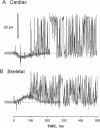
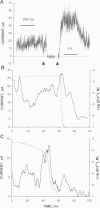
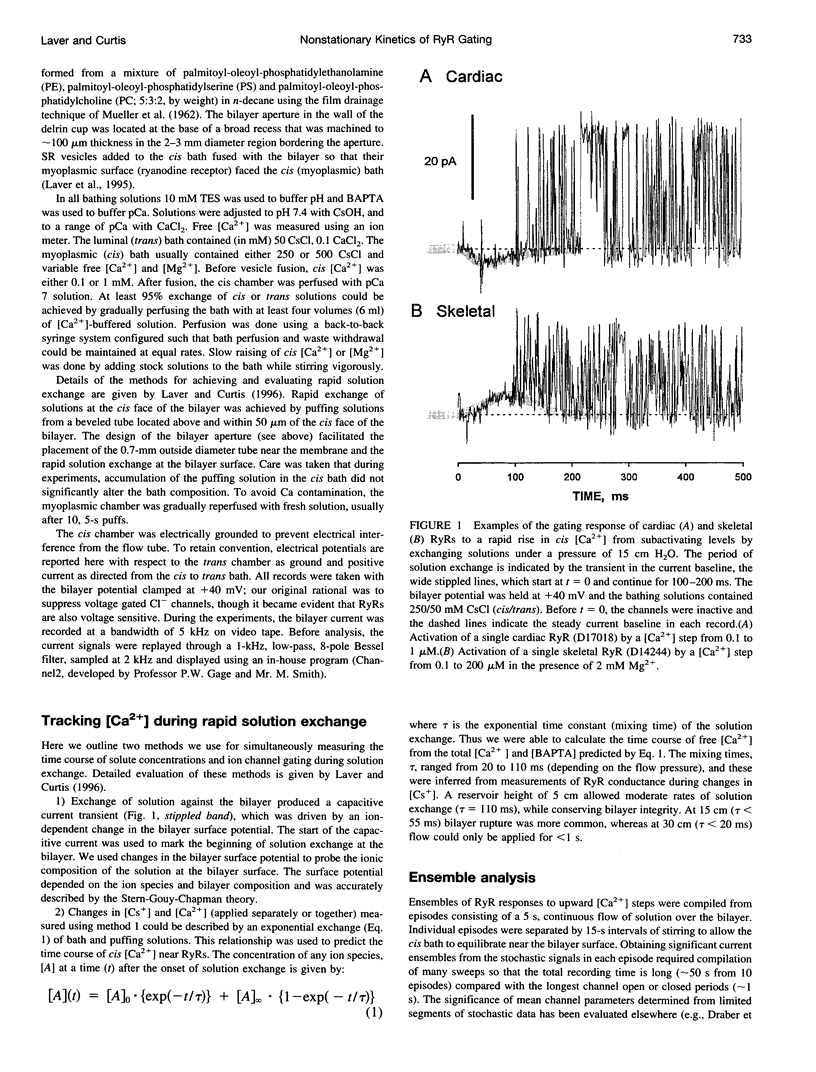
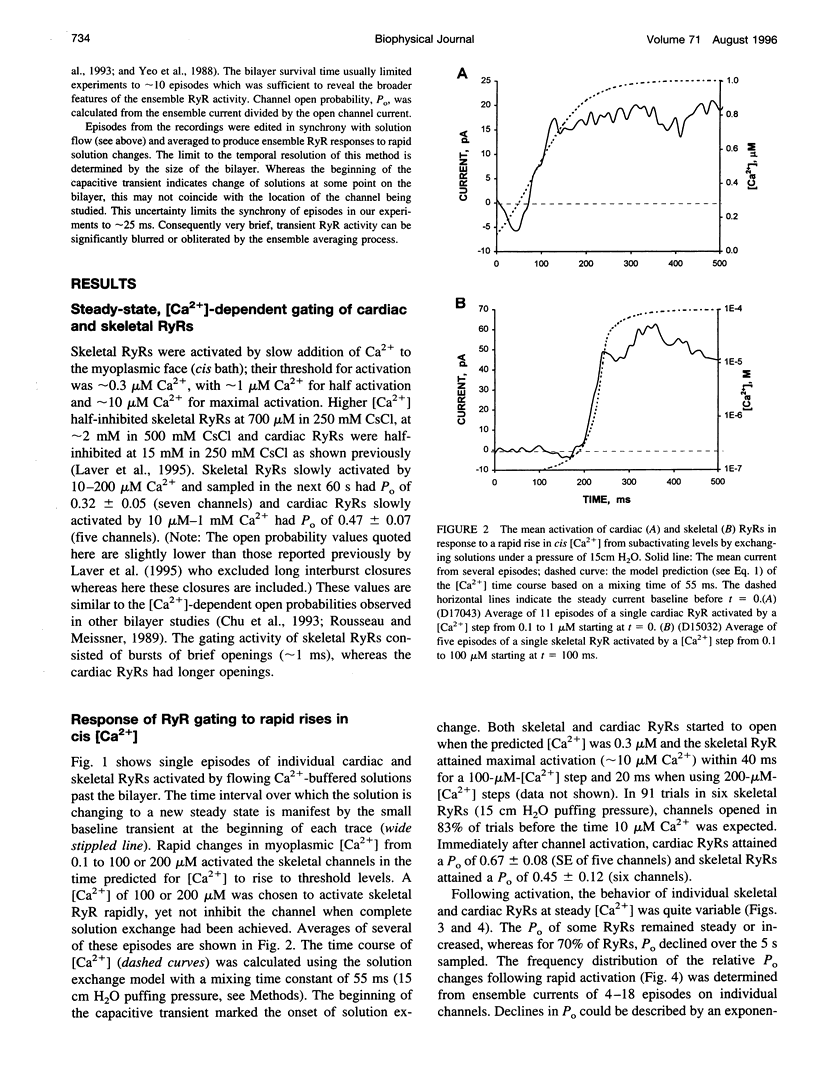
Abstract
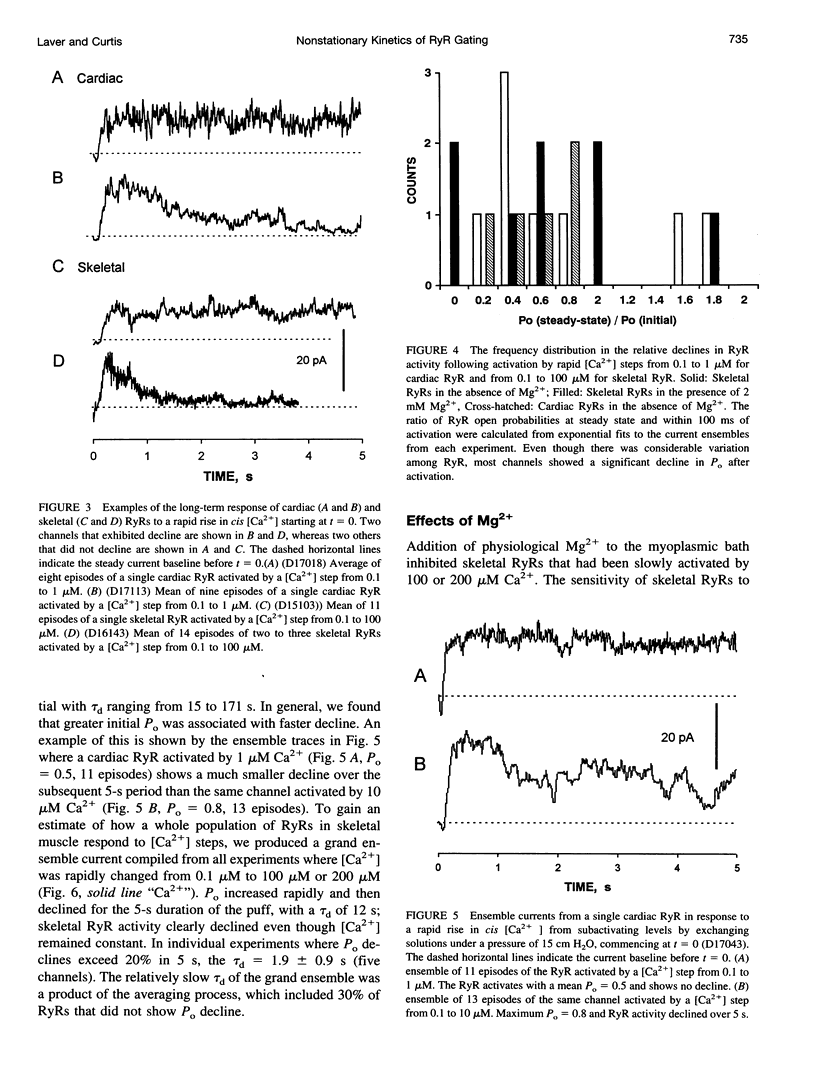
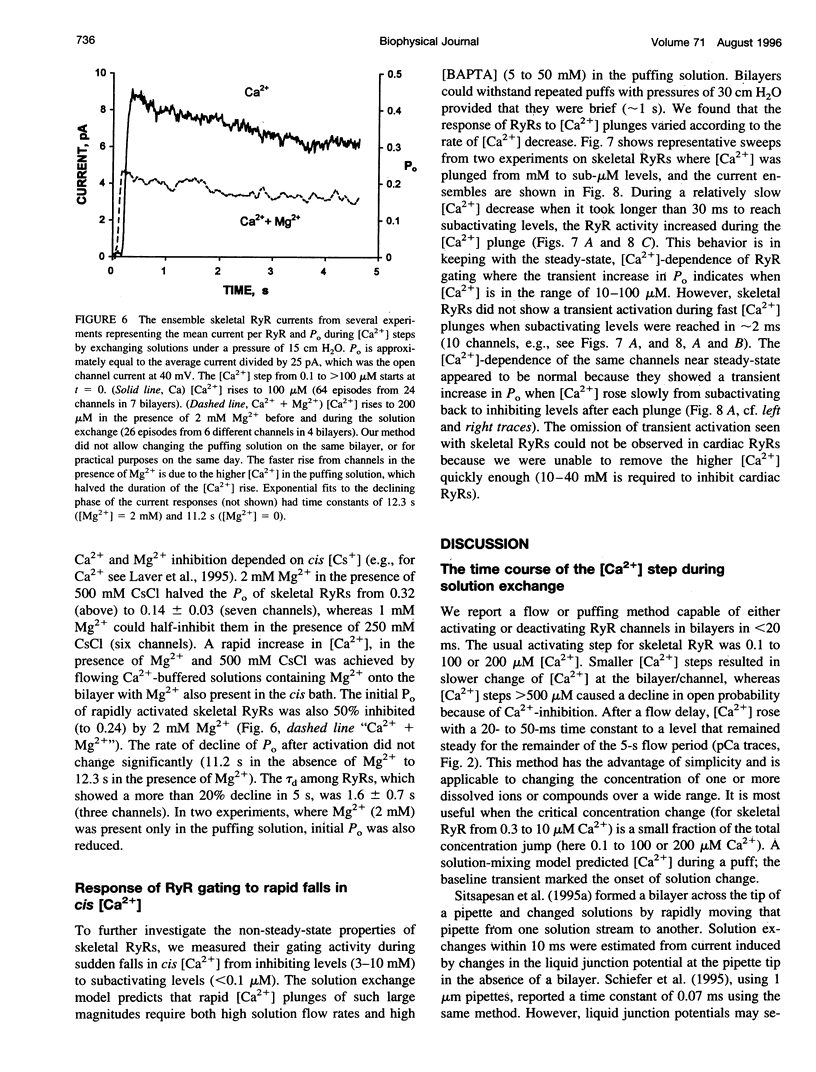
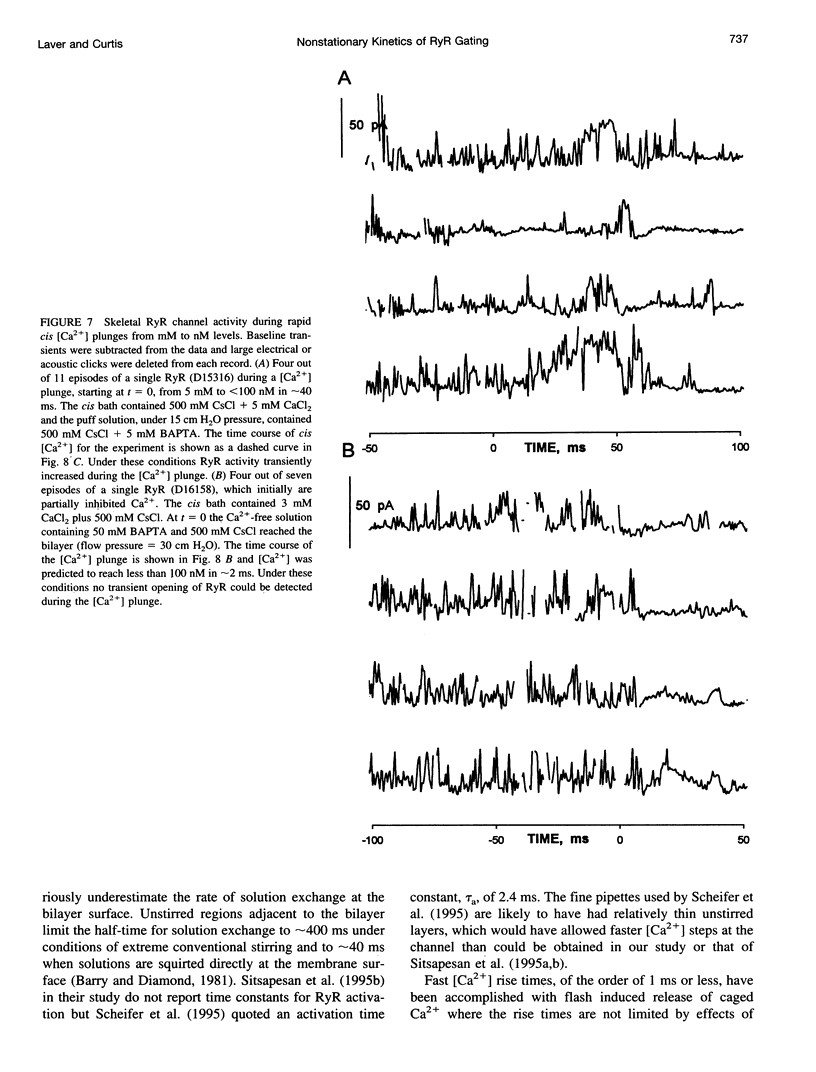
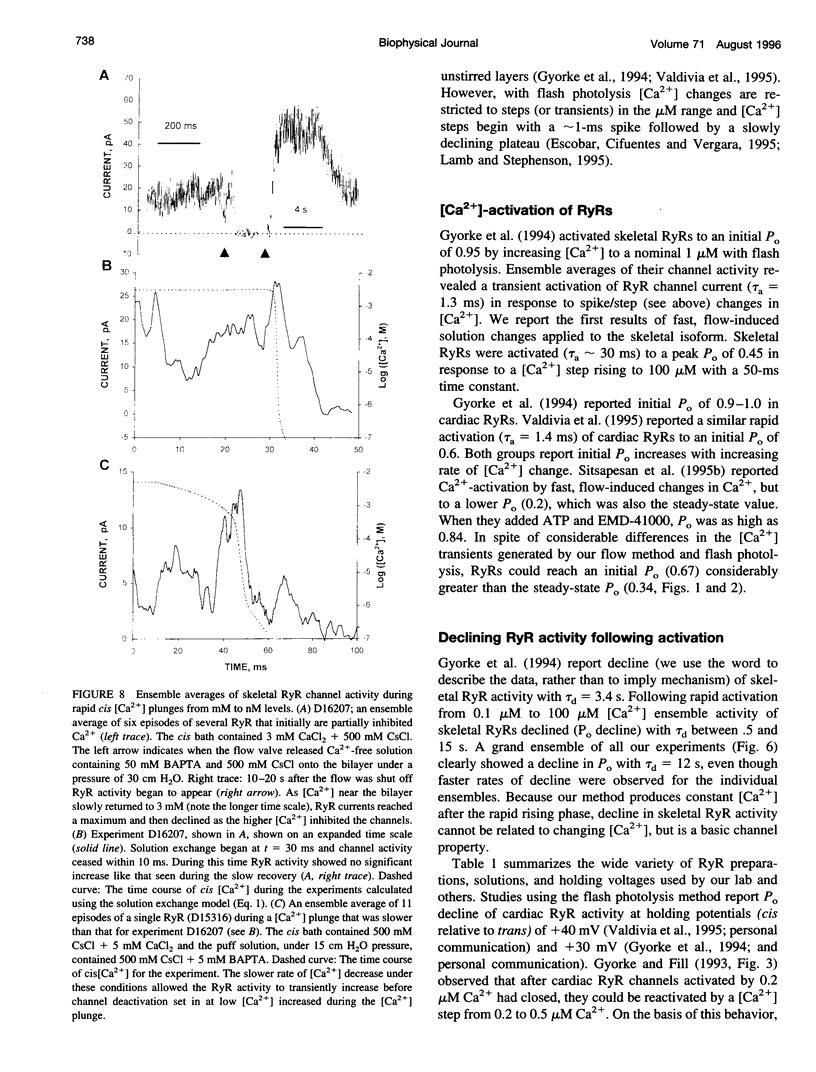
We used a flow method for Ca2+ activation of sheep cardiac and rabbit skeletal ryanodine receptor (RyR) channels in lipid bilayers, which activated RyRs in < 20 ms and maintained a steady [Ca2+] for 5 s. [Ca2+] was rapidly altered by flowing Ca(2+)-buffered solutions containing 100 or 200 microM Ca2+ from a perfusion tube inserted in the cis, myoplasmic chamber above the bilayer. During steps from 0.1 to 100 microM, [Ca2+] reached 0.3 microM (activation threshold) and 10 microM (maximum Po) in times consistent with predictions of a solution exchange model. Immediately following rapid RyR activation, Po was 0.67 (cardiac) and 0.45 (skeletal) at a holding voltage of +40 mV (cis/trans). Po then declined (at constant [Ca2+]) in 70% of channels (n = 25) with time constants ranging from .5 to 15 s. The mechanism for Po decline, whether it be adaptation or inactivation, was not determined in this study. cis, 2 mM Mg2+ reduced the initial Po for skeletal RyRs to 0.21 and marginally slowed the declining phase. During very rapid falls in [Ca2+] from mM (inhibited) to sub-microM (sub-activating) levels, skeletal RyR did not open. We conclude the RyR gates responsible for Ca(2+)-dependent activation and inhibition of skeletal RyRs can gate independently.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C., Mulligan I. P., Lea T. J. Ca2+ and activation mechanisms in skeletal muscle. Q Rev Biophys. 1991 Feb;24(1):1–73. doi: 10.1017/s0033583500003267. [DOI] [PubMed] [Google Scholar]
- Barry P. H., Diamond J. M. Effects of unstirred layers on membrane phenomena. Physiol Rev. 1984 Jul;64(3):763–872. doi: 10.1152/physrev.1984.64.3.763. [DOI] [PubMed] [Google Scholar]
- Boraso A., Williams A. J. Modification of the gating of the cardiac sarcoplasmic reticulum Ca(2+)-release channel by H2O2 and dithiothreitol. Am J Physiol. 1994 Sep;267(3 Pt 2):H1010–H1016. doi: 10.1152/ajpheart.1994.267.3.H1010. [DOI] [PubMed] [Google Scholar]
- Chu A., Fill M., Stefani E., Entman M. L. Cytoplasmic Ca2+ does not inhibit the cardiac muscle sarcoplasmic reticulum ryanodine receptor Ca2+ channel, although Ca(2+)-induced Ca2+ inactivation of Ca2+ release is observed in native vesicles. J Membr Biol. 1993 Jul;135(1):49–59. doi: 10.1007/BF00234651. [DOI] [PubMed] [Google Scholar]
- Draber S., Schultze R., Hansen U. P. Cooperative behavior of K+ channels in the tonoplast of Chara corallina. Biophys J. 1993 Oct;65(4):1553–1559. doi: 10.1016/S0006-3495(93)81194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar A. L., Cifuentes F., Vergara J. L. Detection of Ca(2+)-transients elicited by flash photolysis of DM-nitrophen with a fast calcium indicator. FEBS Lett. 1995 May 15;364(3):335–338. doi: 10.1016/0014-5793(95)00425-9. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke S., Fill M. Ryanodine receptor adaptation: control mechanism of Ca(2+)-induced Ca2+ release in heart. Science. 1993 May 7;260(5109):807–809. doi: 10.1126/science.8387229. [DOI] [PubMed] [Google Scholar]
- Györke S., Vélez P., Suárez-Isla B., Fill M. Activation of single cardiac and skeletal ryanodine receptor channels by flash photolysis of caged Ca2+. Biophys J. 1994 Jun;66(6):1879–1886. doi: 10.1016/S0006-3495(94)80981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemond V., Schneider M. F. Low myoplasmic Mg2+ potentiates calcium release during depolarization of frog skeletal muscle fibers. J Gen Physiol. 1992 Jul;100(1):137–154. doi: 10.1085/jgp.100.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D. Ca2+ inactivation, Mg2+ inhibition and malignant hyperthermia. J Muscle Res Cell Motil. 1993 Dec;14(6):554–556. doi: 10.1007/BF00141551. [DOI] [PubMed] [Google Scholar]
- Lamb G. D., Stephenson D. G. Activation of ryanodine receptors by flash photolysis of caged Ca2+. Biophys J. 1995 Mar;68(3):946–948. doi: 10.1016/S0006-3495(95)80270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D., Stephenson D. G. Effect of Mg2+ on the control of Ca2+ release in skeletal muscle fibres of the toad. J Physiol. 1991 Mar;434:507–528. doi: 10.1113/jphysiol.1991.sp018483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D., Stephenson D. G. Effects of intracellular pH and [Mg2+] on excitation-contraction coupling in skeletal muscle fibres of the rat. J Physiol. 1994 Jul 15;478(Pt 2):331–339. doi: 10.1113/jphysiol.1994.sp020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver D. R., Curtis B. A. Surface potentials measure ion concentrations near lipid bilayers during rapid solution changes. Biophys J. 1996 Aug;71(2):722–731. doi: 10.1016/S0006-3495(96)79271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver D. R., Roden L. D., Ahern G. P., Eager K. R., Junankar P. R., Dulhunty A. F. Cytoplasmic Ca2+ inhibits the ryanodine receptor from cardiac muscle. J Membr Biol. 1995 Sep;147(1):7–22. doi: 10.1007/BF00235394. [DOI] [PubMed] [Google Scholar]
- MUELLER P., RUDIN D. O., TIEN H. T., WESCOTT W. C. Reconstitution of cell membrane structure in vitro and its transformation into an excitable system. Nature. 1962 Jun 9;194:979–980. doi: 10.1038/194979a0. [DOI] [PubMed] [Google Scholar]
- Mack M. M., Molinski T. F., Buck E. D., Pessah I. N. Novel modulators of skeletal muscle FKBP12/calcium channel complex from Ianthella basta. Role of FKBP12 in channel gating. J Biol Chem. 1994 Sep 16;269(37):23236–23249. [PubMed] [Google Scholar]
- Meissner G., Darling E., Eveleth J. Kinetics of rapid Ca2+ release by sarcoplasmic reticulum. Effects of Ca2+, Mg2+, and adenine nucleotides. Biochemistry. 1986 Jan 14;25(1):236–244. doi: 10.1021/bi00349a033. [DOI] [PubMed] [Google Scholar]
- Mickelson J. R., Litterer L. A., Jacobson B. A., Louis C. F. Stimulation and inhibition of [3H]ryanodine binding to sarcoplasmic reticulum from malignant hyperthermia susceptible pigs. Arch Biochem Biophys. 1990 Apr;278(1):251–257. doi: 10.1016/0003-9861(90)90255-w. [DOI] [PubMed] [Google Scholar]
- Ritucci N. A., Corbett A. M. Effect of Mg2+ and ATP on depolarization-induced Ca2+ release in isolated triads. Am J Physiol. 1995 Jul;269(1 Pt 1):C85–C95. doi: 10.1152/ajpcell.1995.269.1.C85. [DOI] [PubMed] [Google Scholar]
- Rousseau E., Meissner G. Single cardiac sarcoplasmic reticulum Ca2+-release channel: activation by caffeine. Am J Physiol. 1989 Feb;256(2 Pt 2):H328–H333. doi: 10.1152/ajpheart.1989.256.2.H328. [DOI] [PubMed] [Google Scholar]
- Ríos E., Pizarro G. Voltage sensor of excitation-contraction coupling in skeletal muscle. Physiol Rev. 1991 Jul;71(3):849–908. doi: 10.1152/physrev.1991.71.3.849. [DOI] [PubMed] [Google Scholar]
- Schiefer A., Meissner G., Isenberg G. Ca2+ activation and Ca2+ inactivation of canine reconstituted cardiac sarcoplasmic reticulum Ca(2+)-release channels. J Physiol. 1995 Dec 1;489(Pt 2):337–348. doi: 10.1113/jphysiol.1995.sp021055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitsapesan R., Montgomery R. A., Williams A. J. A novel method for incorporation of ion channels into a planar phospholipid bilayer which allows solution changes on a millisecond timescale. Pflugers Arch. 1995 Aug;430(4):584–589. doi: 10.1007/BF00373896. [DOI] [PubMed] [Google Scholar]
- Sitsapesan R., Montgomery R. A., Williams A. J. New insights into the gating mechanisms of cardiac ryanodine receptors revealed by rapid changes in ligand concentration. Circ Res. 1995 Oct;77(4):765–772. doi: 10.1161/01.res.77.4.765. [DOI] [PubMed] [Google Scholar]
- Valdivia H. H., Kaplan J. H., Ellis-Davies G. C., Lederer W. J. Rapid adaptation of cardiac ryanodine receptors: modulation by Mg2+ and phosphorylation. Science. 1995 Mar 31;267(5206):1997–2000. doi: 10.1126/science.7701323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo G. F., Milne R. K., Edeson R. O., Madsen B. W. Statistical inference from single channel records: two-state Markov model with limited time resolution. Proc R Soc Lond B Biol Sci. 1988 Oct 22;235(1278):63–94. doi: 10.1098/rspb.1988.0063. [DOI] [PubMed] [Google Scholar]