) (Fig. 2). ROC analysis identified a cut-off copeptin level of 21 pmol/L for distinguishing the CVID patients with low TAPSE from the CVID patients without low TAPSE, with 80% sensitivity and 94% specificity (area under the curve [AUC] 0.953; 95% CI, 0.900–1.000; p < 0.001) (Fig. 3).
Fig. 2.
Distribution of plasma copeptin concentrations according to subgroup of reduced TAPSE in CVID. Data are presented as medians with 25th and 75th percentiles. TAPSE tricuspid annular plane systolic excursion
Fig. 3.
ROC curves revealing valuable discrimination of CVID patients with reduced TAPSE of < 16 mm by serum concentrations of copeptin. ROC receiver-operating characteristic, TAPSE tricuspid annular plane systolic excursion
Copeptin and echocardiographic right ventricular function parameters
Pearson correlation analysis was used to determine the relationship between copeptin and RVF in CVID patients. A strong negative correlation was found between TAPSE and copeptin (Table 3). In logistic regression analysis on CVID patients, low TAPSE was determined as an independent risk factor for the copeptin elevation (odds ratio (OR) = 0.738; 95% confidence interval (CI) = 0.610–0.910; p = 0.004) (Table 4).
Table 3.
Multiple correlations between echocardiographic right ventricular functions and copeptin
| Parameter | Correlation coefficients (Pearson) |
P |
|---|---|---|
| TAPSE (mm) | r= -0.72 | < 0.001 |
| RVSM (cm/sn) | r = 0.118 | 0.36 |
| RVEM (cm/sn) | r = 0.08 | 0.54 |
| RVAM (cm/sn) | r = 0.07 | 0.6 |
Bold p< 0.05 value indicate statistically significant differences
TAPSE tricuspid annular plane systolic excursion, RVSM systolic myocardial velocity, RVEM early diastolic myocardial velocity, RVAM late diastolic myocardial velocity
Table 4.
Logistic regression model to evaluate associations with copeptin
| (OR) | (CI) | P | |
|---|---|---|---|
| TAPSE (< 16 mm) | 0.738 | 0.610–0.910 | 0.004 |
| Pulmonary Complications | 0.912 | 0.825–0.968 | 0.006 |
TAPSE tricuspid annular plane systolic excursion, OR odds ratio, CI 95% confidence interval
Demographic and clinical findings of CVID patients with and without pulmonary complications
The patients with CVID were divided into 2 groups: those with pulmonary complications (CVID-P) and those without pulmonary complications. Among all the patients with CVID, 50% (n = 30) had at least one concomitant pulmonary disease. Bronchiectasis (n = 20 [33.3%]) was the most common pulmonary complication (Table 1). More patients with CVID-P were women (n = 18 [60%]); the median (IQR) age of patients with CVID-P was 40.5 years [32–60]. There were no significant differences between patients with CVID-P and patients with CVID without pulmonary complications in terms of sex (p = 0.21), median age (p = 0.110), BMI(p = 0.405), or comorbidity (p = 0.76). Median (IQR) basal immunoglobulin (Ig) G was lower in the CVID-P group, but there was no statistically significant difference (p = 0.093). There was no statistically significant difference between the two groups in terms of other median (IQR) baseline Ig values. The relationship between pulmonary disease and B and T lymphocyte subgroups was examined; no significant difference was found between the two groups (Table 5).
Table 5.
Demographic and clinical characteristics of patients with CVID and with and without pulmonary complications
| Variable | CVID-P n = 30 |
CVID-non P n = 30 |
P* |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 18 (60) | 14 (46.7) | 0.21 |
| Male | 12 (40) | 16 (53.3) | |
| Age, median (IQR), years | 40.5 (32–60) | 38 (27–47) | 0.11 |
| BMI, median (IQR), kg/m2 | 24 (18–32) | 26 (19–33) | 0.405 |
| **Comorbidity | 7 (23.3) | 8 (26.7) | 0.76 |
| Basal IgG, median (IQR), mg/dL | 2.6 (1.5–5.3) | 4.45 (2.4–5.45) | 0.093 |
| Basal IgM, median (IQR), mg/dL | 0.2 (0.18–0.5) | 0.29 (0.17–0.5) | 0.912 |
| Basal IgA, median (IQR), mg/dL | 0.30 (0.2–1.2) | 0.35 (0.25–0.9) | 0.28 |
| Basal IgE, median (IQR), IU/mL | 17.5 (14.7–19.2) | 18 (15.7–19) | 0.88 |
| CD3+ T cell, median, (IQR), % | 77.5 (69.5–85) | 68 (63–80) | 0.71 |
| CD19+ B cell, median, (IQR), % | 6 (2–10) | 7 (1–13) | 0.57 |
| CD3+CD4+ T cell, median, (IQR), % | 33 (22–42) | 35 (29–45) | 0.188 |
| CD3+ CD8+ T cell, median, (IQR), % | 42.5 (33.7–53) | 38 (30–49) | 0.151 |
| CD3− CD16+ CD56+ NK cells, median, (IQR), % | 7 (5.7–9.2) | 7 (5-12.5) | 0.84 |
| Copeptin, median (IQR), (pmol/L) | 19 (14–22) | 10 (7–13) | < 0.001 |
CVID-P common variable immunodeficiency with pulmonary complications, CVID-non P common variable immunodeficiency without pulmonary complications, IQR interquartile range, BMI body mass index, IgG immunoglobulin G, IgM immunoglobulin M, IgA immunoglobulin A, IgE immunoglobulin E, NK natural killer
*Intergroup comparisons were conducted using the chi-square, and Mann–Whitney U tests, as appropriate
**Comorbidity; Hypertension, diabetes mellitus, cardiovascular diseases, hyperlipidemia, etc.
Relationship between copeptin and pulmonary complications
In the patients with CVID-P, the median (IQR) serum copeptin level was 19 pmol/L [14–22]. Copeptin levels were significantly higher in the patients with CVID-P than in CVID patients without pulmonary disease (p < 0.001) (Fig. 4).
Fig. 4.
Distribution of plasma copeptin concentrations in CVID with and without pulmonary complications. Data are presented as medians with 25th and 75th percentiles. CVID-P, common variable immunodeficiency with pulmonary complications; CVID-non P, common variable immunodeficiency without pulmonary complications
ROC analysis identified a cut-off copeptin level of 11 pmol/L for distinguishing the patients with CVID-P from the CVID patients without pulmonary disease, with 70% sensitivity and 96% specificity (area under the curve [AUC] 0.881; 95% CI, 0.812–0.951; p < 0.001) (Fig. 5). Pearson correlation analysis was used to determine the relationship between copeptin and pulmonary diseases in CVID patients. A moderate positive correlation was found between copeptin and the development of pulmonary complications (r = 0.553, P < 0.001).
Fig. 5.
ROC curves revealing valuable discrimination of CVID with pulmonary complications by serum concentrations of copeptin. ROC, receiver-operating characteristic; CVID, common variable immunodeficiency
In a logistic regression analysis of CVID patients, the presence of pulmonary complications was identified as an independent risk factor for copeptin elevation (odds ratio (OR) = 0.912; 95% confidence interval (CI) = 0.825–0.968; P = 0.006) (Table 4).
Relationship between immune parameters and copeptin
The relationship between copeptin and B and T lymphocyte subgroups was examined. However, there was no statistically significant difference (Table 6).
Table 6.
Multivariable linear regression model to evaluate associations with copeptin and
immunological parameters
| Parameter | B | Standard error | Beta | T | P | |
|---|---|---|---|---|---|---|
| Creatinin | -13.902 | 16.593 | -0.196 | –0.838 | 0.406 | |
| GFR | -0.127 | 0.092 | -0.281 | -1.382 | 0.173 | |
| IgG | -0.714 | 1.703 | − 0.0.083 | -0.419 | 0.677 | |
| IgM | -2.019 | 3.994 | -0.079 | -0.505 | 0.616 | |
| IgA | 5.596 | 5.518 | 0.228 | 1.014 | 0.316 | |
| IgE | 0.135 | 0.154 | 0.143 | 0.877 | 0.385 | |
| CD3+ T cell | 0.099 | 0.442 | 0.066 | 0.224 | 0.824 | |
| CD3+CD4+ T cell | -0.008 | 0.333 | -0.007 | -0.023 | 0.981 | |
| CD3+ CD8+ T cell | -0.025 | 0.322 | -0.019 | -0.076 | 0.940 | |
| CD19+ B cell | -0.107 | 0.532 | -0.039 | -0.202 | 0.841 | |
| NK cells | 0.319 | 0.648 | 0.091 | 0.492 | 0.625 | |
GFR glomerular filtration rate, IgG immunoglobulin G, IgM immunoglobulin M, IgA immunoglobulin A, IgE immunoglobulin E, NK CD3- CD16+ CD56+ natural killer
Discussion
In this study, serum copeptin levels were significantly higher in CVID patients with low TAPSE values compared to CVID patients without low TAPSE values. The presence of lower than normal TAPSE values in the patients with CVID constituted an independent risk factor for the copeptin rise. Another remarkable finding of our study is that serum copeptin levels were significantly higher in the patients with CVID-P than in the patients with CVID and without pulmonary complications. The presence of pulmonary disease in the patients with CVID constituted an independent risk factor for the copeptin rise.
The arginine vasopressin (AVP) system is a neuroendocrine structure that plays a key role in maintaining fluid-electrolyte balance and haemodynamic stability in the body. AVP is synthesised in the paraventricular and supraoptic nuclei of the hypothalamus and transported to the posterior pituitary (neurohypophysis) via axons. Here, it is stored for release into the circulation in response to physiological signals such as changes in plasma osmolality or stimuli related to the circulatory system. AVP is derived from a precursor molecule called preprovasopressin. This precursor structure also contains copeptin along with AVP. The conversion of the hormone into its active components occurs through a multi-step maturation mechanism involving a series of enzymatic processes. In the first step of this process, the signal peptide is cleaved off, followed by the separation of AVP and copeptin into their functional forms. As a result, AVP and kopeptin are released into the circulation simultaneously in response to physiological stimuli [19, 20]. Under physiological conditions, the release of AVP is primarily regulated by plasma osmolality [21]. However, it is not only osmotic changes; vasoactive substances such as angiotensin II and norepinephrine can also directly stimulate AVP secretion [22]. This situation shows that the AVP system is closely related not only to fluid balance but also to cardiovascular stability. Copeptin is a stable biomarker representative of the hormone AVP [19]. However, AVP is difficult to measure directly; therefore, copeptin, which is secreted together with it, is used as a more reliable and easily measurable alternative [20]. Copeptin is stable in EDTA plasma at room temperature for up to 14 days and in citrate and heparin plasma for 7 days [23]. No complex preanalytical steps are required for its detection [24]. Copeptin can be easily measured ex vivo by manually or fully automatically chemiluminescence [25]. The test requires a minimum of serum or plasma and analysis time 20 to 30 min [24].
In inflammatory conditions, inflammatory mediators such as interleukin (IL)-1 and tumor necrosis factor-α can trigger AVP secretion. As a result, copeptin levels also rise [26].
IL-2 and norepinephrine can also stimulate AVP release from the hypothalamus [27].
In a previous study, AVP and, consequently, copeptin levels were higher in patients with severe sepsis and septic shock than in patients with infection without inflammation [26].
In another study conducted with sepsis patients, plasma copeptin levels were higher in septic shock patients than in healthy controls [28]. In a study conducted on individuals with advanced chronic liver disease (ACLD), it was emphasised that plasma copeptin levels showed an independent relationship with IL-6 levels and that systemic inflammation could be one of the underlying factors behind increased AVP levels in decompensated ACLD [29].
Another study reported that increasing doses of intravenous recombinant IL-6 strongly stimulated the hypothalamic-pituitary-adrenal axis and induced systemic AVP secretion [30].For these reasons, copeptin may be considered clinically significant due to its significant relationship with neuroendocrine stress and inflammatory responses in non-cardiovascular conditions.
CVID is a complex disease that is characterised not only by antibody deficiency but also by systemic and organ-specific inflammatory complications. Autoimmune, granulomatous, and interstitial lung diseases, as well as gastrointestinal diseases caused by inflammation, are important in terms of mortality and morbidity. This information supports our study, which suggests that copeptin should not be overlooked in CVID, a disease group that may be accompanied by inflammation.
In healthy controls, median copeptin concentrations were reported 4.7 (IQR 2.9–7.6) pmol/L [31, 32]. Similarly, in this study, the median copeptin was 5.79 pmol/L in HVs.In addition, copeptin shows minimal interindividual differences (age, sex, eGFR, etc.) [32]. The prevalence of HF in the adult population is reported to be around 1–2% [33]. HF is associated with a elevated mortality rate and HF treatment imposes a large economic burden on healthcare systems [34]. In a previous study, the predictive ability of copeptin for the entire spectrum of HF was investigated and it was emphasised that it was independently associated with mortality in all symptomatic stages of HF and was the strongest predictor of death in New York Heart Association (NYHA) class II and III [35]. In another study of patients with HF symptoms, the association between plasma concentrations of copeptin and mortality was evaluated, and elevated copeptin concentrations were associated with an increased risk of all-cause mortality [36].
A wide variety of congenital and structural cardiovascular diseases have been reported in primary immunodeficiencies (Atrial septal defect, ventricular septum defect, patent ductus arteriosus, mitral, tricuspid, and pulmonary insufficiency, HF, bradycardia, ischemic heart disease, etc.). CVID is not usually associated with cardiovascular diseases [5]. The possible association between CVID and cardiac manifestations might be due to immune dysregulation like autoimmunity.
In a case report, a patient with CVID of acute giant cell myocarditis diagnosed by endomyocardial biopsy was mentioned, and it is an autoimmune myocarditis [37].
Cases of acute pericarditis in CVID have been reported in earlier research. It was noted that all patients had a history of recurrent respiratory infections, and in one case, bacterial growth was detected during the episode of acute pericarditis, suggesting a possible infectious etiology in this context [38]. Moreover, prior investigations have proposed a role for autoimmunity in the pathogenesis of pericardial diseases [39, 40].
In a former research with 337 CVID patients, 9.1% with cardiac manifestations were found. Pericardial disease was reported in 1.7%, arrhythmia in 1.1%, and congenital heart disease in 1.1% [41]. Similarly, in this study, cardiac manifestations were found in 10% of CVID patients, arrhythmia in 5%, pericardial disease in 3.3%, and congenital heart disease in 1.6%.
Studies have generally focused on left-sided HF with either reduced or preserved EF; however, RVF were shown to be an independent predictor of survival in a study of patients with stable HF [42].
Transthoracic echocardiography is the standard diagnostic technique for evaluating RVF due to its widespread availability, easy applicability, and low cost [43]. TAPSE is the main parameter for assessing RVF by transthoracic echocardiography [44]. Preceding investigations have shown that TAPSE is strongly correlated with RVF and is the dominant parameter in the evaluation of RVF [45, 46]. A prior research has shown that copeptin concentrations are inversely related to TAPSE. Moreover, it has been shown that clinically significant stages of RV dysfunction, defined by TAPSE below 18–14 mm, were reliably distinguished by copeptin concentrations [15]. Similarly, in the present study, copeptin was found to be elevated in CVID patients with TAPSE values lower than 16 mm and an inverse relationship was found between TAPSE and copeptin. This suggests that copeptin may be a predictive biomarker in the evaluation of RVF in CVID patients.
Chronic lung diseases are the leading cause of mortality and morbidity in patients with CVID [47]. According to earlier studies, the prevalence of chronic lung disease in individuals with CVID ranges from 28 to 62% [48, 49]. Among the pulmonary complications associated with CVID, the most common lung disease diagnosed is %40 bronchiectasis [50]. In previous study, bronchiectasis was reported 28.5% in patients with CVID [51]. In this study, bronchiectasis was detected at a rate of 33.3%. Bronchiectasis results from progressive damage to the bronchial wall secondary to recurrent lower respiratory tract infections and inflammation, progressing before the start of immünoglobulin replacement treatment (IgRT) and sometimes despite adequate IgRT [52]. Pulmonary diseases may progress without signs of infection, therefore monitoring of pulmonary complications with imaging, pulmonary function tests and microbial culture methods is recommended in CVID [53].
There are studies on the effect of copeptin on prediction and prognosis in pulmonary diseases. In a study conducted with acute pulmonary embolism patients, copeptin level was found to be significantly higher than in healthy control group. Moreover, copeptin was significantly higher in PE-related death and who developed RV disfunction at one-year follow-up. Addition, similarly in this study, there was moderate and negative correlation between copeptin and TAPSE [54]. In a earlier study, copeptin was reported to be a prognostic marker in AECOPD [55]. In another study, it was emphasized that copeptin levels were elevated in patients with lower respiratory tract infection, and copeptin levels were higher in patients with AECOPD and acute bronchitis due to lower respiratory tract infection than in the control group [56]. In another multicentre study, copeptin was found to be a predictive biomarker of two-year mortality in stable chronic obstructive pulmonary disease independent of selected pulmonary risk factors [57]. In this study, supporting this informations, it was determined that copeptin may be predictive of pulmonary complications in CVID.
Granulomatous lymphocytic interstitial lung disease (GLILD) is a complex interstitial lung disease characterised by granulomatous and lymphocytic infiltration, seen particularly in patients with CVID (approximately 20%) [58]. There is no specific biomarker unique to GLILID, but certain laboratory, radiological, and immunological findings are considered helpful biomarkers in the diagnosis and monitoring process [59]. Peribronchial and interstitial chronic inflammation are among the most commonly observed histopathological features in GLILD [58]. A study conducted in association with GLILD indicates that this condition frequently co-occurs with granulomatous and inflammatory processes [59]. In this study, two patients had a diagnosis of GLILD. Plasma copeptin levels were 30 pmol/L and 20 pmol/L respectively. These values were above the cut-off values established for pulmonary diseases. However, there was no statistically significant difference in copeptin levels and other parameters between CVID patients diagnosed with GLILD and CVID patients with other pulmonary diseases. This may be related to the small number of patients with GLILD. Nevertheless, this finding supports the importance of copeptin as a biomarker in diffuse lung parenchymal diseases such as GLILD, where diagnosis is challenging in CVID.
Previous studies have described RV dysfunction in patients with chronic lung disease [60]. Hypoxaemia plays an important role in affecting RVF [61]. In patients with bronchiectasis, chronic hypoxaemia may lead to an increase in pulmonary vascular resistance and cause RV dysfunction [62]. In a former research, it was suggested that haemodynamic changes may occur in the lungs of patients with bronchiectasis. When the systemic-pulmonary anastomoses that develop in granulomatous areas at the sites of destruction of the bronchial wall in bronchiectasis expand, oxygenated blood shunts from the high-pressure systemic bronchial and intercostal arteries to the low-pressure pulmonary circulation. Chronic shunt flow can lead to elevated PAPs and thus RV dysfunction [63]. As in this study, these data support that the development of pulmonary complications and RV dysfunction should not be ignored considering that pulmonary diseases are frequently observed in CVID.
For some diseases, diagnosis and prognosis can be very demanding and time-consuming. So clinicians need new biomarkers for faster treatment decisions and prognostic evaluation [13]. A new biomarker should be practical, reliable, easy to measure, fast and cheap. Therefore, copeptin is proposed as a prognostic biomarker that may help early diagnosis and diagnostic accuracy in different diseases. In CVID, an algorithmic diagnostic approach is applied at specific intervals to monitor malignancy, autoimmunity, and granulomatous diseases (Clinical examination, every 3–6 months complete blood count, liver function tests, IgG level, annual lymphadenopathy/splenomegaly check, chest CT scan every 1–2 years, annual gastrointestinal system screening (as needed), etc.) [64].
According to this study, the following new copeptin cut-off values are recommended to contribute to the algorithmic diagnostic approach in CVID: plasma copeptin levels greater than 21 pmol/L (with over 90% specificity for RV dysfunction); plasma copeptin levels greater than 11 pmol/L (with over 90% specificity for pulmonary complications).
These mentioned cases indicate that copeptin may be an important biomarker in the monitoring of the development of RV dysfunction classified with TAPSE < 16 mm in CVID patients and in the follow-up of the development of pulmonary diseases, which is one of the frequently observed complications in CVID.
This study had some limitations. First, this cross-sectional study was a single-center study with a limited sample size. Second, although the study was prospectively planned, copeptin levels and echocardiographic parameters were measured at only one time point. Serial measurements would have better reflected time-dependent changes in disease activity or treatment response. Third, did not include long-term follow-up analyses of any patients. Despite these limitations, our study may shed light on future studies on the role of copeptin as a predictive biomarker for developing pulmonary diseases and RV dysfunction in patients with CVID. However, large-scale multicenter studies are needed to re-evaluate our findings.
Conclusion
Early diagnosis and treatment are important for reducing CVID-related complications. Thus, there is a need to monitor the development of pulmonary and cardiac complications to prevent diagnostic delays and provide adequate pharmacologic treatment to patients with CVID, both at the time of diagnosis and during clinical follow-up.
Serum copeptin levels were elevated during the development of pulmonary diseases and RV dysfunction in CVID. Measuring serum copeptin concentration may help predict the diagnosis and prognosis of pulmonary diseases and RV dysfunction in CVID. Further studies are needed to elucidate the role of copeptin as a valuable biomarker in patients with CVID.
Acknowledgements
Not applicable.
Abbreviations
- CVID
Common variable immunodeficiency
- HVs
Healthy volunteers
- HF
Heart failure
- IQR
Interquartile range
- BMI
Body mass index
- AVP
Arginine vasopressin
- EF
Ejection fraction
- RV
Right ventricular
- AECOPD
Chronic obstructive pulmonary disease
- RVF
Right ventricular functions
- MDRD
The modification of the diet in renal disease
- OR
Odds ratio
- Egfr
Estimate the glomerular filtration rate
- ROC
Receiver operating characteristic
- CVID-P
CVID with pulmonary complications
- TAPSE
Tricuspid annular plane systolic excursion
- RVSM
Systolic myocardial velocity
- RVEM
Early diastolic myocardial velocity
- RVAM
Late diastolic myocardial velocity
- IgRT
Immünoglobulin replacement treatment
- SD
Standard deviation
- PAPs
Pulmonary artery systolic pressure
- GLILD
Granulomatous lymphocytic interstitial lung disease
Author contributions
MK and FÇ conceptualized and designed the study. MK and ŞA performed data acquisition and analysis. FSA, RE and EY interpreted the results. BF, MD and YA revised the manuscript critically for intellectual content.ST drafted the initial manuscript. All authors reviewed and approved the final manuscript.
Funding
This study was not supported by any funding source.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was approved by the local ethics committee of Necmettin Erbakan University Medical Faculty Hospital, Konya, Türkiye (decision no. 2020/2962). All procedures were conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bonilla FA, Barlan I, Chapel H, Costa-Carvalho BT, Cunningham-Rundles C, de la Morena MT, et al. International consensus document (ICON): common variable immunodeficiency disorders. J Allergy Clin Immunol Pract. 2016;4(1):38–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knight AK, Cunningham-Rundles C. Inflammatory and autoimmune complications of common variable immune deficiency. Autoimmun Rev. 2006;5(2):156–9. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham-Rundles C, Maglione PJ. Common variable immunodeficiency. J Allergy Clin Immunol. 2012;129(5):1425–e63. [DOI] [PubMed] [Google Scholar]
- 4.Yazdani R, Habibi S, Sharifi L, Azizi G, Abolhassani H, Olbrich P, et al. Common variable immunodeficiency: epidemiology, pathogenesis, clinical manifestations, diagnosis, classification, and management. J Investig Allergol Clin Immunol. 2020;30(1):14–34. [DOI] [PubMed] [Google Scholar]
- 5.Human A, Murguia-Favela L, Benson L, Roifman I, Grunebaum E. Cardiovascular abnormalities in primary immunodeficiency diseases. LymphoSign J. 2015;2(3):107–34. [Google Scholar]
- 6.Wang J, Tan GJ, Han LN, Bai YY, He M, Liu HB. Novel biomarkers for cardiovascular risk prediction. J Geriatr Cardiol. 2017;14(2):135–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taçoy G, Çengel A. The light of current knowledge right ventricle. Turk Kardiyol Dern Ars. 2014;42(6):574–84. [DOI] [PubMed] [Google Scholar]
- 8.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251–9. [DOI] [PubMed] [Google Scholar]
- 9.Busse PJ, Farzan S, Cunningham-Rundles C. Pulmonary complications of common variable immunodeficiency. Ann Allergy Asthma Immunol. 2007;98(1):1–8. quiz– 11, 43. [DOI] [PubMed] [Google Scholar]
- 10.Johnston SL, Hill SJ, Lock RJ, Dwight JF, Unsworth DJ, Gompels MM. Echocardiographic abnormalities in primary antibody deficiency. Postgrad Med J. 2004;80(942):214–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation. 2010;121(18):2045–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52(1):112–9. [DOI] [PubMed] [Google Scholar]
- 13.Dobsa L, Edozien KC. Copeptin and its potential role in diagnosis and prognosis of various diseases. Biochem Med (Zagreb). 2013;23(2):172–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antonescu-Turcu AL, Tomic R. C-reactive protein and copeptin: prognostic predictors in chronic obstructive pulmonary disease exacerbations. Curr Opin Pulm Med. 2009;15(2):120–5. [DOI] [PubMed] [Google Scholar]
- 15.Harbrücker M, Natale M, Kim SH, Müller J, Ansari U, Huseynov A, et al. Copeptin reliably reflects longitudinal right ventricular function. Ann Clin Biochem. 2021;58(4):270–9. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, et al. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53(4):766–72. [DOI] [PubMed] [Google Scholar]
- 17.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–70. [DOI] [PubMed] [Google Scholar]
- 18.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American society of echocardiography endorsed by the European association of echocardiography, a registered branch of the European society of cardiology, and the Canadian society of echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. quiz 86– 8. [DOI] [PubMed] [Google Scholar]
- 19.Morgenthaler NG, Struck J, Jochberger S, Dünser MW. Copeptin: clinical use of a new biomarker. Trends Endocrinol Metabolism. 2008;19(2):43–9. [DOI] [PubMed] [Google Scholar]
- 20.Lee CR, Watkins ML, Patterson JH, Gattis W, O’Connor CM, Gheorghiade M, et al. Vasopressin: a new target for the treatment of heart failure. Am Heart J. 2003;146(1):9–18. [DOI] [PubMed] [Google Scholar]
- 21.Barat C, Simpson L, Breslow E. Properties of human vasopressin precursor constructs: inefficient monomer folding in the absence of copeptin as a potential contributor to diabetes insipidus. Biochemistry. 2004;43(25):8191–203. [DOI] [PubMed] [Google Scholar]
- 22.Chatterjee K. Neurohormonal activation in congestive heart failure and the role of vasopressin. Am J Cardiol. 2005;95(9):8–13. [DOI] [PubMed] [Google Scholar]
- 23.Stoiser B, Mörtl D, Hülsmann M, Berger R, Struck J, Morgenthaler NG, et al. Copeptin, a fragment of the vasopressin precursor, as a novel predictor of outcome in heart failure. Eur J Clin Invest. 2006;36(11):771–8. [DOI] [PubMed] [Google Scholar]
- 24.Lippi G, Plebani M, Di Somma S, Monzani V, Tubaro M, Volpe M, et al. Considerations for early acute myocardial infarction rule-out for emergency department chest pain patients: the case of copeptin. Clin Chem Lab Med. 2012;50(2):243–53. [DOI] [PubMed] [Google Scholar]
- 25.Nickel CH, Bingisser R, Morgenthaler NG. The role of copeptin as a diagnostic and prognostic biomarker for risk stratification in the emergency department. BMC Med. 2012;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jochberger S, Dörler J, Luckner G, Mayr VD, Wenzel V, Ulmer H, et al. The vasopressin and copeptin response to infection, severe sepsis, and septic shock. Crit Care Med. 2009;37(2):476–82. [DOI] [PubMed] [Google Scholar]
- 27.Raber J, Bloom FE. IL-2 induces vasopressin release from the hypothalamus and the amygdala: role of nitric oxide-mediated signaling. J Neurosci. 1994;14(10):6187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Struck J, Morgenthaler NG, Bergmann A. Copeptin, a stable peptide derived from the vasopressin precursor, is elevated in serum of sepsis patients. Peptides. 2005;26(12):2500–4. [DOI] [PubMed] [Google Scholar]
- 29.Hartl L, Hintersteininger M, Simbrunner B, Jachs M, Hofer BS, Bauer DJM et al. The vasopressin biomarker copeptin is linked to systemic inflammation and refines prognostication in decompensated cirrhosis. Clin Gastroenterol Hepatol. 2025;30(25):461–66. [DOI] [PubMed]
- 30.Mastorakos G, Weber JS, Magiakou M, Gunn H, Chrousos GP. Hypothalamic-pituitary-adrenal axis activation and stimulation of systemic vasopressin secretion by Recombinant interleukin-6 in humans: potential implications for the syndrome of inappropriate vasopressin secretion. J Clin Endocrinol Metab. 1994;79(4):934–9. [DOI] [PubMed] [Google Scholar]
- 31.van Gastel MD, Meijer E, Scheven LE, Struck J, Bakker SJ, Gansevoort RT. Modifiable factors associated with copeptin concentration: a general population cohort. Am J Kidney Dis. 2015;65(5):719–27. [DOI] [PubMed] [Google Scholar]
- 32.Bhandari SS, Loke I, Davies JE, Squire IB, Struck J, Ng LL. Gender and renal function influence plasma levels of copeptin in healthy individuals. Clin Sci (Lond). 2009;116(3):257–63. [DOI] [PubMed] [Google Scholar]
- 33.Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93(9):1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5-Year outcomes. J Am Coll Cardiol. 2017;70(20):2476–86. [DOI] [PubMed] [Google Scholar]
- 35.Neuhold S, Huelsmann M, Strunk G, Stoiser B, Struck J, Morgenthaler NG, et al. Comparison of copeptin, B-type natriuretic peptide, and amino-terminal pro-B-type natriuretic peptide in patients with chronic heart failure: prediction of death at different stages of the disease. J Am Coll Cardiol. 2008;52(4):266–72. [DOI] [PubMed] [Google Scholar]
- 36.Alehagen U, Dahlström U, Rehfeld JF, Goetze JP. Association of copeptin and N-terminal probnp concentrations with risk of cardiovascular death in older patients with symptoms of heart failure. JAMA. 2011;305(20):2088–95. [DOI] [PubMed] [Google Scholar]
- 37.Laufs H, Nigrovic PA, Schneider LC, Oettgen H, Del NP, Moskowitz IP, et al. Giant cell myocarditis in a 12-year-old Girl with common variable immunodeficiency. Mayo Clin Proc. 2002;77(1):92–6. [DOI] [PubMed] [Google Scholar]
- 38.Ramzi N, Yazdani S, Talakoob H, Jamee M, Karim H, Azizi G. Acute pericarditis: A peculiar manifestation of common variable immune deficiency. Allergol Immunopathol (Madr). 2021;49(3):115–9. [DOI] [PubMed] [Google Scholar]
- 39.Payandeh P, Khoshkhui M, Jabbari Azad F, Farid Hosseini R. A patient with common variable immunodeficiency and pericardial effusion: A case report and review of literature. J Pediatr Rev. 2019;7(3):177–80. [Google Scholar]
- 40.Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J, et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases: the task force for the diagnosis and management of pericardial diseases of the European society of cardiology (ESC)Endorsed by: the European association for Cardio-Thoracic surgery (EACTS). Eur Heart J. 2015;36(42):2921–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramzi N, Yazdani S, Talakoob H, Rasouli SE, Karim H, Azizi G. Cardiac complications in patients with common variable immune deficiency: A longitudinal study. Immunol Genet J. 2022;4(2):87–94
- 42.de Groote P, Millaire A, Foucher-Hossein C, Nugue O, Marchandise X, Ducloux G, et al. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J Am Coll Cardiol. 1998;32(4):948–54. [DOI] [PubMed] [Google Scholar]
- 43.Lu KJ, Chen JX, Profitis K, Kearney LG, DeSilva D, Smith G, et al. Right ventricular global longitudinal strain is an independent predictor of right ventricular function: a multimodality study of cardiac magnetic resonance imaging, real time three-dimensional echocardiography and speckle tracking echocardiography. Echocardiography. 2015;32(6):966–74. [DOI] [PubMed] [Google Scholar]
- 44.Schneider M, Binder T. Echocardiographic evaluation of the right heart. Wien Klin Wochenschr. 2018;130(13–14):413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vizzardi E, Bonadei I, Sciatti E, Pezzali N, Farina D, D’Aloia A, et al. Quantitative analysis of right ventricular (RV) function with echocardiography in chronic heart failure with no or mild RV dysfunction: comparison with cardiac magnetic resonance imaging. J Ultrasound Med. 2015;34(2):247–55. [DOI] [PubMed] [Google Scholar]
- 46.Scrutinio D, Catanzaro R, Santoro D, Ammirati E, Passantino A, Oliva F, et al. Tricuspid annular plane systolic excursion in acute decompensated heart failure: relevance for risk stratification. Can J Cardiol. 2016;32(8):963–9. [DOI] [PubMed] [Google Scholar]
- 47.Chapel H, Cunningham-Rundles C. Update in Understanding common variable immunodeficiency disorders (CVIDs) and the management of patients with these conditions. Br J Haematol. 2009;145(6):709–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Resnick ES, Moshier EL, Godbold JH, Cunningham-Rundles C. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood. 2012;119(7):1650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bintalib HM, Grigoriadou S, Patel SY, Mutlu L, Sooriyakumar K, Vaitla P, et al. Investigating pulmonary and non-infectious complications in common variable immunodeficiency disorders: a UK National multi-centre study. Front Immunol. 2024;15:1451813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edgar JD, Buckland M, Guzman D, Conlon NP, Knerr V, Bangs C, et al. The united Kingdom primary immune deficiency (UKPID) registry: report of the first 4 years’ activity 2008–2012. Clin Exp Immunol. 2014;175(1):68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Janssen LMA, van der Flier M, de Vries E. Lessons learned from the clinical presentation of common variable immunodeficiency disorders: A systematic review and Meta-Analysis. Front Immunol. 2021;12:620709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quinti I, Soresina A, Guerra A, Rondelli R, Spadaro G, Agostini C, et al. Effectiveness of Immunoglobulin replacement therapy on clinical outcome in patients with primary antibody deficiencies: results from a multicenter prospective cohort study. J Clin Immunol. 2011;31(3):315–22. [DOI] [PubMed] [Google Scholar]
- 53.van Zeggeren L, van de Ven AA, Terheggen-Lagro SW, Mets OM, Beek FJ, van Montfrans JM, et al. High-resolution computed tomography and pulmonary function in children with common variable immunodeficiency. Eur Respir J. 2011;38(6):1437–43. [DOI] [PubMed] [Google Scholar]
- 54.Ozmen C, Deveci OS, Karaaslan MB, Baydar O, Akray A, Deniz A et al. Predictive value of plasma copeptin level for diagnosis and mortality of pulmonary embolism. Rev Assoc Med Bras (1992). 2020;66(12):1645-50. [DOI] [PubMed]
- 55.Winther JA, Brynildsen J, Høiseth AD, Strand H, Følling I, Christensen G, et al. Prognostic and diagnostic significance of copeptin in acute exacerbation of chronic obstructive pulmonary disease and acute heart failure: data from the ACE 2 study. Respir Res. 2017;18(1):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Müller B, Morgenthaler N, Stolz D, Schuetz P, Müller C, Bingisser R, et al. Circulating levels of copeptin, a novel biomarker, in lower respiratory tract infections. Eur J Clin Invest. 2007;37(2):145–52. [DOI] [PubMed] [Google Scholar]
- 57.Boeck L, Soriano JB, Brusse-Keizer M, Blasi F, Kostikas K, Boersma W, et al. Prognostic assessment in COPD without lung function: the B-AE-D indices. Eur Respir J. 2016;47(6):1635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rao N, Mackinnon AC, Routes JM. Granulomatous and lymphocytic interstitial lung disease: a spectrum of pulmonary histopathologic lesions in common variable immunodeficiency—histologic and immunohistochemical analyses of 16 cases. Hum Pathol. 2015;46(9):1306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hurst JR, Verma N, Lowe D, Baxendale HE, Jolles S, Kelleher P, et al. British lung foundation/united Kingdom primary immunodeficiency network consensus statement on the definition, diagnosis, and management of granulomatous-lymphocytic interstitial lung disease in common variable immunodeficiency disorders. J Allergy Clin Immunology: Pract. 2017;5(4):938–45. [DOI] [PubMed] [Google Scholar]
- 60.Ozer N, Tokgözoglu L, Cöplü L, Kes S. Echocardiographic evaluation of left and right ventricular diastolic function in patients with chronic obstructive pulmonary disease. J Am Soc Echocardiogr. 2001;14(6):557–61. [DOI] [PubMed] [Google Scholar]
- 61.Huez S, Retailleau K, Unger P, Pavelescu A, Vachiéry JL, Derumeaux G, et al. Right and left ventricular adaptation to hypoxia: a tissue doppler imaging study. Am J Physiol Heart Circ Physiol. 2005;289(4):H1391–8. [DOI] [PubMed] [Google Scholar]
- 62.Gencer M, Ceylan E, Yilmaz R, Gur M. Impact of bronchiectasis on right and left ventricular functions. Respir Med. 2006;100(11):1933–43. [DOI] [PubMed] [Google Scholar]
- 63.Liebow AA, Hales MR, Lindskog GE. Enlargement of the bronchial arteries, and their anastomoses with the pulmonary arteries in bronchiectasis. Am J Pathol. 1949;25(2):211–31. [PMC free article] [PubMed] [Google Scholar]
- 64.Bonilla FA, Barlan I, Chapel H, Costa-Carvalho BT, Cunningham-Rundles C, de la Morena MT, et al. International consensus document (ICON): common variable immunodeficiency disorders. J Allergy Clin Immunol Pract. 2015;4(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.