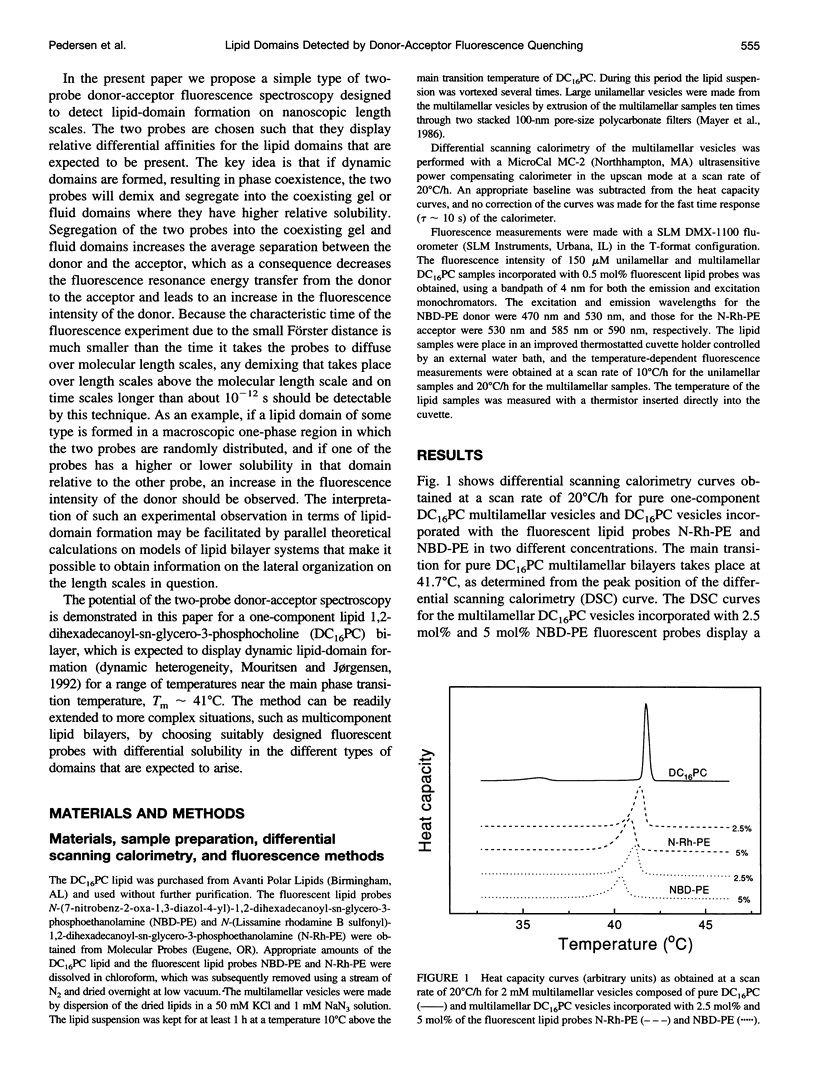
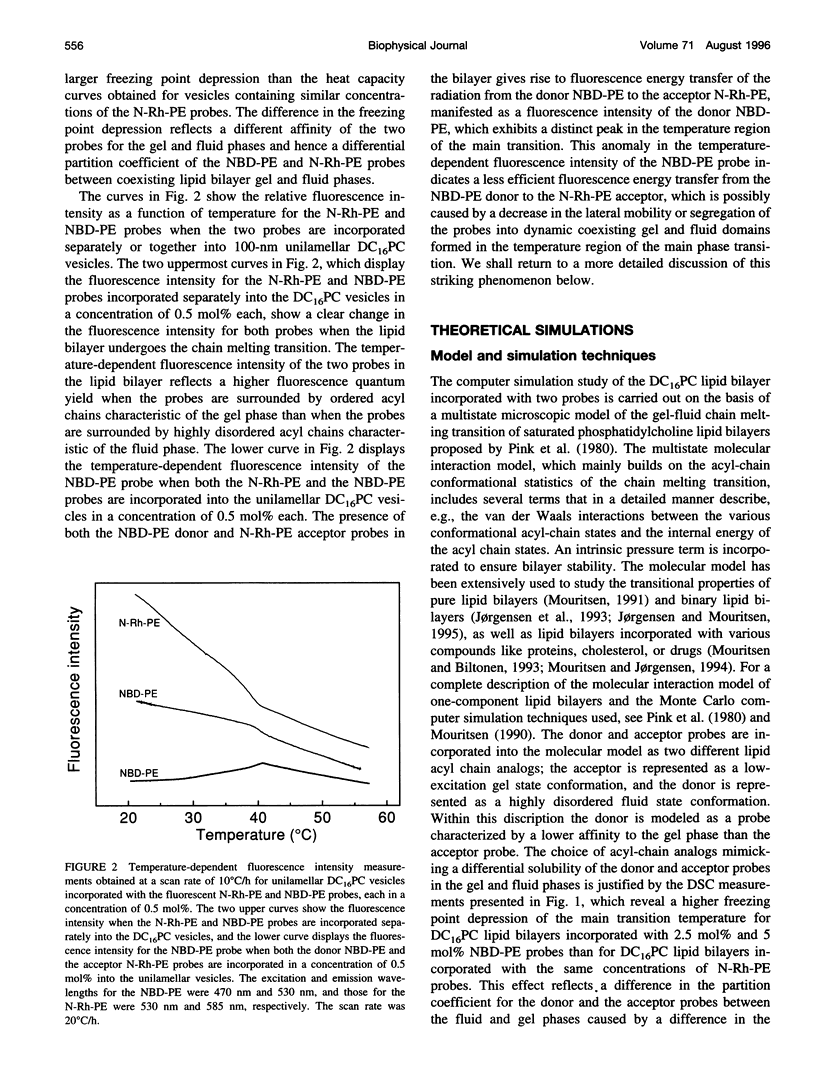
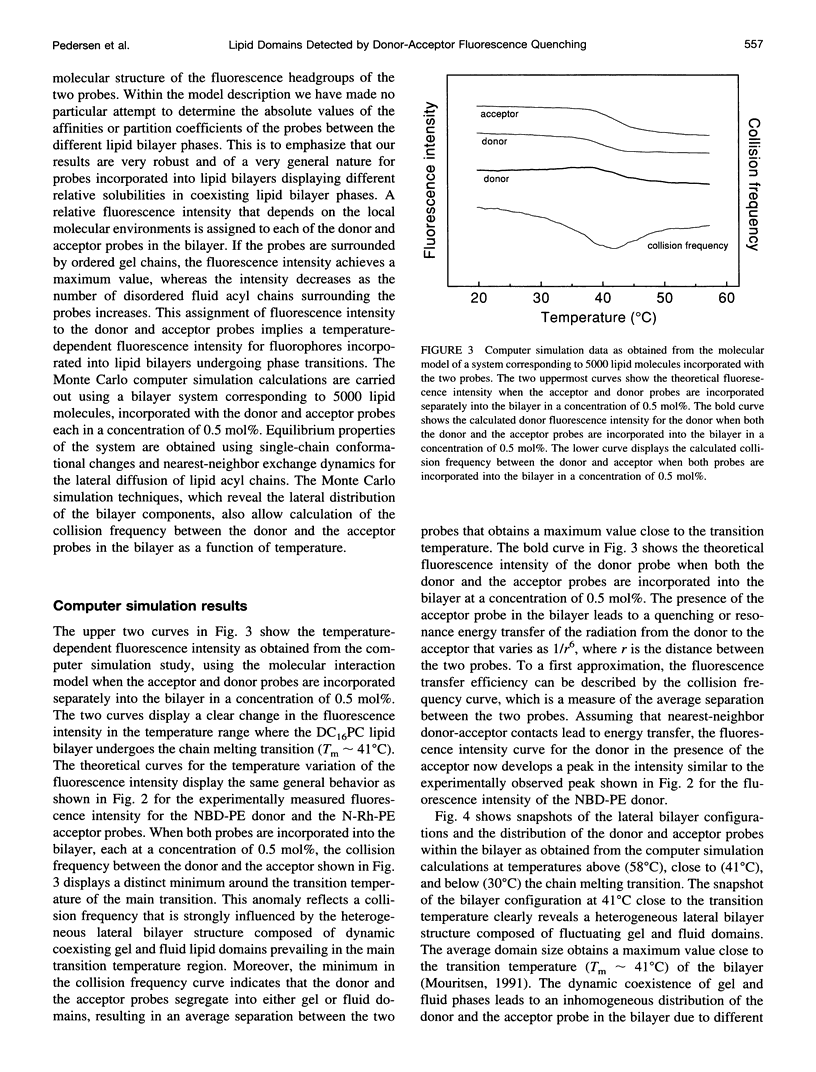
Abstract
The fluorescence energy transfer between two lipid probes, N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-1, 2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine (donor) and N-(Lissamine rhodamine B sulfonyl)-1, 2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine (acceptor), incorporated into 1,2-dihexadecanoyl-sn-glycero-3-phosphocholine unilamellar and multilamellar lipid bilayers, is studied in the temperature region of the main phase transition. The two probes display different relative solubilities in the gel and fluid lipid-bilayer phases. A distinct maximum in the fluorescence intensity of the donor is observed in the transition region, indicating that the two probes are demixing and hence increasing their average separation. The observation is interpreted in terms of dynamic segregation of the two probes into coexisting gel and fluid lipid domains that are formed dynamically in the transition region due to strong density fluctuations. The interpretation of the experimental observations is supported by a detailed theoretical calculation using computer simulation of a microscopic model that takes full account of diffusion of the two probes and the fluctuations of gel and fluid lipid domains characteristic of the main phase transition.
Full text
PDF
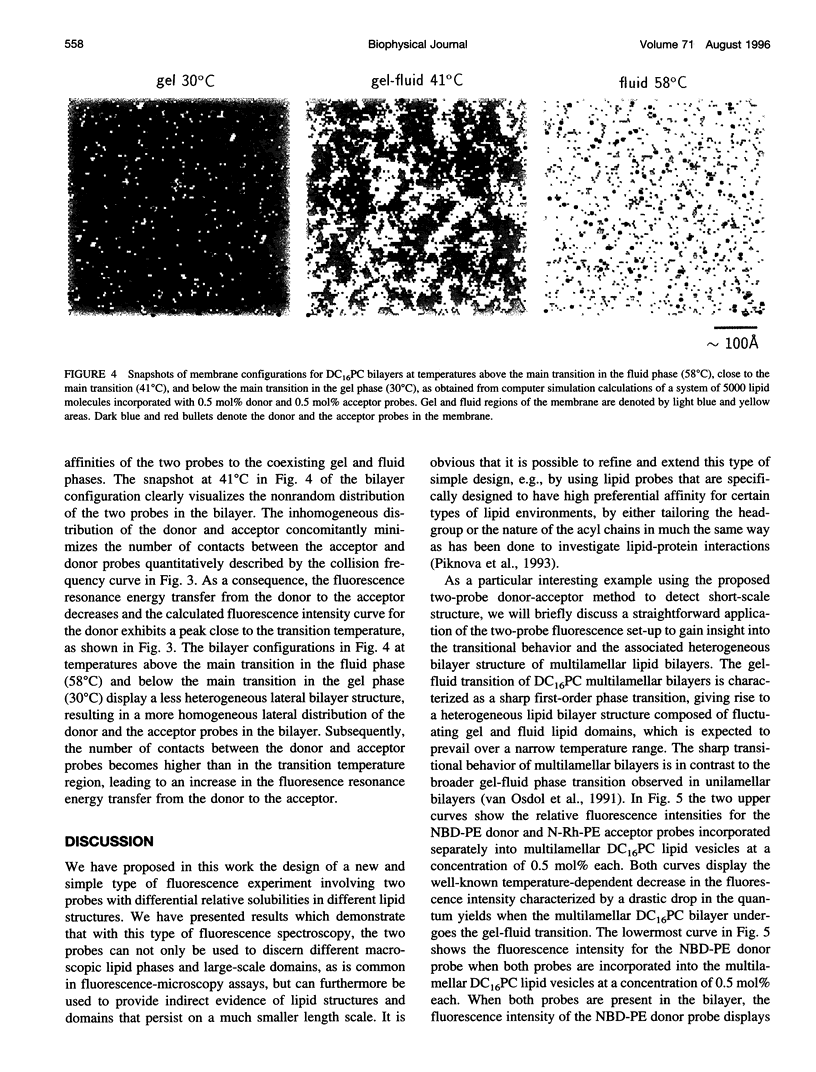
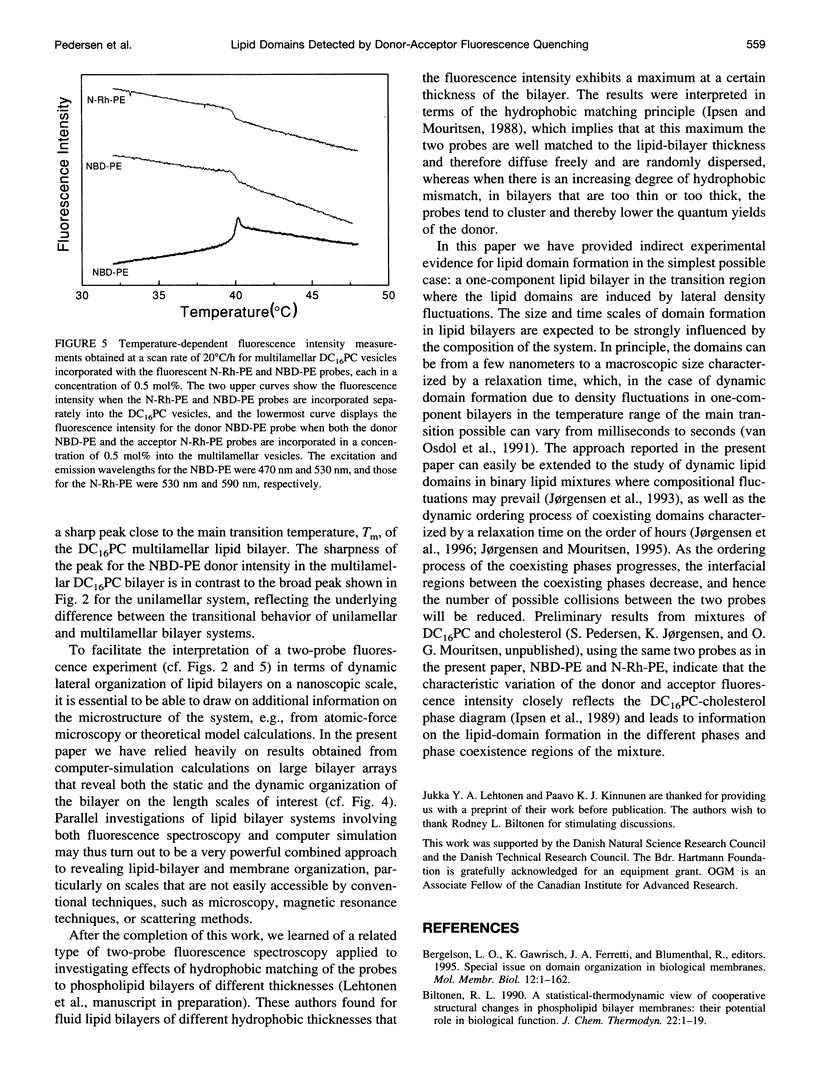

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Freire E., Biltonen R. Estimation of molecular averages and equilibrium fluctuations in lipid bilayer systems from the excess heat capacity function. Biochim Biophys Acta. 1978 Dec 4;514(1):54–68. doi: 10.1016/0005-2736(78)90076-7. [DOI] [PubMed] [Google Scholar]
- Ipsen J. H., Mouritsen O. G. Modelling the phase equilibria in two-component membranes of phospholipids with different acyl-chain lengths. Biochim Biophys Acta. 1988 Oct 6;944(2):121–134. doi: 10.1016/0005-2736(88)90425-7. [DOI] [PubMed] [Google Scholar]
- Ipsen J. H., Mouritsen O. G., Zuckermann M. J. Theory of thermal anomalies in the specific heat of lipid bilayers containing cholesterol. Biophys J. 1989 Oct;56(4):661–667. doi: 10.1016/S0006-3495(89)82713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen K., Mouritsen O. G. Phase separation dynamics and lateral organization of two-component lipid membranes. Biophys J. 1995 Sep;69(3):942–954. doi: 10.1016/S0006-3495(95)79968-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen K., Sperotto M. M., Mouritsen O. G., Ipsen J. H., Zuckermann M. J. Phase equilibria and local structure in binary lipid bilayers. Biochim Biophys Acta. 1993 Oct 10;1152(1):135–145. doi: 10.1016/0005-2736(93)90240-z. [DOI] [PubMed] [Google Scholar]
- Knoll W., Ibel K., Sackmann E. Small-angle neutron scattering study of lipid phase diagrams by the contrast variation method. Biochemistry. 1981 Oct 27;20(22):6379–6383. doi: 10.1021/bi00525a015. [DOI] [PubMed] [Google Scholar]
- Lehtonen J. Y., Holopainen J. M., Kinnunen P. K. Evidence for the formation of microdomains in liquid crystalline large unilamellar vesicles caused by hydrophobic mismatch of the constituent phospholipids. Biophys J. 1996 Apr;70(4):1753–1760. doi: 10.1016/S0006-3495(96)79738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer L. D., Hope M. J., Cullis P. R. Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim Biophys Acta. 1986 Jun 13;858(1):161–168. doi: 10.1016/0005-2736(86)90302-0. [DOI] [PubMed] [Google Scholar]
- Melo E. C., Lourtie I. M., Sankaram M. B., Thompson T. E., Vaz W. L. Effects of domain connection and disconnection on the yields of in-plane bimolecular reactions in membranes. Biophys J. 1992 Dec;63(6):1506–1512. doi: 10.1016/S0006-3495(92)81735-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouritsen O. G., Jørgensen K. Dynamic lipid-bilayer heterogeneity: a mesoscopic vehicle for membrane function? Bioessays. 1992 Feb;14(2):129–136. doi: 10.1002/bies.950140211. [DOI] [PubMed] [Google Scholar]
- Mouritsen O. G., Jørgensen K. Dynamical order and disorder in lipid bilayers. Chem Phys Lipids. 1994 Sep 6;73(1-2):3–25. doi: 10.1016/0009-3084(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Mouritsen O. G., Jørgensen K. Micro-, nano- and meso-scale heterogeneity of lipid bilayers and its influence on macroscopic membrane properties. Mol Membr Biol. 1995 Jan-Mar;12(1):15–20. doi: 10.3109/09687689509038490. [DOI] [PubMed] [Google Scholar]
- Mouritsen O. G. Theoretical models of phospholipid phase transitions. Chem Phys Lipids. 1991 Mar;57(2-3):179–194. doi: 10.1016/0009-3084(91)90075-m. [DOI] [PubMed] [Google Scholar]
- Mustonen P., Virtanen J. A., Somerharju P. J., Kinnunen P. K. Binding of cytochrome c to liposomes as revealed by the quenching of fluorescence from pyrene-labeled phospholipids. Biochemistry. 1987 Jun 2;26(11):2991–2997. doi: 10.1021/bi00385a006. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Piknová B., Pérochon E., Tocanne J. F. Hydrophobic mismatch and long-range protein/lipid interactions in bacteriorhodopsin/phosphatidylcholine vesicles. Eur J Biochem. 1993 Dec 1;218(2):385–396. doi: 10.1111/j.1432-1033.1993.tb18388.x. [DOI] [PubMed] [Google Scholar]
- Pink D. A., Green T. J., Chapman D. Raman scattering in bilayers of saturated phosphatidylcholines. Experiment and theory. Biochemistry. 1980 Jan 22;19(2):349–356. doi: 10.1021/bi00543a016. [DOI] [PubMed] [Google Scholar]
- Ruggiero A., Hudson B. Critical density fluctuations in lipid bilayers detected by fluorescence lifetime heterogeneity. Biophys J. 1989 Jun;55(6):1111–1124. doi: 10.1016/S0006-3495(89)82908-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackmann E. The seventh Datta Lecture. Membrane bending energy concept of vesicle- and cell-shapes and shape-transitions. FEBS Lett. 1994 Jun 6;346(1):3–16. doi: 10.1016/0014-5793(94)00484-6. [DOI] [PubMed] [Google Scholar]
- van Osdol W. W., Johnson M. L., Ye Q., Biltonen R. L. Relaxation dynamics of the gel to liquid-crystalline transition of phosphatidylcholine bilayers. Effects of chainlength and vesicle size. Biophys J. 1991 Apr;59(4):775–785. doi: 10.1016/S0006-3495(91)82290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]