Abstract
Background and purpose
Liver fibrosis, a progressive liver disease arising from viral or metabolic causes, poses a major global health challenge due to its potential progression to cirrhosis and hepatocellular carcinoma. Due to the complex aetiology and epidemiology of liver fibrosis, most therapies fail in the clinic, and very few drugs have been approved by the US FDA.
Approach
This review highlights the pathophysiological features of liver fibrosis, with a focus on novel targets in hepatic stellate cells (HSCs), key players in the fibrogenesis process, to develop successful therapeutic approaches using both pharmacological agents and active targeting strategies. The review also examines current therapeutic strategies targeting liver fibrosis, both in preclinical lab setups and clinical trials. Furthermore, various receptors involved in HSC-mediated liver fibrosis and active drug delivery targeting strategies are reviewed to enhance therapeutic outcomes. This article also integrates existing knowledge to identify research gaps and guide future investigations and clinical translation in liver fibrosis treatment. In addition, novel pathways pertaining to liver fibrosis, such as the RSPO3-LGR4/5-β-catenin cascade, the CD47/YAP/TEAD4 signalling axis, and HAb18G/CD147, are briefly elaborated in the context of therapeutic approaches for arresting HSC activation. Single-cell RNA sequencing of HSCs is presented to provide a clearer picture of liver fibrosis.
Conclusion
The review highlights critical research gaps in liver fibrosis therapy and promising active targeting strategies and pharmacological interventions to improve therapeutic outcomes. Overall, this review provides a robust foundation for scientists and clinicians to advance active targeting of the disease pathology and to develop new pharmaceutical formulations that are pharmacologically safer and more efficacious.
Keywords: Retinoid receptors, non-alcoholic fatty liver disease, resmetirom, liposomes, active targeting, clinical trials
1. Introduction
One out of every twenty-five deaths globally is caused by liver fibrosis, thus causing a huge health burden worldwide. Globally, the prevalence of advanced liver fibrosis in the general population was estimated to be 3.3 % (range 2.4 to 4.2 % with 95 % CI ) [1]. There are various aetiologies involved in inducing liver injuries such as alcohol, cholestasis, non-alcoholic fatty liver disease, autoimmune steatohepatitis, and drug-induced liver injury reactions [2], primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC); viral hepatitis (hepatitis B and hepatitis C); autoimmune hepatitis (AIH) [3]. These factors lead to a dynamic process of cells and tissues, responsible for extracellular matrix (ECM) deposition via the activation of hepatic stellate and myofibroblast cells.
Liver fibrosis develops as a result of the interplay of various cell types, i.e. hepatocytes, cholangiocytes, natural killer cells (NK cells), T helper cells, Kupffer cells, liver sinusoidal and epithelial cells, along with HSC. Although the main functions of hepatocytes include the clearance of hepatitis viruses, toxic metabolites, and xenobiotic compounds, as well as the secretion of lipids and proteins, apoptotic hepatocytes in an injured liver succumb, leading to the activation of myofibroblasts. In addition, inflammatory mediators, including TGF-β1, TNF-α, EGF, and IGF, are also released by injured hepatocytes and activated hepatic stellate cells, ultimately activating T cells [4], which are pivotal to both TH2-mediated fibrosis and TH17-mediated inflammation in liver fibrosis. Cholangiocytes are specialized epithelial cells lining bile ducts that play a vital role in liver regeneration, especially when hepatocyte regeneration is compromised [5]. They also activate Kupffer cells and release free radicals, TNF-α, Endothelin-1, and PDGF. Monocytes also play a very crucial role in various liver diseases, both by inflammatory functions and the resolution of inflammation. Inflammatory mediators, such as CCL2, facilitate the recruitment of LY-6Chi and CCR2+ monocytes into the injured liver [6]. Collagen is produced by LY-6C+ cells, which also activate HSC and release PDGF, NKF β, CD14+, and CD16+ [7].
Liver sinusoidal and epithelial cells also have a focal role in liver fibrosis after liver injury, as these cells release endothelin-1 (ET-1) and nitric oxide (NO) [8,9]. Fenestrae, i.e. non-diaphragmed pores present in them, play an important role in maintaining the quiescent state of HSC and Kupffer cells, essential for a healthy liver. Natural killer cells present in the liver tissue act on pathogens and tumour substances that come through blood vessels and also play an important role in the regulation of inflammation and liver fibrosis [10]. Kupffer cells in the liver show phagocytic action towards pathogens and any hazardous substances and also perform erythrophagocytosis for senescent red blood cells. After activation, Kupffer cells release free radicals and inflammatory mediators, i.e. TGF-β1, TNF-α, and IGF [11]. Recently, the role of Kupffer cell pyroptosis has also been explored by researchers, demonstrating its role in promoting liver injury and inflammation, leading to liver fibrosis via the NOX2/NLRP3 inflammasome pathway [12]. In CLD associated with HBV and HCV, TH17 lymphocytes, a subgroup of T helper cells that release the pro-inflammatory IL-17A, may possess pro-fibrogenic properties. Besides, the appearance of oxidative stress, either as a consequence of excessive production of reactive oxygen species or deficiency of the antioxidant defence system, can wreak havoc in the form of liver fibrosis upon activating the HSCs. The activated HSCs initiated by oxidative damage can be detrimental, and, hence, another related phenomenon, mitochondrial dysfunction, comes into play to exacerbate the condition due to disrupted energy generation and metabolic processes, leading to apoptosis and liver damage [13].
Overall, this review presents recent and novel advancements in understanding the pathogenesis of liver fibrosis, emphasizing the major cell types involved, with a particular focus on hepatic stellate cells (HSCs). It consolidates recent clinical trial data, as well as clinical and experimental approaches that target fibrosis. Additionally, it explores delivery systems through active and passive targeting mechanisms. Lastly, future perspectives are also discussed, highlighting promising strategies for the development of effective antifibrotic therapies.
Role of hepatic stellate cells in healthy liver and liver fibrosis
In a healthy liver, hepatic stellate cells (HSCs) play a crucial role in liver development, homeostasis, and regeneration. Vitamin A is stored by HSCs in the form of retinyl esters, located near the nucleus in the form of lipid droplets. The gut absorbs dietary retinoids, which are then carried to hepatocytes as retinyl esters and broken down into free retinol. Vitamin A is then delivered to HSCs for re-esterification, with perilipins coating these lipid droplets [14]. Adipose-differentiation-related protein is also expressed by HSCs, although its expression declines as the cells produce and shed retinoid droplets [15]. Furthermore, during liver injury, matrix remodelling is regulated by matrix metalloproteinases (MMPs) secreted by HSCs. However, tissue inhibitors of metalloproteinases (TIMPs), which are also released by activated HSCs (aHSCs), downregulate MMP activity, leading to extracellular matrix accumulation and fibrosis [16,17]. Additionally, TMPs promote the formation of collagen I in HSCs by activating TGF-β, a potent stimulant [18]. HSCs also secrete growth factors such as vascular endothelial growth factor (VEGF), which plays a key role in endothelial and epithelial cell division [19]. HSCs also express neurotrophins, including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin-3, which are important for liver function and regeneration [20,21]. Additionally, HSCs contribute to ethanol detoxification by expressing alcohol- and acetaldehyde dehydrogenases, although their role in this process is limited compared to hepatocytes [22]. Overall, in both healthy and injured livers, HSCs control ECM remodelling, play a role in tissue healing, and maintain liver homeostasis.
HSC activation is divided into two main phases: initiation, often referred to as the pre-inflammatory stage, and perpetuation. They may return to a quiescent state if liver injury is healed [23]. During the initiation phase, endothelial cells in the sinusoids play a critical role by secreting fibronectin [24] and activating latent TGF-β [25]. Platelets contribute to this process by producing TGF-β, EGF, and PDGF. Further, the latter is a potent mitogen for HSCs [26]. Additionally, various immune cells, including T cells, dendritic cells, and macrophages, interact with HSCs to modulate inflammation and fibrosis [27]. For instance, macrophages secrete TGF-β, TNF-α, MMP-9, and ROS, which contribute to HSC activation [28]. Apoptotic bodies derived from hepatocytes also play a role in stellate cell activation, without triggering an inflammatory response, and directly promote fibrogenesis [29]. Hepatocyte-expressed P450-2E1 further contributes to stellate cell activation by generating ROS during xenobiotic metabolism [30]. In translational and post-transcriptional mechanisms, including miRNAs and epigenetic regulation, stellate cell responses are modulated [31]. In the perpetuation phase, HSC activation involves cellular processes such as proliferation, fibrogenesis, chemotaxis, and matrix degradation. Upregulation of PDGF receptor expression via TNF-α enhances the fibrogenic phenotype of stellate cells [32]. Matrix metalloproteinases (MMPs), such as MMP-2 and MMP-9, play dual roles in fibrosis progression; while they regulate ECM turnover, their antifibrotic expression declines as fibrosis advances [33]. Moreover, stellate cells also express a variety of chemokine receptors that are important in cell migration and subsequent activation, including CXCR4 and CCR5 [34,35]. TGF-β1 stimulates ECM production through autocrine and paracrine pathways, while connective tissue growth factor (CTGF) amplifies profibrotic signals via a G-protein-coupled receptor [36]. Micro iRNAs, especially miR-29b, play a major function in regulating collagen synthesis via inhibiting TGF-β-induced collagen production [37]. Crucially, mitochondrial ROS and HIF-1α-mediated hypoxia-induced signalling pathways, such as ERK1/2 and JNK1/2, further promote HSC migration and activation, underscoring the intricate interactions of molecular pathways implicated in liver fibrosis [38]. Regulatory T cells (Tregs) mitigate hepatic fibrogenesis by producing IL-10, which inhibits HSC activation and the production of TH17-derived IL-17, while also limiting Kupffer cell activation [39].
Although initially believed to play protective roles in NAFLD, mucosal-associated invariant T cells (MAIT) depletion in mice has been connected to fibrosis resistance. More recently, it has been demonstrated that MAIT cells induce fibrosis by stimulating HSCs through IL-17 and macrophage pro-inflammatory polarization [40,41]. Similarly, γδ T cells, which accumulate in fibrotic livers, can induce HSC apoptosis and enhance natural killer cell-mediated cytotoxicity, thereby limiting fibrosis, though they also contribute to IL-17 production, supporting fibrogenesis [42,43]. Innate lymphoid cells (ILCs), particularly NK cells, play a pivotal role in liver fibrosis, with NK cells exhibiting antifibrotic effects by killing activated HSCs and releasing IFNγ, offering a potential therapeutic strategy by boosting their activity [44,45]The intricate relationships among these many immune cell types highlight the complexity of liver fibrosis, where the resolution or development of the illness is determined by the balance between profibrotic and antifibrotic signalling pathways (see Figure 1).
Figure 1.
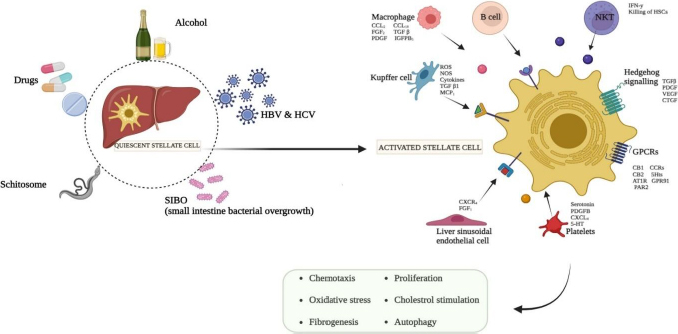
Pathophysiology of hepatic stellate cells. Several dramatic changes occurred during the development of liver fibrosis at cellular level. Liver fibrosis is incurred by several factors, including drugs, schistosomes, SIBO, HCV & HBV, and alcohol followed by HSC activation. Regular injury fosters a chronic liver disease. In activated HSC cells, collagen and extracellular matrix accumulation, vitamin A is lost, and oxidative stress occurs. Overall, cells including Macrophages, Kupffer cells, platelets, B cells, and natural killer cells are involved in the activation of HSC
The RSPO3-LGR4/5 axis in quiescent hepatic stellate cells
Recently, Sugimoto et. al. [46] demonstrated that R-spondin 3 (RSPO3), secreted by quiescent HSCs, plays a crucial role in supporting hepatocyte metabolism, regeneration, and survival. These findings are contrary to the traditional view of HSCs functioning solely as fibrogenic drivers in chronic liver disease (CLD). The study demonstrated that as CLD progresses, RSPO3 expression declines, leading to worsened liver function and fibrosis. The RSPO3-LGR4/5-β-catenin pathway was demonstrated as a crucial communication axis between HSCs, hepatocytes, and endothelial cells, coordinating liver zonation and function. Therapeutically, restoring RSPO3 or reverting HSCs to a quiescent state may simultaneously suppress fibrosis and restore liver function, offering a promising strategy for treating diseases like MASLD and ALD [46].
Single-cell RNA sequencing of hepatic stellate cells in understanding liver fibrosis
A very recently reported study [47] has successfully presented the first single-cell atlas of HSCs across seven liver injury models in mice (using 10 experimental single-cell sequencing data), revealing a conserved HSC activation trajectory that is also preserved in human livers. The group identified three distinct HSC subtypes—quiescent (qHSCs), initiatory, and myofibroblasts— across all injury types, suggesting a universal activation mechanism for liver fibrosis. The outcome of the reported study demonstrated key transcription factors (Rxra, Foxf1, Nr1h4 for qHSCs; Fosl1, Egr3, Nfkb2 for initiatory HSCs; and Wt1, Prrx1, Mef2c for myofibroblasts) and ligands (TGF-β, EGF, SPP1, AGT, and parathyroid hormone) functioning as conserved drivers of HSC activation.
The authors successfully identified [47] and reported COLEC10 as a highly specific HSC marker and potential biomarker for liver fibrosis, outperforming traditional markers such as FIB4 and APRI. This report has paved the way further for the potential for broad application of anti-fibrotic therapies targeting conserved TFs or ligands and markers for their potential role in preclinical drug discovery for liver-related injuries [47].
CD47YAP/TEAD4 pathway in hepatic stellate cells activation
There are additional novel signalling cascades involved in HSC activation, one of which is CD47, a YAP target gene and transcriptional factor that plays a role in cell proliferation, development, and survival. CD47 is also known to prevent phagocytosis by inhibiting macrophage activity, and TEAD4 has a developmental role. Here, YAP and TEAD4 function in a complementary manner to each other in the induction of CD47 transcriptional activation. This pathway contributes to the activation of HSC, and hence, liver fibrosis can be propagated. The CD47/YAP/TEAD4 signalling axis is more inclined to the association of non-alcoholic fatty liver disease (NAFLD). Upregulated CD47, triggered by the YAP and TEAD4 genes, leads to the heightened activation of HSCs, resulting in liver fibrosis. Therefore, silencing or knocking down the CD47/YAP/TEAD4 pathway can inhibit the fibrotic gene expression, thereby attenuating liver fibrosis pathology. In this context, anti-CD47 antibodies have been investigated for their potential to alleviate fibrosis-associated inflammation. Moreover, pharmacological intervention in the communication halting between YAP and TEAD4 will further potentially reduce the chances of HSC activation and liver fibrosis [48].
HAb18G/CD147 role in hepatic stellate cell activation
Activation of the HAb18G/CD147 pathway has been reported to stimulate HSCs and induce the overexpression of fibrogenesis-associated genes. The HAb18G/CD147 protein is expressed on the surfaces of HSCs in cases of liver fibrosis. This phenomenon arises due to extracellular matrix remodelling and collagen production, which are responsible for liver fibrosis. As a therapeutic target, antibodies have been investigated in preclinical animal studies and found to be effective in downregulating the HAb18G/CD147 cascade, thereby alleviating HSC-activated liver fibrosis [49].
Therapeutic approaches
Drugs approved by the US-FDA and in clinical trials
Extensive research has been conducted on various pharmacological methods to combat liver fibrosis, to address the fundamental processes driving fibrosis and to develop effective therapies. One of the most widely explored targets is PPARα and PPARδ. In a recently reported study [50], the activation of PPARα and PPARδ has been observed to alter metabolic pathways and decrease inflammation, both of which are important elements in the development of liver fibrosis. It has been demonstrated that elafibranor effectively enhances markers for liver fibrosis and slows down the progression of the condition in a non-alcoholic steatohepatitis animal model [50]. Pioglitazone, another PPARγ agonist, has also been investigated for its ability to treat liver fibrosis. Huang and colleagues revealed that pioglitazone enhanced liver histology and lowered the expression of fibrogenic genes in an animal model of non-alcoholic steatohepatitis [51]. The FXR, a nuclear receptor crucial for maintaining bile acid equilibrium and regulating liver metabolism, is strongly and selectively stimulated by tropifexor. Furthermore, some researchers observed that tropifexor decreases liver fibrosis and enhances various indicators of liver well-being in an animal model with non-alcoholic fatty liver disease [52]. The researchers propose that emricasan's anti-fibrotic effects result from its ability to inhibit caspase-mediated apoptosis and inflammation, both of which play crucial roles in the development of liver fibrosis [53]. To treat this disease, several medications have been examined, which are discussed in Table 1. Recently, the FDA approved resmetirom for non-cirrhotic MASH with moderate to advanced fibrosis, based on a Phase 3 clinical trial (NCT03900429), which demonstrated MASH resolution with at least one stage improvement in fibrosis [54]. This drug acts by targeting the thyroid hormone receptor (THR)-β, which is present in the liver. The detailed molecular mechanism of the effects is presented in Figure 2. Pirfenidone is an antifibrogenic and anti-inflammatory drug and is approved by the US-FDA for idiopathic pulmonary fibrosis and is currently under investigation for liver fibrosis and various other fibrotic diseases. It acts by affecting multiple molecular targets, notably inhibiting TGF-β and reducing pro-inflammatory cytokines, leading to alleviated fibrosis (Figure 3b). Its hydroxy analog, hydronidone, has been proven better for the therapy of liver fibrosis owing to its high hepatic safety (due to metabolism by Phase 2 transformation instead of Phase I transformation).
Table 1.
Drugs/compounds used in clinics to treat liver fibrosis
| Name | Original indication | Target | Current status / Effects | Ref. |
|---|---|---|---|---|
| Belapectin | Liver fibrosis | Galectin-3 antagonist | NCT02462967 phase 2b: NCT04365868 phase 2/3 prevention of oesophageal varices | [58,59] |
| Saroglitazar | Diabetic dyslipidaemia | PPARα/γ agonist | Phase 2 | [60] |
| Elafibranor | Primary biliary cholangitis | PPARα and PPARδ agonist | Not effective phase 3 | [61] |
| Seladelpar | Primary biliary cholangitis | PPARδ agonist | Accelerated approval US-FDA | [61] |
| Resmetirom Rezdiffra™ | NASH with Fibrosis | THR-β agonist | Accelerated approval US-FDA | [62] |
| Liraglutide | Diabetes and obesity | GLP-1 analog | Diabetes associated liver fibrosis | [63] |
| Semaglutide | Metabolic disorders associated steatohepatitis linked liver fibrosis | [64] | ||
| Obeticholic acid | Liver fibrosis | FXR agonists | Accelerated approval US-FDA currently restricted use | [65] |
| Setanaxib | Liver fibrosis | NOX1, NOX4 inhibitor, NADPH oxidase | Fast track approval, FDA (Orphan) | [66] |
| Pentoxifylline | Peripheral vascular disease | Non-specific phosphodiesterase inhibitor | Benefits in small clinical trials not approved by US-FDA | [67] |
| Hydronidone | NA | Wnt/β-Catenin pathway inhibitor | Demonstrated efficacy in phase 2 and phase 3 trials | [68] |
| Fluorofenidone | NA | Inhibited TGFβ1/Smad and MAPK signalling | Limited clinical trial data | [69] |
| Tirzepatide | Type 2 diabetes | GLP-1 and GIP agonist | Phase 2 resolution of MASH without worsening liver fibrosis | [70] |
| GS-0976 / Firsocostat | NA | Acetyl-CoA carboxylase (ACC) | Phase 2 NASH | [71] |
| Pegbelfermin | NA | FGF21 analogue | Failed Phase 2b | [72] |
| Aldafermin | NA | FGF19 analogue | Phase 2/3 promising results | [73] |
| Aramchol | Metabolic disorders | Inhibitor of de novo lipid synthesis | Effective in Phase 3 ARMOR study | [74] |
Figure 2.
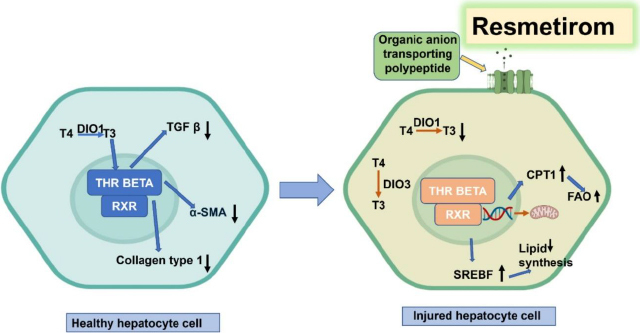
Mechanisms of action of thyroid hormones and resmetirom in regulation of hepatocyte lipid metabolism., Thyroxine (T4), Iodothyronine deiodinase 1 (DIO1) Triiodothyronine (T3), Nuclear thyroid receptor β (THR-β), Retinoid X receptor (RXR) Carnitine palmitoyltransferase I (CPT1), Mitochondrial fatty acid oxidation (FAO), and Sterol regulatory element binding transcription factor 1 (SREBF1) Iodothyronine deiodinase 1 (DIO3), reverse T3 (rT3). Modified from [57], Creative Commons CC BY
Figure 3.
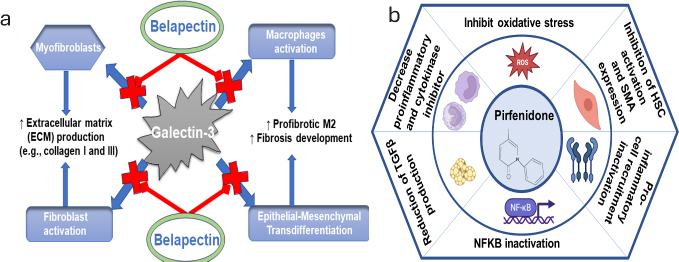
a - targets of belapectin for alleviation of fibrosis; b - Molecular mechanisms of action of pirfenidone. It affects signalling pathways such as the Smad pathway, which is downstream of TGF-β, leading to a decrease of fibrogenic activity. Consequently, it inhibits fibroblast proliferation and increases fibroblast apoptosis
Additionally, the drug leads to enhanced Smad 7 upregulation and binding to TGFβRI, resulting in the inhibition of phosphorylation of profibrotic Smad 2/3 and subsequent degradation of TGFβRI [55]. Furthermore, belapectin is a carbohydrate-based drug developed by galectin Therapeutics that acts on the molecular level by inhibiting the effects of Galectin-3 on various cells (see Figure 3a), leading to reduced extracellular matrix deposition and fibrosis development [56].
Experimental approaches
Recent studies have demonstrated the potential for improving the treatment approaches through the utilization of specific targeting agents and ligands, which focus on particular markers expressed on target cells. Targeted delivery methods have demonstrated encouraging outcomes in enhancing the precision and effectiveness of therapeutic approaches [75]. The receptors playing an important role in liver fibrosis and emerged as potential targets for the therapy of liver fibrosis include nuclear receptors, i.e. liver X receptors (LXR), farnesoid X receptor (FXR), retinoid X receptor (RXR), vitamin D receptor (VDR), cannabinoid receptors, Toll-like receptor (TLR), and peroxisome proliferator-activated receptors (PPARs) (See Table 2).
Table 2.
Experimental approaches of the therapy of liver fibrosis
| Target | Drug/components | Function/pharmacological implication | Ref. |
|---|---|---|---|
| FXR | ID119031166 (ID166) | Regulate bile acid homeostasis and ↓ liver fibrosis and NASH pathology without activating itch-related receptors | [86] |
| GW4064 | Inhibition of NF-KB and decrease cytokine-STAT3 signalling | [87] | |
| INT-787 and OCA | Modulate RECK expression, ↓ MMPs, ADAM10, and ADAM17 activity | [88] | |
| INT-767, OCA | ↓ of collagen and induced MMP2-9 activity | [89] | |
| GCDCA | Activating the NLRP3 inflammasome pathway | [90] | |
| LXR and FXR | Acanthoic Acid | ↓lipid accumulation, ↓SREBP1, ↑AMPK-SIRT1 signaling | [76] |
| LXR | Sesamol | Inhibits autophagy markers MAPLC3α/β, P62 | [91] |
| SR9238 | SR9238 inhibits the activity of LXR | [92] | |
| PPARγ agonist | GSK2033 | Inhibit activity of LXR | [93] |
| PPAR agonist | Ligustrazine | Inhibited HSC migration, adhesion, contraction, and angiogenic cytokine production | [94] |
| PPARα | Methoxyeugenol | Inhibiting HSC activation | [95] |
| Oleoylethanolamide | Block activation of HSC inhibits alpha smooth muscle actin, TGF-β1 | [96] | |
| Pirfenidone | Induced PPAR-α/carnitine O-palmitoyltransferase 1/acyl-CoA oxidase 1 | [97] | |
| PPARα / δ agonist | ZLY16 | Regulating gene expression including COLIA1, TIMP, TGFβ, TNFα and IL6 | [98] |
| PPARα / γ agonist | Saroglitazar | Decrease liver collagen level and cholangiocyte proliferation marker (CK9) | [99] |
| Integrin Subunit Alpha 11 (ITGA11) | MicroRNAs (Eugol) | miR-12135 suppressed Integrin Subunit Alpha 11 (ITGA11) likewise suppress fibrosis | [100] |
| PDGFRβ | Crenolanib | Crenolanib inhibit PDGFR-β inhibit HSC proliferation | [101] |
| Salvianolic acid B | Inhibit HSC activation migration and proliferation | [102] | |
| PDGF | Benzimidazoisoquinoline derivatives | ↓ PDGF and TGFβ-induced proliferation | [103] |
| PDGFRβ | Gomisin D | Inhibit HSC proliferation, activation and promote apoptosis | [104] |
| IGFIIR | Aptamer-20 (SELEX) | High-affinity ligand to receptor for antifibrotic activity | [105] |
| Cannabinoid receptor | AM1241 | Inhibits the TLR4/NF-κB pathway | [106] |
| Cannabigerol | ↓ hepatic inflammation and fibrosis | [107] | |
| Exosomes | Upregulating CB1 receptors and downregulating CB2 receptors | [108] | |
| CB1 receptors | JD5037 | Activated HSCs via β-arrestin1/Akt signalling, which is inhibited by JD5037 | [81] |
| CB2 receptors | AM1241 | Inhibiting the TLR4/miR-155/NFκB p65 pathway | [82] |
| Toll like receptor | ALT-100 | Neutralizing eNAMPT, thereby inhibiting the eNAMPT/TLR4 inflammatory pathway | [84] |
| Rutin | Inhibiting the TLR4 (Toll-like receptor 4) and P2X7r signalling pathways | [109] | |
| Protein tyrosine phosphatase 1B | Luteolin-7-diglucuronide | ↓ TGF-β1-mediated overexpression of collagen 1, α-SMA and fibronectin | [110] |
| NF-κB/NLRP3 inflammasome signaling axis | Mangiferin | ↓NF-κB/NLRP3 inflammasome pathway, ↓ α-SMA hepatic expression and fibrous tissue deposit. ↓ MDA and ↑ GSH and Nrf2 | [111] |
| Apolipoprotein L2 | 12-deoxyphorbol 13-palmitate |
↓APOL2-SERCA2-PERK-HES1 signalling | [112] |
| GSK-3β modulation | Fluorofenidone | Targets GSK-3β/β-catenin pathway, ↓ β-catenin-mediated pro-fibrotic gene expression, ↑ Nrf2/HO-1 pathway ↓ oxidative stress, ↓ hepatocyte apoptosis | [113] |
| AMPK-PPARγ pathway | Arctigenin | ↓ expression of fibrosis-related genes (Col1a1, Col3a1, Acta2), reversal of activated HSCs to quiescent HSCs | [114] |
LXR belongs to the family of nuclear receptors and regulates the accumulation of fat, the level of inflammation, and scarring of liver tissue with fibrosis in NAFLD. LXRα expression was positively correlated with intrahepatic expression of CD36, SREBP-1c, and ABCG5/8. They demonstrated that LXR is an attractive therapeutic target for liver fibrosis. GW3965 is an agonist of LXR and reduces signs of scarring and inflammation, as well as the activation of hepatic stellate cells in mice [76].
FXR is a bile acid-activated nuclear receptor, also known as NR1H4. FXR contributes to preventing HSC activation and retaining their quiescent state. FXR lowers the risk of bile acid-induced liver damage by controlling the expression of genes related to bile acid metabolism. FXR maintains quiescent HSCs and controls bile acid levels, so it plays an important role in controlling liver fibrosis [77,78].
RXR is involved in several biological processes, including the regulation of transcription factors and gene expression. RXR-α, RXR-β, and RXR-γ are the three main types of retinoid X receptor. Ligands activate RXRs, like 9-cis retinoic acid, after activation modulates transcription of genes that control cell proliferation, differentiation, and death, as well as maintain HSCs in their quiescent state. Also, it has been reported that vitamin A (VA) decorated valsartan-loaded liposome therapy (VLC) leads to the re-expression of PPAR-γ, with a significant reduction in fibrogenic mediators, and nearly normalized liver function tests. These liposomes were coupled with VA to specifically target HSCs [79,80].
Cannabinoid receptors (G protein-coupled receptors) are part of the endocannabinoid system, having two subtypes, namely CBR1 (present in hepatocytes) and CBR2 (immune cells present in the liver). Activation of CBR1 has been associated with lipid accumulation in hepatocytes, leading to liver fibrosis [81]. CBR2 displays protective behaviour against liver fibrosis through reducing inflammation and regulating immune response [82].
Likewise, Toll-like receptors are a class of proteins that control the activity of the innate immune system. By focusing on pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), they elicit an immune response. In this context, it has been observed that HSCs activation also gets upregulated by TLR4 signalling and promotes fibrosis by releasing profibrotic factors and pro-inflammatory cytokines [83]. Scientists also reported [84] that controlling TLR signalling is a potential therapeutic approach for preventing the development of liver fibrosis. ALT-100, a monoclonal antibody that neutralizes eNAMPT, significantly reduces NAFLD severity and progression to NASH/hepatic fibrosis by attenuating liver inflamemation, triglyceride accumulation, and fibrosis in both human and mouse models. These results suggested that targeting the eNAMPT/TLR4 pathway could be a promising therapeutic strategy for NAFLD [84].
Furthermore, PPARs (subtypes: PPARα, PPARβ/δ, and PPARγ) agonists have emerged as an important therapeutic option for liver and other metabolic disorders by boosting antioxidant factors and reducing inflammation. PPARα, which regulates lipid metabolism and inflammation and has an indirect role in the activation of HSCs, is an important target for preventing the progression of liver fibrosis.
PPARγ is important in reducing liver fibrosis because it has the potential to inhibit the activation of hepatic stellate cells, regulate lipid metabolism, and reduce inflammation and tumour growth in the liver. On the other hand, the role of PPARβ/δ in preventing liver fibrosis was demonstrated by Zang et al. [85], uncovering a new mechanism by which a PPARβ/δ agonist (GW501516) decreases TGF-β1-led activation of HSCs and fibrosis via AMPK signalling, leading to a reduction in both SMAD3 phosphorylation and p300 levels.
Drug delivery in liver fibrosis
The liver, as an organ, is actively involved in the metabolism of drugs, and most drugs encounter liver tissue at higher concentrations due to the presence of receptors and transporters in hepatocytes. Apart from the presence of a major macrophage population, it also facilitates the accumulation of high-molecular-weight drugs, especially peptides and proteins. However, in the case of liver fibrosis, the involvement of other cell types present in the liver has been demonstrated; hence, targeting cells apart from hepatocytes and macrophages has become an important approach that has been explored recently for therapeutic intervention in liver fibrosis.
Active targeting
Targeted delivery of drugs to specific cell subpopulations in the liver can be a crucial strategy, particularly with a deeper understanding of the function of specific cell subtypes in liver fibrosis. Even in the same organ, this method reduces undesirable side effects and improves the therapeutic outcome of the treatment. Cell-specific targeting employs specific ligands targeting receptors selectively expressed in the desired cell type, in the case of a disease, in addition to the physicochemical properties of nanocarriers. These ligands are attached chemically to the nanocarrier surface, enabling active targeting and increasing the specificity of the therapy. A summary overview of active targeting drug delivery systems and their associated ligands in liver fibrosis is presented in Figure 4.
Figure 4.
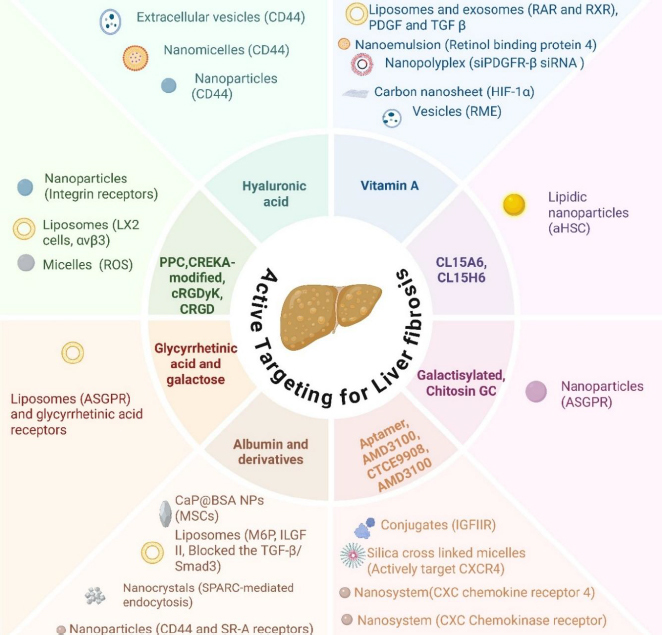
Major drug delivery systems with active targeting agents use for the therapy of liver fibrosis (Created in BioRender. Awasthi, R. (2025) https://BioRender.com/obrtwsc)
Recent studies demonstrated utilization of innovative nanocarriers for targeting hepatic stellate cells (HSCs) in liver fibrosis. Many carriers, such as silica cross-linked micelles coloaded with silibinin and sorafenib modified with the CTCE9908 peptide, CD44 receptor-targeted nanoparticles co-loaded with probucol and collagenase I, palmitic acid-modified albumin nanoparticles, and Endothelin receptor antagonist (CH948, a derivative of PD-156707) conjugated SPIONS MRI, have also been explored (Table 3).
Table 3.
Experimental approaches for active targeting of liver fibrosis
| Molecular targets/receptors | Targeting ligand | Carrier | Drug | Outcomes | Ref. |
|---|---|---|---|---|---|
| PDGFR-β / RARs | Vitamin A (VA) | Nanopolyplex | siPDGFR-β siRNA | Inhibited HSC activation; ↓ pro-fibrogenic proteins and hepatic collagen; restored liver function in mice | [120] |
| ECM | Collagenase | Chitosan nanoparticles | siRNA | Enhanced liver uptake and cell-specific delivery with collagenase pre-treatment | [121] |
| aHSC | CL15A6, CL15H6 | Lipid nanoparticles | siRNA cocktail | Knockdown of Hh and TGFβ1 reversed fibrosis; reprogrammed aHSCs to qHSCs | [122] |
| HIF-1α+ HSCs | VA-PEG | CN@GQDs nanosheets | HIF-1α siRNA | TGF-β1/Smad inhibition; effective siRNA delivery under hypoxia | [123] |
| CXCR4 | CTCE9908 peptide | Silica micelles | Silibinin + Sorafenib | ↓ Inflammation/collagen; ↓ HSC proliferation; ↑ apoptosis. | [124] |
| IGFIIR | Aptamer | Conjugate | siRNA | Diagnostic and therapeutic tool with high affinity | [106] |
| CD44 CD44 RBPR |
Hyaluronic acid Retinoic acid cRGDyK |
Nanoparticle | Curcumin | Promising antifibrotic effect via HA-PLA NPs. | [125] |
| Nanomicelles | Silibinin | Strong antifibrotic effects in HA-based nanomicelles | [126] | ||
| Nanoparticles | Galangin | ↑ Solubility; anti-fibrotic via RA targeting | [127] | ||
| αvβ3 integrin | Liposomes | Vismodegib | Targeted delivery to aHSCs over qHSCs | [128] | |
| LX-2 cells | CREKA peptide | Liposomes | Sorafenib | Reduced ECM and HSC activation | [129] |
| RARs | Vitamin A | Cationic liposomes | TLR4 shRNA | Effective aHSC targeting; antifibrotic outcome | [130] |
| PDGF + TGFβ | Vitamin A | Liposomes | Imatinib | Better antifibrotic effects with VA-conjugation | [131] |
| ROS | cRGD | Micelles | Resveratrol | ROS-responsive release in aHSCs | [132] |
| M6P/IGF-II receptor | M6P-albumin | Liposomes | Hesperidin | ↑ Targeting; ↓ required dose. | [133] |
| GA receptor + CD44 | GA + HA | Liposomes | Curcumin + Berberine | Synergistic anti-aHSC and pro-apoptotic effect | [134] |
| RARs + RXRs | Vitamin A | Liposomes + exosomes | Hydroxychloroquine | Hybrid system effectively targets fibrosis | [135] |
| CXCR4 | AMD3100 | Liposomes | Pirfenidone | ↓ CXCR4, TGFβ, α-SMA; effective antifibrotic response | [136] |
| HSCs SPARC receptor |
Collagenase I + retinol albumin |
Nanodrill micelles | Nilotinib | Deep tissue penetration; strong antifibrotic effect | [80] |
| Nanocrystals | Silibinin | High bioavailability; PDI < 0.15; ~60 nm |
[137] | ||
| PDGFR-β / RARs | Vitamin A (VA) | Nanopolyplex | siPDGFR-β siRNA | Inhibited HSC activation; ↓ pro-fibrogenic proteins and hepatic collagen; restored liver function in mice | [138] |
| ECM | Collagenase | Chitosan nanoparticles | siRNA | Enhanced liver uptake and cell-specific delivery with collagenase pre-treatment | [139] |
| aHSC | CL15A6, CL15H6 | Lipid nanoparticles | siRNA cocktail | Knockdown of Hh and TGFβ1; reversed fibrosis; reprogrammed aHSCs to qHSCs | [140] |
| HIF-1α+ HSCs | VA-PEG | CN@GQDs nanosheets | HIF-1α siRNA | TGF-β1/Smad inhibition; effective siRNA delivery under hypoxia | [141] |
| SPARC receptor | Albumin | Albumin coated liposomes | Naringenin | better antifibrotic effects in mouse model | [142] |
| IGFIIR | Aptamer | Conjugate | siRNA | Diagnostic and therapeutic tool with high affinity | [143] |
| CD44 | Hyaluronic acid | Nanoparticle | Curcumin | Promising antifibrotic effect via HA-PLA NPs | [79] |
| CD44 | Hyaluronic acid | Nanomicelles | Silibinin | Strong antifibrotic effects in HA-based nanomicelles | [144] |
| RBPR | Retinoic acid | Nanoparticles | Galangin | ↑ Solubility; anti-fibrotic via RA targeting | [145] |
| αvβ3 integrin | cRGDyK | Liposomes | Vismodegib | Targeted delivery to aHSCs over qHSCs. | [146] |
| LX-2 cells RARs | CREKA peptide | Liposomes | Sorafenib | Reduced ECM and HSC activation | [124] |
| Vitamin A | Cationic liposomes | TLR4 shRNA | Effective aHSC targeting; antifibrotic outcome | [147] | |
| PDGF + TGFβ | Vitamin A | Liposomes | Imatinib | Better antifibrotic effects with VA-conjugation | [148] |
| ROS | cRGD | Micelles | Resveratrol | ROS-responsive release in aHSCs. | [149] |
| M6P/IGF-II receptor | M6P-albumin | Liposomes | Hesperidin | ↑ Targeting; ↓ required dose. | [150] |
| GA receptor + CD44 | GA + HA | Liposomes | Curcumin + Berberine | Synergistic anti-aHSC and pro-apoptotic effect | [151] |
| Asialoglycoprotein receptor + GA receptor | Galactose-PEG2000-NH2 (GalNH2) + Glycyrrhetinic acid | Nanostructured Lipid Carriers | Dehydrocavidine | ↑ liver targeting, ↑ uptake by aHSCs, apoptosis induction ↓fibrosis markers | [152] |
| Cation-independent mannose-6-phosphate receptor (CI-M6PR) | Ligands targeting TGF-β Receptor I and CI-M6PR | Polydopamine (PDA) Nanoparticles | Ligand targeting TGF-β Receptor I | ↑Accumulation in fibrotic liver tissue ↓ ROS ↓Transforming growth factor-beta (TGF-β) production targeted lysosomal degradation of TGF-β Inhibition of TGF-β-Smad2/3 signaling pathway ↓ECM secretion Significant attenuation of liver fibrosis |
[153] |
| Collagen I (ECM component) |
Succinylated Gelatin | Succinylated gelatin -coated Liposomes (SG-lip) | Pirfenidone | Prolonged retention, enzyme-responsive release, ↓ fibroblast proliferation, invasion, and myofibroblast differentiation | [154] |
| CD44 | Chondroitin Sulfate-Hexadecylamine conjugate (CS-HDA) |
Amphiphilic CS-HDA-based nanoparticles | Imatinib | ↑ uptake by aHSCs; fibrosis resolution in mouse model | [155] |
| Asialoglycoprotein receptor | DSPE-PEG-galactose | Self-assembled SHG nanoparticles | Sorafenib and hederagenin derivative (Hed) | ↓ Collagen deposition by 57.5 ± 2.3% (vs. 24.8 ± 1.8% with sorafenib alone); ↑ uptake in ASGPR-overexpressing HSCs; ↑ anti-fibrotic efficacy (in vivo) | [156] |
| Retinol-binding protein receptor (RBPR) | Vitamin A (VA) | Vitamin A-PEG-PNTC(poly(ethylene glycol)-poly(nitrate carbonate) Micelle | Benazepril (BN) | Targeted delivery to activated HSCs; controlled NO release led to ↓ α-SMA, induction of apoptosis, and ↓ fibrosis | [157] |
| TGF-β1 | Galactose | Bilosomes | Sofosbuvir | Drug loaded bilosomes improved liver function (↓ AST, ALT, ALP), ↓ TGF-β1 levels, and regressed liver fibrosis score in animal model |
[158] |
| α-SMA, COL1A1 | IGFBP-3-targeting moiety | Xanthosomes | TMEM219 (Transmembrane protein 219) | ↓ α-SMA, ↓ COL1A1, ↓ IGFBP-3, ↓ TGF-β1, ↓ p-AKT; ↓ALT, AST); ↓ necrosis, inflammation, and fibrogenesis in BDL-induced fibrosis | [159] |
| FAP-α | Hepatic fibrosis-targeting peptide (FAP-α-responsive) | FAP-α- nano shells (PFD@ns) | Pirfenidone (PFD) | Targeted delivery to fibrotic liver, ↓ TGF-β1-induced collagen production, ↓ HSC activation, ↓ inflammation, modulation of PI3K/AKT/mTOR pathway, ↑ antifibrotic efficacy, ↓ off-target effects | [160] |
| Retinoid receptors | Retinoid (Vitamin A) | Retinoid decorated RBC membrane encapsulated polydopamine nanoparticles | Sorafenib | ↑ aHSC targeting, ↓ HIF-1α & glycolysis, reversal of myofibroblast phenotype, ↓ liver inflammation and fibrosis in CCl4-induced mice |
[161] |
| HA receptor (e.g. CD44 on activated HSCs) | Hyaluronic acid (HA) | Cu2+-coordinated mesoporous | Curcumin | Effective liver targeting, ROS scavenging, ↓ HSC proliferation, collagen deposition, ↑ antioxidant activity, pH-responsive drug release, and alleviation of liver fibrosis | [162] |
Advances in active targeting
Inherent redundancy in fibrogenic signalling pathways means that targeting a single molecular pathway often fails to halt disease progression. This fact has recently led to the development of various experimental therapeutic approaches targeting multiple targets using a combination of two or more drugs and targeting approaches (see Table 3).
Recently, a reported study demonstrated a novel targeted therapeutic strategy for liver fibrosis using mesenchymal stromal cells (MSCs) engineered with a high-affinity peptide ligand (pPB) specific for the platelet-derived growth factor receptor beta (PDGFRβ), which is overexpressed on activated hepatic stellate cells (aHSCs). This target was identified through an analysis of previously published differential cell expression data and single-cell RNA expression data, utilising in silico methods [116]. The investigators successfully functionalized MSC membranes [116] with the pPB peptide via hydrophobic insertion, a rapid and clinically amenable technique that preserves viability, cellular membrane integrity, and functionality. Notably, in vitro and in vivo analyses revealed a significantly enhanced binding affinity of pPB-MSCs for aHSCs compared to unmodified MSCs. The group also successfully demonstrated therapeutic efficacy in a murine model of liver fibrosis, indicated by marked [116] improvements in liver histopathology and a significant reduction in serum levels of hepatic injury biomarkers (alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase), attenuated extracellular matrix (ECM) deposition and reduce the population of α-SMA-positive aHSCs compared to unmodified MSCs, corroborating in vitro findings indicative of inhibited HSC activation. pPB-MSCs restored mitochondrial ultrastructure in fibrotic hepatocytes, suggesting a potential mechanism involving mitochondrial transfer. In other reported research both passive (Ultrasound mediated ECM degradation) and active targeting (Hyaluronic acid modification) were utilized to develop porous coordination network nanoparticles (PCN-NPs) remodel the ECM under ultrasound, enhancing drug access to activated hepatic stellate cells (aHSCs) delivering V-9302 (glutamine metabolism inhibitor) lead to resolution of fibrosis by disrupting hepatocyte-HSC pro-inflammatory crosstalk [117].
In another study [119], a multi-target approach, a nanocarrier composed of circular spherical DNA (cSNA) with surface decoration of circular PDGF-BB aptamer (a nuclease-resistant aptamer blocking PDGF-BB/PDGFR-β signal) and core composed of Collagenase I and ML290 (for reprogramming of pro-fibrogenic into pro-resolution macrophages by activating RXFP1 signalling). The cSNA releases collagenase I in fibrotic liver to degrade collagen and enhance nanoparticle penetration. The cSNA enables both upstream disease blockade and downstream tissue restoration, offering a novel therapeutic approach for fibrosis [119].
Conclusion
Liver fibrosis has emerged as a major pathological outcome of various liver disorders, often progressing to hepatocellular carcinoma and contributing significantly to increased mortality, morbidity, and economic burden. Although numerous clinical trials have been conducted to address liver fibrosis associated with diverse etiologies, the majority were discontinued during Phase 2 or 3 due to a lack of therapeutic efficacy (e.g. Simtuzumab). Only a few drugs, such as Adafermin and Aramchol, have shown promising results in Phase 2 and 3 trials. Notably, Setanaxib is currently the only molecule approved by the U.S. FDA for the treatment of liver fibrosis.
In recent years, hepatic stellate cells (HSCs) have emerged as key mediators and therapeutic targets in liver fibrosis. Pharmacological interventions targeting liver fibrosis have demonstrated encouraging results in experimental models; however, clinical trials remain limited. Among these approaches, the active targeting of HSCs has shown significant promise in preclinical studies for the resolution of liver fibrosis. Combining active targeting strategies with antifibrotic agents in clinical trials may represent a promising avenue to enhance therapeutic success and clinical translation. Ultimately, such advancements aim to improve patient outcomes and overall quality of life.
Future directions
HSCs have emerged as a promising and important target for treating liver fibrosis, leading to a complex yet promising area of research. HSCs play a central role in fibrogenesis after activation in response to liver insult, and express excess extracellular matrix proteins. However, potential challenges impede the development of effective therapies. Primarily, because HSCs share surface markers with other cell types, leading to potential off-target effects. Another challenge arises because HSCs are diverse cells that exhibit unique phenotypes depending on their location in the tissue and the disease state, which complicates the design and development of universal therapeutic options. Furthermore, the reversibility of fibrosis varies, with advanced stages posing greater resistance to therapeutic intervention. Additionally, efficient drug delivery to liver tissue is hindered by the liver’s complex anatomy and the presence of varying levels of the fibrotic matrix within the tissue. The major hurdle in the treatment monitoring aspect is the lack of reliable, non-invasive biomarkers for HSC activation, resulting in poor treatment monitoring. Moreover, the redundancy in fibrogenic signalling pathways leads to the fact that targeting a single molecular pathway often fails to halt disease progression. This is a crucial factor influencing the translation of promising experimental therapies into a clinical setting. This is evident by analysing the successful progression of clinical trials through various stages and regulatory approval for clinical use. Researchers are exploring ways to control HSC-immune cell interactions, restore HSC quiescence, or trigger their apoptosis to combat liver fibrosis. Artificial intelligence (AI) and computational tools are enhancing therapy design, while improved models like humanized mice and organ-on-chip systems are advancing drug testing. These strategies aim to develop more effective and targeted treatments.
To address these challenges, future research should focus on several innovative strategies, including precision delivery systems such as actively targeted nanocarriers loaded with more than one therapeutic molecule, nanoparticles, and antibody-drug conjugates, which are being developed to selectively target activated HSCs. Gene editing tools, such as CRISPR/Cas9 and RNA-based therapeutics, offer potential for modulating key fibrogenic genes and can be used to target multiple pathways, thereby mitigating liver fibrosis and its progression to liver cancer. Advances in single-cell transcriptomics and spatial omics studies can be helpful in two ways, as these studies can improve understanding of HSC heterogeneity, leading to a better understanding of unique phenotypes based upon location and tissue, paving the way for personalized treatments. Combination therapies that target multiple pathways or integrate anti-inflammatory and regenerative approaches may enhance efficacy and improve therapeutic outcomes. Additionally, modulating HSC-immune cell interactions and promoting either the reversion of HSCs to a quiescent state or their selective apoptosis are being explored. Computational modelling and AI will also be crucial for analysing data generated from single-cell sequencing data for the identification of potential novel targets, and a better understanding of the disease mechanism will also be an important aspect in optimizing therapeutic strategies.
In brief, future directions will be guided by advanced techniques, including single-cell transcriptome analysis and AI and machine learning, to identify novel therapeutic targets and options for treating liver fibrosis.
Acknowledgment
The authors are thankful to UPES Dehradun, India, for providing a PhD fellowship to Ms. Alka
Funding Statement
The authors have received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Footnotes
Author Contributions: All authors contributed to the study conception and design. Data collection and analysis were performed by Alka and Ansab Akhtar. Prashant Shukla supervised all phases of the study, including manuscript writing. All authors have read and agreed to the published version of the manuscript.
Ethics approval and consent to participate: Not applicable.
Consent to participate: Not applicable.
Consent to publish: Not applicable
Data availability: Not applicable due to the review article.
Conflicts of Interest: The authors declare no conflicts of interest.
References
- 1.Zamani M., Alizadeh-Tabari S., Ajmera V., Singh S., Murad M.H., Loomba R.. Global Prevalence of Advanced Liver Fibrosis and Cirrhosis in the General Population: A Systematic Review and Meta-analysis. Clinical Gastroenterology and Hepatology 23 (2025) 1123-1134. https://doi.org/10.1016/J.CGH.2024.08.020 10.1016/J.CGH.2024.08.020 [DOI] [PubMed] [Google Scholar]
- 2.Tiwari V., Shandily S., Albert J., Mishra V., Dikkatwar M., Singh R., Sah S.K., Chand S.. Insights into medication-induced liver injury: Understanding and management strategies. Toxicology Reports 14 (2025) 101976. https://doi.org/10.1016/J.TOXREP.2025.101976 10.1016/J.TOXREP.2025.101976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai J.C.T., Liang L.Y., Lai-Hung Wong G.. Noninvasive tests for liver fibrosis in 2024: are there different scales for different diseases? Gastroenterology Report 12 (2023) goae096. https://doi.org/10.1093/GASTRO/GOAE024 10.1093/GASTRO/GOAE024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seo W., Jeong W.I.. Hepatic non-parenchymal cells: Master regulators of alcoholic liver disease? World Journal of Gastroenterology 22 (2016) 1348-1356. https://doi.org/10.3748/wjg.v22.i4.1348 10.3748/wjg.v22.i4.1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banales J.M., Huebert R.C., Karlsen T., Strazzabosco M., LaRusso N.F., Gores G.J.. Cholangiocyte pathobiology. Nature Reviews Gastroenterology and Hepatology 16 (2019) 269-281. https://doi.org/10.1038/s41575-019-0125-y 10.1038/s41575-019-0125-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behmoaras J., Mulder K., Ginhoux F., Petretto E.. The spatial and temporal activation of macrophages during fibrosis. Nature Reviews Immunology (2025). https://doi.org/10.1038/s41577-025-01186-x 10.1038/s41577-025-01186-x [DOI] [PubMed] [Google Scholar]
- 7.Wan Y., Meng F., Wu N., Zhou T., Venter J., Francis H., Kennedy L., Glaser T., Bernuzzi F., Invernizzi P., Glaser S., Huang Q., Alpini G.. Substance P increases liver fibrosis by differential changes in senescence of cholangiocytes and hepatic stellate cells. Hepatology 66 (2017) 528-541. https://doi.org/10.1002/hep.29138 10.1002/hep.29138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunt N.J., McCourt P.A.G., Le Couteur D.G., Cogger V.C.. Novel targets for delaying aging: The importance of the liver and advances in drug delivery. Advanced Drug Delivery Reviews 135 (2018) 39-49. https://doi.org/10.1016/j.addr.2018.09.006 10.1016/j.addr.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 9.Gracia-Sancho J., Caparrós E., Fernández-Iglesias A., Francés R.. Role of liver sinusoidal endothelial cells in liver diseases. Nature Reviews Gastroenterology and Hepatology 18 (2021) 411-431. https://doi.org/10.1038/s41575-020-00411-3 10.1038/s41575-020-00411-3 [DOI] [PubMed] [Google Scholar]
- 10.Wei Y., Bingyu W., Lei Y., Xingxing Y.. The antifibrotic role of natural killer cells in liver fibrosis. Experimental Biology and Medicine 247 (2022) 1235-1243. https://doi.org/10.1177/15353702221092672 10.1177/15353702221092672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slevin E., Baiocchi L., Wu N., Ekser B., Sato K., Lin E., Ceci L., Chen L., Lorenzo S.R., Xu W., Kyritsi K., Meadows V., Zhou T., Kundu D., Han Y., Kennedy L., Glaser S., Francis H., Alpini G., Meng F.. Kupffer Cells: Inflammation Pathways and Cell-Cell Interactions in Alcohol-Associated Liver Disease. The American Journal of Pathology 190 (2020) 2185-2193. https://doi.org/10.1016/j.ajpath.2020.08.014 10.1016/j.ajpath.2020.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wan Y., Zhang W., Huang C., Jian J., Zhang Y., Liu Q., Chen P., Zhu X.. Ursolic acid alleviates Kupffer cells pyroptosis in liver fibrosis by the NOX2/NLRP3 inflammasome signaling pathway. International Immonopharmacology 113 (2022) 109321. https://doi.org/10.1016/j.intimp.2022.109321 10.1016/j.intimp.2022.109321 [DOI] [PubMed] [Google Scholar]
- 13.Zhou Y., Long D., Zhao Y., Li S., Liang Y., Wan L., Zhang J., Xue F., Feng L.. Oxidative stress-mediated mitochondrial fission promotes hepatic stellate cell activation via stimulating oxidative phosphorylation. Cell Death Disease 13 (2022) 689. https://doi.org/10.1038/s41419-022-05088-x 10.1038/s41419-022-05088-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plakkal Ayyappan J., Paul A., Goo Y.H.. Lipid droplet-associated proteins in atherosclerosis (Review). Molecular Medicine Reports 13 (2016) 4527-4534. https://doi.org/10.3892/mmr.2016.5099 10.3892/mmr.2016.5099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee T.F., Mak K.M., Rackovsky O., Lin Y.L., Kwong A.J., Loke J.C., Friedman S.L.. Downregulation of hepatic stellate cell activation by retinol and palmitate mediated by adipose differentiation-related protein (ADRP). Journal of Cellular Physiology 223 (2010) 648-657. https://doi.org/10.1002/jcp.22063 10.1002/jcp.22063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitropoulou G., Kompoura V., Saffioti F., Mavroeidis V.K.. The Role of Matrix Metalloproteinases in Liver Function and Disease. Frontiers in Bioscience (Landmark Edition) 30 (2025) 27127. https://doi.org/10.31083/FBL27127 10.31083/FBL27127 [DOI] [PubMed] [Google Scholar]
- 17.Sato M., Suzuki S., Senoo H.. Hepatic stellate cells: Unique characteristics in cell biology and phenotype. Cells Structure and Function 28 (2003) 105-112. https://doi.org/10.1247/csf.28.105 10.1247/csf.28.105 [DOI] [PubMed] [Google Scholar]
- 18.Tan Q., Xia D., Ying X.. miR-29a in Exosomes from Bone Marrow Mesenchymal Stem Cells Inhibit Fibrosis during Endometrial Repair of Intrauterine Adhesion. International Journal of Stem Cells 13 (2020) 414-423. https://doi.org/10.15283/ijsc20049 10.15283/ijsc20049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwabe R.F., Brenner D.A.. Hepatic stellate cells: balancing homeostasis, hepatoprotection and fibrogenesis in health and disease. Nature Reviews Gastroenterology Hepatology 22 (2025) 481-499. https://doi.org/10.1038/s41575-025-01068-6 10.1038/s41575-025-01068-6 [DOI] [PubMed] [Google Scholar]
- 20.Trinh V.Q.H., Lee T.F., Lemoinne S., Ray K.C., Ybanez M.D., Tsuchida T., Carter J.K., Agudo J., Brown B.D., Akat K.M., Friedman S.L., Lee Y.A.. Hepatic stellate cells maintain liver homeostasis through paracrine neurotrophin-3 signaling that induces hepatocyte proliferation. Science Signaling 16 (2023) eadf6696. https://doi.org/10.1126/scisignal.adf6696 10.1126/scisignal.adf6696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cassiman D., Denef C., Desmet V.J., Roskams T.. Human and rat hepatic stellate cells express neurotrophins and neurotrophin receptors. Hepatology (Baltimore, Md.) 33 (2001) 148-158. https://doi.org/10.1053/jhep.2001.20793 10.1053/jhep.2001.20793 [DOI] [PubMed] [Google Scholar]
- 22. Patidar, Hirani N., Bharti S., Baig M.S.. Key regulators of hepatic stellate cell activation in alcohol liver Disease: A comprehensive review. International Immunopharmacology 141 (2024) 112938. https://doi.org/10.1016/j.intimp.2024.112938 10.1016/j.intimp.2024.112938 [DOI] [PubMed] [Google Scholar]
- 23.Geng Y., Schwabe R.F.. Hepatic stellate cell heterogeneity: Functional aspects and therapeutic implications. Hepatology (2025) 1386. https://doi.org/10.1097/HEP.0000000000001386 10.1097/HEP.0000000000001386 [DOI] [PubMed] [Google Scholar]
- 24.McConnell M.J., Kostallari E., Ibrahim S.H., Iwakiri Y.. The evolving role of liver sinusoidal endothelial cells in liver health and disease. Hepatology 78 (2023) 649. https://doi.org/10.1097/HEP.0000000000000207 10.1097/HEP.0000000000000207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poisson J., Lemoinne S., Boulanger C., Durand F., Moreau R., Valla D., Rautou P.-E.. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. Journal of Hepatology 66 (2017) 212-227. https://doi.org/10.1016/j.jhep.2016.07.009 10.1016/j.jhep.2016.07.009 [DOI] [PubMed] [Google Scholar]
- 26.Dai Q., Ain Q., Seth N., Zhao H., Rooney M., Zipprich A.. Aging-Associated Liver Sinusoidal Endothelial Cells Dysfunction Aggravates the Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease. Aging Cell 24 (2025) e14502. https://doi.org/10.1111/acel.14502 10.1111/acel.14502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao B., Radaeva S.. Natural killer and natural killer T cells in liver fibrosis. Biochimica et Biophysica Acta 1832 (2013) 1061-1069. https://doi.org/10.1016/j.bbadis.2012.09.008 10.1016/j.bbadis.2012.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramachandran P., Iredale J.P.. Macrophages: Central regulators of hepatic fibrogenesis and fibrosis resolution. Journal of Hepatology 56 (2012) 1417-1419. https://doi.org/10.1016/j.jhep.2011.10.026 10.1016/j.jhep.2011.10.026 [DOI] [PubMed] [Google Scholar]
- 29.Canbay A., Taimr P., Torok N., Higuchi H., Friedman S., Gores G.J.. Apoptotic Body Engulfment by a Human Stellate Cell Line Is Profibrogenic. Laboratory Investigation 83 (2003) 655-663. https://doi.org/10.1097/01.LAB.0000069036.63405.5C 10.1097/01.LAB.0000069036.63405.5C [DOI] [PubMed] [Google Scholar]
- 30.Nieto N., Friedman S.L., Cederbaum A.I.. Cytochrome P450 2E1-derived reactive oxygen species mediate paracrine stimulation of collagen I protein synthesis by hepatic stellate cells. The Journal of Biological 277 (2002) 9853-9864. https://doi.org/10.1074/jbc.M110506200 10.1074/jbc.M110506200 [DOI] [PubMed] [Google Scholar]
- 31.Tsukamoto H.. Epigenetic mechanism of stellate cell trans-differentiation. Journal of Hepatology 46 (2007) 352-353. https://doi.org/10.1016/j.jhep.2006.11.002 10.1016/j.jhep.2006.11.002 [DOI] [PubMed] [Google Scholar]
- 32.Tarrats N., Moles A., Morales A., García-Ruiz C., Fernández-Checa J.C., Marí M.. Critical role of tumor necrosis factor receptor 1, but not 2, in hepatic stellate cell proliferation, extracellular matrix remodeling, and liver fibrogenesis. Hepatology (Baltimore, Md.) 54 (2011) 319-327. https://doi.org/10.1002/hep.24388 10.1002/hep.24388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chuliá-Peris L., Carreres-Rey C., Gabasa M., Alcaraz J., Carretero J., Pereda J.. Matrix Metalloproteinases and Their Inhibitors in Pulmonary Fibrosis: EMMPRIN/CD147 Comes into Play. International Journal of Molecular Sciences 23 (2022) 6894. https://doi.org/10.3390/ijms23136894 10.3390/ijms23136894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urtasun R., Lopategi A., George J., Leung T.M., Lu Y., Wang X., Ge X., Fiel M.I., Nieto N.. Osteopontin, an oxidant stress sensitive cytokine, up-regulates collagen-I via integrin α(V)β(3) engagement and PI3K/pAkt/NFκB signaling. Hepatology (Baltimore, Md.) 55 (2012) 594-608. https://doi.org/10.1002/hep.24701 10.1002/hep.24701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seki E., De Minicis S., Gwak G.Y., Kluwe J., Inokuchi S., Bursill C.A., Llovet J.M., Brenner D.A., Schwabe R.F.. CCR1 and CCR5 promote hepatic fibrosis in mice. The Journal of Clinical Investigation 119 (2009) 1858-1870. https://doi.org/10.1172/jci37444 10.1172/jci37444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rebolledo D.L., Lipson K.E., Brandan E.. Driving fibrosis in neuromuscular diseases: Role and regulation of Connective tissue growth factor (CCN2/CTGF). Matrix Biology Plus 11 (2021) 100059. https://doi.org/10.1016/j.mbplus.2021.100059 10.1016/j.mbplus.2021.100059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J., Chu E. S. H., Chen H.-Y., Man K., Go M. Y. Y., Huang X.R., Lan H.Y., Sung J. J. Y., Yu J., microRNA-29b prevents liver fibrosis by attenuating hepatic stellate cell activation and inducing apoptosis through targeting PI3K/AKT pathway. Oncotarget 6 (2015) 7325-7338. https://doi.org/10.18632/oncotarget.2621 10.18632/oncotarget.2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Novo E., Povero D., Busletta C., Paternostro C., Di Bonzo L.V., Cannito S., Compagnone A., Bandino A., Marra F., Colombatto S., David E., Pinzani M., Parola M.. The biphasic nature of hypoxia-induced directional migration of activated human hepatic stellate cells. Journal of Pathology 226 (2012) 588-597. https://doi.org/10.1002/path.3005 10.1002/path.3005 [DOI] [PubMed] [Google Scholar]
- 39.Li J., Qiu S.J., She W.M., Wang F.P., Gao H., Li L., Tu C.T., Wang J.Y., Shen X.Z., Jiang W.. Significance of the balance between regulatory T (Treg) and T helper 17 (Th17) cells during hepatitis B virus related liver fibrosis. Plos One 7(7) (2012) e39307. https://doi.org/10.1371/journal.pone.0039307 10.1371/journal.pone.0039307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y., Huang B., Jiang X., Chen W., Zhang J., Wei Y., Chen Y., Lian M., Bian Z., Miao Q., Peng Y., Fang J., Wang Q., Tang R., Gershwin M.E., Ma X.. Mucosal-Associated Invariant T Cells Improve Nonalcoholic Fatty Liver Disease Through Regulating Macrophage Polarization. Frontiers in Immunology 9 (2018) 1994. https://doi.org/10.3389/fimmu.2018.01994 10.3389/fimmu.2018.01994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hegde P., Weiss E., Paradis V., Wan J., Mabire M., Sukriti S., Rautou P.E., Albuquerque M., Picq O., Gupta A.C., Ferrere G., Gilgenkrantz H., Kiaf B., Toubal A., Beaudoin L., Lettéron P., Moreau R., Lehuen A., Lotersztajn S.. Mucosal-associated invariant T cells are a profibrogenic immune cell population in the liver. Nature Communications 9 (2018) 2146. https://doi.org/10.1038/s41467-018-04450-y 10.1038/s41467-018-04450-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu M., Hu Y., Yuan Y., Tian Z., Zhang C.. γδT Cells Suppress Liver Fibrosis via Strong Cytolysis and Enhanced NK Cell-Mediated Cytotoxicity Against Hepatic Stellate Cells. Frontiers In Immunology 10 (2019) 477. https://doi.org/10.3389/fimmu.2019.00477 10.3389/fimmu.2019.00477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seo W., Eun H.S., Kim S.Y., Yi H.S., Lee Y.S., Park S.H., Jang M.J., Jo E., Kim S.C., Han Y.M., Park K.G., Jeong W.I.. Exosome-mediated activation of toll-like receptor 3 in stellate cells stimulates interleukin-17 production by γδ T cells in liver fibrosis. Hepatology (Baltimore, Md.) 64 (2016) 616-631. https://doi.org/10.1002/hep.28644 10.1002/hep.28644 [DOI] [PubMed] [Google Scholar]
- 44.Gilgenkrantz H., Sayegh R.A., Lotersztajn S.. Immunoregulation of Liver Fibrosis: New Opportunities for Antifibrotic Therapy. Annual Review of Pharmacology and Toxicology 65 (2025) 281-299. https://doi.org/10.1146/annurev-pharmtox-020524-012013 10.1146/annurev-pharmtox-020524-012013 [DOI] [PubMed] [Google Scholar]
- 45.Wijaya R.S., Read S.A., Schibeci S., Eslam M., Azardaryany M.K., El-Khobar K., van der Poorten D., Lin R., Yuen L., Lam V., George J., Douglas M.W., Ahlenstiel G.. KLRG1+ natural killer cells exert a novel antifibrotic function in chronic hepatitis B. Journal of Hepatology 71 (2019) 252-264. https://doi.org/10.1016/j.jhep.2019.03.012 10.1016/j.jhep.2019.03.012 [DOI] [PubMed] [Google Scholar]
- 46.Sugimoto A., Saito Y., Wang G., Sun Q., Yin C., Lee K.H., Geng Y., Rajbhandari P., Hernandez C., Steffani M., Qie J., Savage T., Goyal D.M., Ray K.C., Neelakantan T.V., Yin D., Melms J., Lehrich B.M., Yasaka T.M., Liu S., Oertel M., Lan T., Guillot A., Peiseler M., Filliol A., Kanzaki H., Fujiwara N., Ravi S., Izar B., Brosch M., Hampe J., Remotti H., Argemi J., Sun Z., Kendall T.J., Hoshida Y., Tacke F., Fallowfield J.A., Blockley-Powell S.K., Haeusler R.A., Steinman J.B., Pajvani U.B., Monga S.P., Bataller R., Masoodi M., Arpaia N., Lee Y.A., Stockwell B.R., Augustin H.G., Schwabe R.F.. Hepatic stellate cells control liver zonation, size and functions via R-spondin 3. Nature 640 (2025) 752-761. https://doi.org/10.1038/s41586-025-08677-w 10.1038/s41586-025-08677-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merens V., Knetemann E., Gürbüz E., De Smet V., Messaoudi N., Reynaert H., Verhulst S., van Grunsven L. A.. Hepatic stellate cell single cell atlas reveals a highly similar activation process across liver disease aetiologies. JHEP Reports 7 (2025) 101223. https://doi.org/10.1016/J.JHEPR.2024.101223 10.1016/J.JHEPR.2024.101223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang X., Zhao W., Shen B., Han Y., Chen K.. CD47-mediated regulation of glucose and lipid metabolism: implications for the pathogenesis of MASLD. Frontiers In Endocrinology 16 (2025). https://doi.org/10.3389/fendo.2025.1535382 10.3389/fendo.2025.1535382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang D.W., Zhao Y.X., Wei D., Li Y.L., Zhang Y., Wu J., Xu J., Chen C., Tang H., Zhang W., Gong L., Han Y., Chen Z.N., Bian H.. HAb18G/CD147 promotes activation of hepatic stellate cells and is a target for antibody therapy of liver fibrosis. Journal of Hepatology 57 (2012) 1283-1291. https://doi.org/10.1016/J.JHEP.2012.07.042 10.1016/J.JHEP.2012.07.042 [DOI] [PubMed] [Google Scholar]
- 50.Pirola L.. Elafibranor, a dual PPARα and PPARδ agonist, reduces alcohol-associated liver disease: Lessons from a mouse model. World Journal of Gastroenterology 31 (2025) 99312. https://doi.org/10.3748/wjg.v31.i4.99312 10.3748/wjg.v31.i4.99312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang G., Zhang M., Wang M., Xu W., Duan X., Han X., Ren J., Pioglitazone. a peroxisome proliferator-activated receptor γ agonist, induces cell death and inhibits the proliferation of hypoxic HepG2 cells by promoting excessive production of reactive oxygen species. Oncology Letters 27 (2024) 160. https://doi.org/10.3892/ol.2024.14294 10.3892/ol.2024.14294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fiorucci S., Biagioli M., Baldoni M., Ricci P., Sepe V., Zampella A., Distrutti E.. The identification of farnesoid X receptor modulators as treatment options for nonalcoholic fatty liver disease. Expert Opinion on Drug Discovery 16 (2021) 1193-1208. https://doi.org/10.1080/17460441.2021.1916465 10.1080/17460441.2021.1916465 [DOI] [PubMed] [Google Scholar]
- 53.Lekakis V., Cholongitas E.. The impact of emricasan on chronic liver diseases: current data. Clinical Journal of Gastroenterology 15 (2022) 271-285. https://doi.org/10.1007/s12328-021-01585-2 10.1007/s12328-021-01585-2 [DOI] [PubMed] [Google Scholar]
- 54.Harrison S.A., Bedossa P., Guy C.D., Schattenberg J.M., Loomba R., Taub R., Labriola D., Moussa S.E., Neff G.W., Rinella M.E., Anstee Q.M., Abdelmalek M.F., Younossi Z., Baum S.J., Francque S., Charlton M.R., Newsome P.N., Lanthier N., Schiefke I., Mangia A., Pericàs J.M., Patil R., Sanyal A.J., Noureddin M., Bansal M.B., Alkhouri N., Castera L., Rudraraju M., Ratziu V.. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. New England Journal of Medicine 390 (2024) 497-509. https://doi.org/10.1056/NEJMOA2309000 10.1056/NEJMOA2309000 [DOI] [PubMed] [Google Scholar]
- 55.Xu X., Guo Y., Luo X., Shen Z., Sun Z., Shen B., Zhou C., Wang J., Lu J., Zhang Q., Ye Y., Luo Y., Qu Y., Cai X., Dong H., Lu L.. Hydronidone ameliorates liver fibrosis by inhibiting activation of hepatic stellate cells via Smad7-mediated degradation of TGFβRI. Liver International 43 (2023) 2523-2537. https://doi.org/10.1111/LIV.15715 10.1111/LIV.15715 [DOI] [PubMed] [Google Scholar]
- 56.Chalasani N., Abdelmalek M.F., Garcia-Tsao G., Vuppalanchi R., Alkhouri N., Rinella M., Noureddin M., Pyko M., Shiffman M., Sanyal A., Allgood A., Shlevin H., Horton R., Zomer E., Irish W., Goodman Z., Harrison S.A., Traber P.G., Balart L., Borg B., Charlton M., Conjeevaram H., Fuchs M., Ghalib R., Gholam P., Halegoua-De Marzio D., Harrison S., Jue C., Kemmer N., Kowdley K., Lai M., Lawitz E., Loomba R., Paredes A., Rockey D., Rodriguez M., Rubin R., Ryan M., Scanga A., Sepe T., Tetri B., Thuluvath P., Torres D., Vierling J., Wattacheril J., Weiland A., Zogg D.. Effects of Belapectin, an Inhibitor of Galectin-3, in Patients with Nonalcoholic Steatohepatitis with Cirrhosis and Portal Hypertension. Gastroenterology 158 (2020) 1334-1345.e5. https://doi.org/10.1053/J.GASTRO.2019.11.296 10.1053/J.GASTRO.2019.11.296 [DOI] [PubMed] [Google Scholar]
- 57.Petta S., Targher G., Romeo S., Pajvani U.B., Zheng M.H., Aghemo A., Valenti L.V.C.. The first MASH drug therapy on the horizon: Current perspectives of resmetirom. Liver International 44 (2024) 1526-1536. https://doi.org/10.1111/LIV.15930 10.1111/LIV.15930 [DOI] [PubMed] [Google Scholar]
- 58.Kram M.. Galectin-3 inhibition as a potential therapeutic target in non-alcoholic steatohepatitis liver fibrosis. World Journal of Hepatology 15 (2023) 201-207. https://doi.org/10.4254/wjh.v15.i2.201 10.4254/wjh.v15.i2.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nasir S.A., Mangla A., Taneja V., Berger T., Pandya D., Gupta V., Lim J.K.. Advances in Novel Drug Therapy for Metabolic Dysfunction-associated Steatohepatitis Cirrhosis. Journal of Translational Gastroenterology 3 (2025) 9-17. https://doi.org/10.14218/JTG.2024.00040 10.14218/JTG.2024.00040 [DOI] [Google Scholar]
- 60.Ezhilarasan D.. Deciphering the molecular pathways of saroglitazar: A dual PPAR α/γ agonist for managing metabolic NAFLD. Metabolism 155 (2024) 155912. https://doi.org/10.1016/j.metabol.2024.155912 10.1016/j.metabol.2024.155912 [DOI] [PubMed] [Google Scholar]
- 61.Hayes C.M., Gallucci G.M., Boyer J.L., Assis D.N., Ghonem N.S.. PPAR agonists for the treatment of cholestatic liver diseases: Over a decade of clinical progress. Hepatology Communications 9 (2024). https://doi.org/10.1097/HC9.0000000000000612 10.1097/HC9.0000000000000612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keam S.J.. Resmetirom: First Approval. Drugs 84 (2024) 729-735. https://doi.org/10.1007/s40265-024-02045-0 10.1007/s40265-024-02045-0 [DOI] [PubMed] [Google Scholar]
- 63.Yabut J.M., Drucker D.J.. Glucagon-like Peptide-1 Receptor-based Therapeutics for Metabolic Liver Disease. Endocrine Reviews 44 (2023) 14-32. https://doi.org/10.1210/endrev/bnac018 10.1210/endrev/bnac018 [DOI] [PubMed] [Google Scholar]
- 64.Sanyal A.J., Newsome P.N., Kliers I., Østergaard L.H., Long M.T., Kjær M.S., Cali A.M.G., Bugianesi E., Rinella M.E., Roden M., Ratziu V.. Phase 3 Trial of Semaglutide in Metabolic Dysfunction-Associated Steatohepatitis. New England Journal of Medicine 392 (2025) 2089-2099. https://doi.org/10.1056/NEJMOA2413258 10.1056/NEJMOA2413258 [DOI] [PubMed] [Google Scholar]
- 65.Azizsoltani A., Niknam B., Taghizadeh-Teymorloei M., Ghoodjani E., Dianat-Moghadam H., Alizadeh E.. Therapeutic implications of obeticholic acid, a farnesoid X receptor agonist, in the treatment of liver fibrosis. Biomedicine Pharmacotherapy 189 (2025) 11829. https://doi.org/10.1016/j.biopha.2025.118249 10.1016/j.biopha.2025.118249 [DOI] [PubMed] [Google Scholar]
- 66.Thannickal V.J., Jandeleit-Dahm K., Szyndralewiez C., Török N.J.. Pre-clinical evidence of a dual NADPH oxidase 1/4 inhibitor (setanaxib) in liver, kidney and lung fibrosis. Journal of Cellular and Molecular Medicine 27 (2023) 471-481. https://doi.org/10.1111/jcmm.17649 10.1111/jcmm.17649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.El-Haggar S.M., Hegazy S.K., Abd-Elsalam S.M., Bahaa M.M.. Pentoxifylline, a nonselective phosphodiesterase inhibitor, in adjunctive therapy in patients with irritable bowel syndrome treated with mebeverine. Biomedicine Pharmacotherapy 145 (2022) 112399. https://doi.org/10.1016/j.biopha.2021.112399 10.1016/j.biopha.2021.112399 [DOI] [PubMed] [Google Scholar]
- 68.Cai X., Liu X., Xie W., Ma A., Tan Y., Shang J., Zhang J., Chen C., Yu Y., Qu Y., Zhang L., Luo Y., Yin P., Cheng J., Lu L.. Hydronidone for the Treatment of Liver Fibrosis Related to Chronic Hepatitis B: A Phase 2 Randomized Controlled Trial. Clinical Gastroenterology and Hepatology 21 (2023) 1893-1901.e7. https://doi.org/10.1016/j.cgh.2022.05.056 10.1016/j.cgh.2022.05.056 [DOI] [PubMed] [Google Scholar]
- 69.Zhang J., Wang Q., Zhou N., Liu J., Tao L., Peng Z., Hu G., Wang H., Fu L., Peng S.. Fluorofenidone attenuates choline-deficient, l-amino acid-defined, high-fat diet-induced metabolic dysfunction-associated steatohepatitis in mice. Scientific Reports 15 (2025) 9863. https://doi.org/10.1038/s41598-025-94401-7 10.1038/s41598-025-94401-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma Z., Jin K., Yue M., Chen X., Chen J.. Research Progress on the GIP/GLP-1 Receptor Coagonist Tirzepatide, a Rising Star in Type 2 Diabetes. Journal of Diabetes Research 2023 (2023) 5891532. https://doi.org/10.1155/2023/5891532 10.1155/2023/5891532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alkhouri N., Lawitz E., Noureddin M., DeFronzo R., Shulman G.I.. GS-0976 (Firsocostat): an investigational liver-directed acetyl-CoA carboxylase (ACC) inhibitor for the treatment of non-alcoholic steatohepatitis (NASH). Expert Opinion on Investigational Drugs 29 (2020) 135-141. https://doi.org/10.1080/13543784.2020.1668374 10.1080/13543784.2020.1668374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Loomba R., Sanyal A.J., Nakajima A., Neuschwander-Tetri B.A., Goodman Z.D., Harrison S.A., Lawitz E.J., Gunn N., Imajo K., Ravendhran N., Akahane T., Boone B., Yamaguchi M., Chatterjee A., Tirucherai G.S., Shevell D.E., Du S., Charles E.D., Abdelmalek M.F.. Pegbelfermin in Patients With Nonalcoholic Steatohepatitis and Stage 3 Fibrosis (FALCON 1): A Randomized Phase 2b Study. Clinical Gastroenterology and Hepatology 22 (2024) 102-112.e9. https://doi.org/10.1016/j.cgh.2023.04.011 10.1016/j.cgh.2023.04.011 [DOI] [PubMed] [Google Scholar]
- 73.Rinella M.E., Lieu H.D., Kowdley K.V., Goodman Z.D., Alkhouri N., Lawitz E., Ratziu V., Abdelmalek M.F., Wong V.W.S., Younes Z.H., Sheikh A.M., Brannan D., Freilich B., Membreno F., Sinclair M., Melchor-Khan L., Sanyal A.J., Ling L., Harrison S.A.. A randomized, double-blind, placebo-controlled trial of aldafermin in patients with NASH and compensated cirrhosis. Hepatology 79 (2024) 674-689. https://doi.org/10.1097/HEP.0000000000000607 10.1097/HEP.0000000000000607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ratziu V., Yilmaz Y., Lazas D., Friedman S.L., Lackner C., Behling C., Cummings O.W., Chen L., Petitjean M., Gilgun-Sherki Y., Gorfine T., Kadosh S., Eyal E., Sanyal A.J.. Aramchol improves hepatic fibrosis in metabolic dysfunction-associated steatohepatitis: Results of multimodality assessment using both conventional and digital pathology. Hepatology 81 (2025) 932. https://doi.org/10.1097/HEP.0000000000000980 10.1097/HEP.0000000000000980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ciftci F., Özarslan A.C., Kantarci İ.C., Yelkenci A., Tavukcuoglu O., Ghorbanpour M.. Advances in Drug Targeting, Drug Delivery, and Nanotechnology Applications: Therapeutic Significance in Cancer Treatment. Pharmaceutics 17 (2025) 121. https://doi.org/10.3390/pharmaceutics17010121 10.3390/pharmaceutics17010121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Han X., Cui Z.Y., Song J., Piao H.Q., Lian L.H., Hou L.S., Wang G., Zheng S., Dong X.X., Nan J.X., Wu Y.L.. Acanthoic acid modulates lipogenesis in nonalcoholic fatty liver disease via FXR/LXRs-dependent manner. Chemico-Biological Interactions 311 (2019) 108794. https://doi.org/10.1016/j.cbi.2019.108794 10.1016/j.cbi.2019.108794 [DOI] [PubMed] [Google Scholar]
- 77.Azizsoltani A., Hatami B., Zali M.R., Mahdavi V., Baghaei K., Alizadeh E.. Obeticholic acid-loaded exosomes attenuate liver fibrosis through dual targeting of the FXR signaling pathway and ECM remodeling. Biomedicine Pharmacotherapy 168 (2023) 115777. https://doi.org/10.1016/j.biopha.2023.115777 10.1016/j.biopha.2023.115777 [DOI] [PubMed] [Google Scholar]
- 78.Girisa S., Aswani B.S., Manickasamy M.K., Hegde M., Alqahtani M.S., Abbas M., Sethi G., Kunnumakkara A.B.. Restoring FXR expression as a novel treatment strategy in liver cancer and other liver disorders. Expert Opinion on Therapeutic Targets 29 (2025) 193-221. https://doi.org/10.1080/14728222.2025.2487465 10.1080/14728222.2025.2487465 [DOI] [PubMed] [Google Scholar]
- 79.El-Mezayen N.S., El-Hadidy W.F., El-Refaie W.M., Shalaby T.I., Khattab M.M., El-Khatib A.S.. Oral vitamin-A-coupled valsartan nanomedicine: High hepatic stellate cell receptors accessibility and prolonged enterohepatic residence. Journal of Controlled Release 283 (2018) 32-44. https://doi.org/10.1016/j.jconrel.2018.05.021 10.1016/j.jconrel.2018.05.021 [DOI] [PubMed] [Google Scholar]
- 80.Zhang Y.W., Hou L.S., Xing J.H., Zhang T.R., Zhou S.Y., Zhang B.L.. Two-Membrane Hybrid Nanobiomimetic Delivery System for Targeted Autophagy Inhibition of Activated Hepatic Stellate Cells To Synergistically Treat Liver Fibrosis. ACS Applied Materials Interfaces 15 (2023) 50863-50877. https://doi.org/10.1021/acsami.3c11046 10.1021/acsami.3c11046 [DOI] [PubMed] [Google Scholar]
- 81.Tan S., Liu H., Ke B., Jiang J., Wu B.. The peripheral CB receptor antagonist JD5037 attenuates liver fibrosis via a CB receptor/β-arrestin1/Akt pathway. British Journal of Pharmacology 177 (2020) 2830-2847. https://doi.org/10.1111/bph.15010 10.1111/bph.15010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ali A.M., El-Tawil O.S., Al-Mokaddem A.K., Abd El-Rahman S.S.. Promoted inhibition of TLR4/miR-155/ NFkB p65 signaling by cannabinoid receptor 2 agonist (AM1241), aborts inflammation and progress of hepatic fibrosis induced by thioacetamide. Chemo-Biological Interactions 336 (2021) 109398. https://doi.org/10.1016/j.cbi.2021.109398 10.1016/j.cbi.2021.109398 [DOI] [PubMed] [Google Scholar]
- 83.Li B., Yan C., Wu J., Stephane K., Dong X., Zhang Y.Z., Zhang Y., Yu Q., Zheng K.Y.. Clonorchis sinensis ESPs enhance the activation of hepatic stellate cells by a cross-talk of TLR4 and TGF-β/Smads signaling pathway. Acta Tropica 205 (2020) 105307. https://doi.org/10.1016/j.actatropica.2019.105307 10.1016/j.actatropica.2019.105307 [DOI] [PubMed] [Google Scholar]
- 84.Sun B.L., Sun X., Kempf C.L., Song J.H., Casanova N.G., Camp S.M., Reyes Hernon V., Fallon M., Bime C., Martin D.R., Travelli C., Zhang D.D., Garcia J.G.N.. Involvement of eNAMPT/TLR4 inflammatory signaling in progression of non-alcoholic fatty liver disease, steatohepatitis, and fibrosis. The FASEB Journal 37 (2023) e22825. https://doi.org/10.1096/fj.202201972RR 10.1096/fj.202201972RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang M., Barroso E., Peña L., Rada P., Valverde Á.M., Wahli W., Palomer X., Vázquez-Carrera M.. PPARβ/δ attenuates hepatic fibrosis by reducing SMAD3 phosphorylation and p300 levels via AMPK in hepatic stellate cells. Biomedicine and Pharmacotherapy 179 (2024) 117303. https://doi.org/10.1016/j.biopha.2024.117303 10.1016/j.biopha.2024.117303 [DOI] [PubMed] [Google Scholar]
- 86.Moon A.N., Briand F., Breyner N., Song D.K., Madsen M.R., Kim H., Choi K., Lee Y., Namkung W.. Improvement of NASH and liver fibrosis through modulation of the gut-liver axis by a novel intestinal FXR agonist. Biomedicine Pharmacotherapy 173 (2024) 116331. https://doi.org/10.1016/j.biopha.2024.116331 10.1016/j.biopha.2024.116331 [DOI] [PubMed] [Google Scholar]
- 87.Liu H.M., Lee T.Y., Liao J.F.. GW4064 attenuates lipopolysaccharide-induced hepatic inflammation and apoptosis through inhibition of the Toll-like receptor 4-mediated p38 mitogen-activated protein kinase signaling pathway in mice. International Journal of Molecular Medicine 41 (2018) 1455-1462. https://doi.org/10.3892/ijmm.2018.3366 10.3892/ijmm.2018.3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Di Pasqua L.G., Cagna M., Palladini G., Croce A.C., Cadamuro M., Fabris L., Perlini S., Adorini L., Ferrigno A., Vairetti M.. FXR agonists INT-787 and OCA increase RECK and inhibit liver steatosis and inflammation in diet-induced ob/ob mouse model of NASH. Liver International 44 (2024) 214-227. https://doi.org/10.1111/liv.15767 10.1111/liv.15767 [DOI] [PubMed] [Google Scholar]
- 89.Anfuso B., Tiribelli C., Adorini L., Rosso N.. Obeticholic acid and INT-767 modulate collagen deposition in a NASH in vitro model. Scientific Reports 10 (2020) 1699. https://doi.org/10.1038/s41598-020-58562-x 10.1038/s41598-020-58562-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feng S., Xie X., Li J., Xu X., Chen C., Zou G., Lin G., Huang T., Hu R., Ran T., Han L., Zhang Q., Li Y., Zhao X.. Bile acids induce liver fibrosis through the NLRP3 inflammasome pathway and the mechanism of FXR inhibition of NLRP3 activation. Hepatology International 18 (2024) 1040-1052. https://doi.org/10.1007/s12072-023-10610-0 10.1007/s12072-023-10610-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiang Y., Hou L., Dou J., Xuan M., Cui Z., Lian L., Nan J., Wu Y.. Sesamol as a potential candidate for the treatment of hepatic fibrosis, based on its regulation of FXR/LXR axis-mediated inhibition of autophagy through crosstalk between hepatic cells and macrophage. Phytomedicine 123 (2024) 155145. https://doi.org/10.1016/j.phymed.2023.155145 10.1016/j.phymed.2023.155145 [DOI] [PubMed] [Google Scholar]
- 92.Sengupta M., Griffett K., Flaveny C.A., Burris T.P.. Inhibition of Hepatotoxicity by a LXR Inverse Agonist in a Model of Alcoholic Liver Disease. ACS Pharmacology Translational Science 1 (2018) 50-60. https://doi.org/10.1021/acsptsci.8b00003 10.1021/acsptsci.8b00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Griffett K., Burris T.P.. Promiscuous activity of the LXR antagonist GSK2033 in a mouse model of fatty liver disease. Biochemical and Biophysical Research Communications 479 (2016) 424-428. https://doi.org/10.1016/j.bbrc.2016.09.036 10.1016/j.bbrc.2016.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang F., Lu S., He J., Jin H., Wang F., Wu L., Shao J., Chen A., Zheng S.. Ligand Activation of PPARγ by Ligustrazine Suppresses Pericyte Functions of Hepatic Stellate Cells via SMRT-Mediated Transrepression of HIF-1α. Theranostics 8 (2018) 610-626. https://doi.org/10.7150/thno.22237 10.7150/thno.22237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.de Souza Basso B., Haute G.V., Ortega-Ribera M., Luft C., Antunes G.L., Bastos M.S., Carlessi L.P., Levorse V.G., Cassel E., Fagundes Donadio M.V., Santarém E.R., Gracia-Sancho J., Rodrigues de Oliveira J.. Methoxyeugenol deactivates hepatic stellate cells and attenuates liver fibrosis and inflammation through a PPAR-ɣ and NF-kB mechanism. Journal of Ethnopharmacology 280 (2021) 114433. https://doi.org/10.1016/j.jep.2021.114433 10.1016/j.jep.2021.114433 [DOI] [PubMed] [Google Scholar]
- 96.Chen L., Li L., Chen J., Li L., Zheng Z., Ren J., Qiu Y.. Oleoylethanolamide, an endogenous PPAR-α ligand, attenuates liver fibrosis targeting hepatic stellate cells. Oncotarget 6 (2015) 42530-42540. https://doi.org/10.18632/oncotarget.6466 10.18632/oncotarget.6466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sandoval-Rodriguez A., Monroy-Ramirez H.C., Meza-Rios A., Garcia-Bañuelos J., Vera-Cruz J., Gutiérrez-Cuevas J., Silva-Gomez J., Staels B., Dominguez-Rosales J., Galicia-Moreno M., Vazquez-Del Mercado M., Navarro-Partida J., Santos-Garcia A., Armendariz-Borunda J.. Pirfenidone Is an Agonistic Ligand for PPARα and Improves NASH by Activation of SIRT1/LKB1/pAMPK. Hepatology Communications 4 (2020) 434-449. https://doi.org/10.1002/hep4.1474 10.1002/hep4.1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou Z., Deng L., Hu L., Ren Q., Cai Z., Wang B., Li Z., Zhang L.. Hepatoprotective effects of ZLY16, a dual peroxisome proliferator-activated receptor α/δ agonist, in rodent model of nonalcoholic steatohepatitis. European Journal of Pharmacology 882 (2020) 173300. https://doi.org/10.1016/j.ejphar.2020.173300 10.1016/j.ejphar.2020.173300 [DOI] [PubMed] [Google Scholar]
- 99.Honda A., Kamata S., Satta C., Machida Y., Uchii K., Terasawa K., Nemoto A., Oyama T., Ishii I.. Structural basis for anti-non-alcoholic fatty liver disease and diabetic dyslipidemia drug saroglitazar as a PPAR α/γ dual agonist. Biological and Pharmaceutical Bulletin 44 (2021) 1210. https://doi.org/10.1248/bpb.b21-00232 10.1248/bpb.b21-00232 [DOI] [PubMed] [Google Scholar]
- 100.Kumazoe M., Miyamoto E., Oka C., Kondo M., Yoshitomi R., Onda H., Shimada Y., Fujimura Y., Tachibana H.. miR-12135 ameliorates liver fibrosis accompanied with the downregulation of integrin subunit alpha 11. IsciencE 27 (2024) 108730. https://doi.org/10.1016/j.isci.2023.108730 10.1016/j.isci.2023.108730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reichert D., Adolph L., Köhler J.P., Buschmann T., Luedde T., Häussinger D., Kordes C.. Improved recovery from liver fibrosis by crenolanib. Cells 10 (2021) 804. https://doi.org/10.3390/cells10040804 10.3390/cells10040804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu F., Li S., Chen P., Gu Y., Wang S., Wang L., Chen C., Wang R., Yuan Y.. Salvianolic acid B inhibits hepatic stellate cell activation and liver fibrosis by targeting PDGFRβ. International Immunopharmacology 122 (2023) 110550. https://doi.org/10.1016/j.intimp.2023.110550 10.1016/j.intimp.2023.110550 [DOI] [PubMed] [Google Scholar]
- 103.Mekala S., Sukumar G., Chawla S., Geesala R., Prashanth J., Reddy B.J.M., Mainkar P., Das A.. Therapeutic Potential of Benzimidazoisoquinoline Derivatives in Alleviating Murine Hepatic Fibrosis. Chemistry Biodiversity 21 (2024) e202301429. https://doi.org/10.1002/cbdv.202301429 10.1002/cbdv.202301429 [DOI] [PubMed] [Google Scholar]
- 104.Wang R., Liu F., Chen P., Li S., Gu Y., Wang L., Chen C., Yuan Y.. Gomisin D alleviates liver fibrosis through targeting PDGFRβ in hepatic stellate cells. International Journal of Biological Macromolecules 235 (2023) 123639. https://doi.org/10.1016/j.ijbiomac.2023.123639 10.1016/j.ijbiomac.2023.123639 [DOI] [PubMed] [Google Scholar]
- 105.Chen Z., Liu H., Jain A., Zhang L., Liu C., Cheng K.. Discovery of aptamer ligands for hepatic stellate cells using SELEX. Theranostics 7 (2017) 2982-2995. https://doi.org/10.7150/thno.19374 10.7150/thno.19374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mohamed A., El-Tawil O., El-Rahman S.. Inhibited TLR-4/NF- κB Pathway Mediated by Cannabinoid Receptor 2 Activation Curbs Ongoing Liver Fibrosis in Bile Duct Ligated Rats. Advances in Animal and Veterinary Sciences 9 (2020) 253-264. https://doi.org/10.17582/journal.aavs/2021/9.2.253.264 10.17582/journal.aavs/2021/9.2.253.264 [DOI] [Google Scholar]
- 107.Aljobaily N., Krutsinger K., Viereckl M.J., Joly R., Menlove B., Cone B., Suppes A., Han Y.. Low-Dose Administration of Cannabigerol Attenuates Inflammation and Fibrosis Associated with Methionine/Choline Deficient Diet-Induced NASH Model via Modulation of Cannabinoid Receptor. Nutrients 15(15) (2023) 178. https://doi.org/10.3390/nu15010178 10.3390/nu15010178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.De Nunzio V., Carrieri L., Scavo M.P., Lippolis T., Cofano M., Caponio G.R., Tutino V., Rizzi F., Depalo N., Osella A.R., Notarnicola M.. Plasma-Derived Exosomes from NAFLD Patients Modulate the Cannabinoid Receptors’ Expression in Cultured HepaRG Cells. International Journal of Molecular Sciences 24(24) (2023) 1739. https://doi.org/10.3390/ijms24021739 10.3390/ijms24021739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hou L.S., Cui Z.Y., Sun P., Piao H.Q., Han X., Song J., Wang G., Zheng S., Dong X.X., Gao L., Zhu Y., Lian L.H., Nan J.X., Wu Y.L.. Rutin mitigates hepatic fibrogenesis and inflammation through targeting TLR4 and P2X7 receptor signaling pathway in vitro and in vivo. Journal of Functional Foods 64 (2020) 103700. https://doi.org/10.1016/j.jff.2019.103700 10.1016/j.jff.2019.103700 [DOI] [Google Scholar]
- 110.Tang B.X., Zhang Y., Sun D.D., Liu Q.Y., Li C., Wang P.P., Gao L.X., Zhang X.M., Li J., Zhu W.L., Zang Y.. Luteolin-7-diglucuronide, a novel PTP1B inhibitor, ameliorates hepatic stellate cell activation and liver fibrosis in mice. Acta Pharmacologica Sinica 46 (2024) 122-133. https://doi.org/10.1038/S41401-024-01351-3 10.1038/S41401-024-01351-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.El-Sayed N.M., Menze E.T., Tadros M.G., Hanna D.M.F.. Mangiferin mitigates methotrexate-induced liver injury and suppresses hepatic stellate cells activation in rats: Imperative role of Nrf2/NF-κB/NLRP3 signaling axis. Journal of Ethnopharmacology 340 (2025) 119296. https://doi.org/10.1016/J.JEP.2024.119296 10.1016/J.JEP.2024.119296 [DOI] [PubMed] [Google Scholar]
- 112.Gan L., Jiang Q., Huang D., Wu X., Zhu X., Wang L., Xie W., Huang J., Fan R., Jing Y., Tang G., Li X.D., Guo J., Yin S.. A natural small molecule alleviates liver fibrosis by targeting apolipoprotein L2. Nature Chemical Biology 21 (2024) 80-90. https://doi.org/10.1038/s41589-024-01704-3 10.1038/s41589-024-01704-3 [DOI] [PubMed] [Google Scholar]
- 113.Huang L., Chen Y., Fan X., Zhang X., Wang X., Liu L., Liu T., Wang P., Xu A., Zhao X., Cong M.. Fluorofenidone mitigates liver fibrosis through GSK-3β modulation and hepatocyte protection in a 3D tissue-engineered model. International Immunopharmacology 149 (2025) 114209. https://doi.org/10.1016/J.INTIMP.2025.114209 10.1016/J.INTIMP.2025.114209 [DOI] [PubMed] [Google Scholar]
- 114.Lv M., Chen S., Shan M., Si Y., Huang C., Chen J., Gong L.. Arctigenin induces activated HSCs quiescence via AMPK-PPARγ pathway to ameliorate liver fibrosis in mice. European Journal of Pharmacology 974 (2024) 176629. https://doi.org/10.1016/J.EJPHAR.2024.176629 10.1016/J.EJPHAR.2024.176629 [DOI] [PubMed] [Google Scholar]
- 115.ten Hove M., Smyris A., Booijink R., Wachsmuth L., Hansen U., Alic L., Faber C., Höltke C., Bansal R.. Engineered SPIONs functionalized with endothelin a receptor antagonist ameliorate liver fibrosis by inhibiting hepatic stellate cell activation. Bioactive Materials 39 (2024) 406-426. https://doi.org/10.1016/j.bioactmat.2024.05.034 10.1016/j.bioactmat.2024.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yuan M., Yin Z., Wang Z., Xiong Z., Chen P., Yao L., Liu P., Sun M., Shu K., Li L., Jiang Y.. Modification of MSCs with aHSCs-targeting peptide pPB for enhanced therapeutic efficacy in liver fibrosis. Biomaterials 321 (2025) 123295. https://doi.org/10.1016/J.BIOMATERIALS.2025.123295 10.1016/J.BIOMATERIALS.2025.123295 [DOI] [PubMed] [Google Scholar]
- 117.Yang P., Liu W., Chen Z., Duan D., Xu J., An X., Xie A., Rao Z., Xia Y., Zhang R., Ning P., Qiao C., Zhang X., Wang Z.. Sono-mediated glutamine metabolic nanoplatform against liver fibrosis via breaking the vicious self-injury of activated hepatic stellate cells. Nano Today 61 (2025) 102641. https://doi.org/10.1016/J.NANTOD.2025.102641 10.1016/J.NANTOD.2025.102641 [DOI] [Google Scholar]
- 118.Xu Y., Fan Y., Zhao Z., Hu W., Qian Y., Hu Y.. Circularized Supramolecular Spherical Nucleic Acids Alleviates Liver Fibrosis through Blocking Upstream Activation and Reversing Activation State of Hepatic Stellate Cells. ACS Nano 19(19) (2025) 15444-15456. https://doi.org/10.1021/ACSNANO.4C15562 10.1021/ACSNANO.4C15562 [DOI] [PubMed] [Google Scholar]
- 119.Huang J., Huang H., Wang Y., Xu B., Lin M., Han S., Yuan Y., Wang Y., Shuai X.. Retinol-binding protein-hijacking nanopolyplex delivering siRNA to cytoplasm of hepatic stellate cell for liver fibrosis alleviation. Biomaterials 299 (2023) 122134. https://doi.org/10.1016/j.biomaterials.2023.122134 10.1016/j.biomaterials.2023.122134 [DOI] [PubMed] [Google Scholar]
- 120.Azzam M., El Safy S., Abdelgelil S.A., Weiskirchen R., Asimakopoulou A., de Lorenzi F., Lammers T., Mansour S., Tammam S.. Targeting Activated Hepatic Stellate Cells Using Collagen-Binding Chitosan Nanoparticles for siRNA Delivery to Fibrotic Livers. Pharmaceutics 12(12) (2020) 590. https://doi.org/10.3390/pharmaceutics12060590 10.3390/pharmaceutics12060590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Younis M.A., Sato Y., Elewa Y.H.A., Harashima H.. Reprogramming activated hepatic stellate cells by siRNA-loaded nanocarriers reverses liver fibrosis in mice. Journal of Controlled Release 361 (2023) 592-603. https://doi.org/10.1016/j.jconrel.2023.08.021 10.1016/j.jconrel.2023.08.021 [DOI] [PubMed] [Google Scholar]
- 122.Liu M.X., Xu L., Cai Y.T., Wang R.J., Gu Y.Y., Liu Y.C., Zou Y.J., Zhao Y.M., Chen J., Zhang X.L.. Carbon Nitride-Based siRNA Vectors with Self-Produced O(2) Effects for Targeting Combination Therapy of Liver Fibrosis via HIF-1α-Mediated TGF-β1/Smad Pathway. Advanced Healthcare Materials 12 (2023) e2301485. https://doi.org/10.1002/adhm.202301485 10.1002/adhm.202301485 [DOI] [PubMed] [Google Scholar]
- 123.Wang L., Zhou J., Wang J., Wang X., Dong H., Zhao L., Wu J., Peng J.. Hepatic Stellate Cell-Targeting Micelle Nanomedicine for Early Diagnosis and Treatment of Liver Fibrosis. Advanced Healthcare Materials 13(13) (2024) 2303710. https://doi.org/10.1002/adhm.202303710 10.1002/adhm.202303710 [DOI] [PubMed] [Google Scholar]
- 124.Chen Y.N., Hsu S.L., Liao M.Y., Liu Y.T., Lai C.H., Chen J.F., Nguyen M.H.T., Su Y.H., Chen S.T., Wu L.C.. Ameliorative effect of curcumin-encapsulated hyaluronic acid-PLA nanoparticles on thioacetamide-induced murine hepatic fibrosis. International Journal of Environmental Research and Public Health 14(14) (2017) 11. https://doi.org/10.3390/ijerph14010011 10.3390/ijerph14010011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu Y., Chen S., Huang H., Midgley A.C., Han Z., Han Z.C., Li Q., Li Z.. Ligand-Tethered Extracellular Vesicles Mediated RNA Therapy for Liver Fibrosis. Advanced Healthcare Materials 14 (2025) 2403068. https://doi.org/10.1002/ADHM.202403068 10.1002/ADHM.202403068 [DOI] [PubMed] [Google Scholar]
- 126.Li W., Zhou C., Fu Y., Chen T., Liu X., Zhang Z., Gong T.. Targeted delivery of hyaluronic acid nanomicelles to hepatic stellate cells in hepatic fibrosis rats. Acta Pharmaceutica Sinica B 10 (2020) 693-710. https://doi.org/10.1016/j.apsb.2019.07.003 10.1016/j.apsb.2019.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang X., Zhang W., Zeng S., Wang L., Wang B.. Collagenase Type I and Probucol-Loaded Nanoparticles Penetrate the Extracellular Matrix to Target Hepatic Stellate Cells for Hepatic Fibrosis Therapy. Acta Biomaterialia 175 (2024) 262-278. https://doi.org/10.1016/j.actbio.2023.12.027 10.1016/j.actbio.2023.12.027 [DOI] [PubMed] [Google Scholar]
- 128.Xiong Y., Wu B., Guo X., Shi D., Xia H., Xu H., Liu X.. Galangin delivered by retinoic acid-modified nanoparticles targeted hepatic stellate cells for the treatment of hepatic fibrosis. RSC Advances 13 (2023) 10987-11001. https://doi.org/10.1039/d2ra07561j 10.1039/d2ra07561j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li Y., Pu S., Liu Q., Li R., Zhang J., Wu T., Chen L., Li H., Yang X., Zou M., Xiao J., Xie W., He J.. An integrin-based nanoparticle that targets activated hepatic stellate cells and alleviates liver fibrosis. Journal of Controlled Release 303 (2019) 77-90. https://doi.org/10.1016/j.jconrel.2019.04.022 10.1016/j.jconrel.2019.04.022 [DOI] [PubMed] [Google Scholar]
- 130.Li R., Zhang J., Liu Q., Tang Q., Jia Q., Xiong Y., He J., Li Y.. CREKA-modified liposomes target activated hepatic stellate cells to alleviate liver fibrosis by inhibiting collagen synthesis and angiogenesis. Acta Biomaterialia 168 (2023) 484-496. https://doi.org/10.1016/j.actbio.2023.06.032 10.1016/j.actbio.2023.06.032 [DOI] [PubMed] [Google Scholar]
- 131.Zhang Y., Li Y., Mu T., Tong N., Cheng P.. Hepatic stellate cells specific liposomes with the Toll-like receptor 4 shRNA attenuates liver fibrosis. Journal of Cellular and Molecular Medicine 25 (2021) 1299-1313. https://doi.org/10.1111/jcmm.16209 10.1111/jcmm.16209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.El-Mezayen N.S., El-Hadidy W.F., El-Refaie W.M., Shalaby Th.I., Khattab M.M., El-Khatib A.S.. Hepatic stellate cell-targeted imatinib nanomedicine versus conventional imatinib: A novel strategy with potent efficacy in experimental liver fibrosis. Journal of Controlled Release 266 (2017) 226-237. https://doi.org/10.1016/j.jconrel.2017.09.035 10.1016/j.jconrel.2017.09.035 [DOI] [PubMed] [Google Scholar]
- 133.Hao Y., Song K., Tan X., Ren L., Guo X., Zhou C., Li H., Wen J., Meng Y., Lin M., Zhang Y., Huang H., Wang L., Zheng W.. Reactive Oxygen Species-Responsive Polypeptide Drug Delivery System Targeted Activated Hepatic Stellate Cells to Ameliorate Liver Fibrosis. ACS Nano 16 (2022) 20739-20757. https://doi.org/10.1021/acsnano.2c07796 10.1021/acsnano.2c07796 [DOI] [PubMed] [Google Scholar]
- 134.Morsy M.A., Nair A.B.. Prevention of rat liver fibrosis by selective targeting of hepatic stellate cells using hesperidin carriers. International Journal of Pharmaceutics 552 (2018) 241-250. https://doi.org/10.1016/j.ijpharm.2018.10.003 10.1016/j.ijpharm.2018.10.003 [DOI] [PubMed] [Google Scholar]
- 135.Wu J., Qi C., Wang H., Wang Q., Sun J., Dong J., Yu G., Gao Z., Zhang B., Tian G.. Curcumin and berberine co-loaded liposomes for anti-hepatocellular carcinoma therapy by blocking the cross-talk between hepatic stellate cells and tumor cells. Frontiers in Pharmacology 13 (2022) 961788. https://doi.org/10.3389/fphar.2022.961788 10.3389/fphar.2022.961788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Omar R., Yang J., Alrushaid S., Burczynski F.J., Minuk G.Y., Gong Y.. Inhibition of BMP4 and Alpha Smooth Muscle Actin Expression in LX-2 Hepatic Stellate Cells by BMP4-siRNA Lipid Based Nanoparticle. Journal of Pharmacy and Pharmaceutical Sciences 21 (2019) 119-134. https://doi.org/10.18433/jpps29584 10.18433/jpps29584 [DOI] [PubMed] [Google Scholar]
- 137.Ullah A., Wang K., Wu P., Oupicky D., Sun M.. CXCR4-targeted liposomal mediated co-delivery of pirfenidone and AMD3100 for the treatment of TGFΒ-induced HSC-T6 cells activation. International Journal of Nanomedicine 14 (2019) 2927-2944. https://doi.org/10.2147/IJN.S171280 10.2147/IJN.S171280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Valentino G., Zivko C., Weber F., Brülisauer L., Luciani P., Synergy of phospholipid-drug formulations significantly deactivates profibrogenic human hepatic stellate cells. Pharmaceutics 11(11) (2019) 676. https://doi.org/10.3390/pharmaceutics11120676 10.3390/pharmaceutics11120676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fan Q.Q., Zhang C.L., Qiao J.B., Cui P.F., Xing L., Oh Y.K., Jiang H.L.. Extracellular matrix-penetrating nanodrill micelles for liver fibrosis therapy. Biomaterials 230 (2020) 119616. https://doi.org/10.1016/j.biomaterials.2019.119616 10.1016/j.biomaterials.2019.119616 [DOI] [PubMed] [Google Scholar]
- 140.Wang M., Zhang M., Fu L., Lin J., Zhou X., Zhou P., Huang P., Hu H., Han Y.. Liver-targeted delivery of TSG-6 by calcium phosphate nanoparticles for the management of liver fibrosis. Theranostics 10 (2020) 36-49. https://doi.org/10.7150/thno.37301 10.7150/thno.37301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang J., Ding Y., Zhou W.. Albumin self-modified liposomes for hepatic fibrosis therapy via SPARC-dependent pathways. International Journal of Pharmaceutics 574 (2020) 118940. https://doi.org/10.1016/j.ijpharm.2019.118940 10.1016/j.ijpharm.2019.118940 [DOI] [PubMed] [Google Scholar]
- 142.Qi C., Wang D., Gong X., Zhou Q., Yue X., Li C., Li Z., Tian G., Zhang B., Wang Q., Wei X., Wu J.. Co-Delivery of Curcumin and Capsaicin by Dual-Targeting Liposomes for Inhibition of aHSC-Induced Drug Resistance and Metastasis. ACS Applied Materials Interfaces 13 (2021) 16019-16035. https://doi.org/10.1021/acsami.0c23137 10.1021/acsami.0c23137 [DOI] [PubMed] [Google Scholar]
- 143.Luo S., Yang Y., Zhao T., Zhang R., Fang C., Li Y., Zhang Z., Gong T.. Albumin-Based Silibinin Nanocrystals Targeting Activated Hepatic Stellate Cells for Liver Fibrosis Therapy. ACS Applied Materials Interfaces 15 (2023) 7747-7758. https://doi.org/10.1021/acsami.2c19269 10.1021/acsami.2c19269 [DOI] [PubMed] [Google Scholar]
- 144.You D.G., Oh B.H., Nguyen V.Q., Lim G.T., Um W., Jung J.M., Jeon J., Choi J.S., Choi Y.C., Jung Y.J., Lee J., Jo D.G., Cho Y.W., Park J.H.. Vitamin A-coupled stem cell-derived extracellular vesicles regulate the fibrotic cascade by targeting activated hepatic stellate cells in vivo. Journal of Controlled Release 336 (2021) 285-295. https://doi.org/10.1016/j.jconrel.2021.06.031 10.1016/j.jconrel.2021.06.031 [DOI] [PubMed] [Google Scholar]
- 145.Tan Y., Wang Z., Guo R., Zhou X., Zhang W., Wu M., Guo C., Gao H., Sun X., Zhang Z., Gong T.. Dual-Targeting Macrophages and Hepatic Stellate Cells by Modified Albumin Nanoparticles for Liver Cirrhosis Treatment. ACS Applied Materials Interfaces 16 (2024) 11239-11250. https://doi.org/10.1021/acsami.3c17670 10.1021/acsami.3c17670 [DOI] [PubMed] [Google Scholar]
- 146.Sun L., Luo X., Zhou C., Zhou Z., Sun M.. Natural polysaccharide-based smart CXCR4-targeted nano-system for magnified liver fibrosis therapy. Chinese Chemical Letters 35 (2024) 108803. https://doi.org/10.1016/j.cclet.2023.108803 10.1016/j.cclet.2023.108803 [DOI] [Google Scholar]
- 147.Wu Z.C., Liu X.Y., Liu J.Y., Piao J.S., Piao M.G.. Preparation of Betulinic Acid Galactosylated Chitosan Nanoparticles and Their Effect on Liver Fibrosis. International Journal of Nanomedicine 17 (2022) 4195-4210. https://doi.org/10.2147/IJN.S373430 10.2147/IJN.S373430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Su X., Zhong H., Zeng Y., Zhang Y., Zhang B., Guo W., Huang Q., Ye Y.. Dual-ligand-functionalized nanostructured lipid carriers as a novel dehydrocavidine delivery system for liver fibrosis therapy. Colloids and Surfaces B 246 (2025) 114376. https://doi.org/10.1016/J.COLSURFB.2024.114376 10.1016/J.COLSURFB.2024.114376 [DOI] [PubMed] [Google Scholar]
- 149.Niu X., Chang G., Xu N., Li R., Niu B., Mao R., Wang S., Li G., Jiang J., Wang L.. Vitamin A-Integrated Cinnamaldehyde Nanoemulsion: A Nanotherapeutic Approach To Counteract Liver Fibrosis via Gut-Liver Axis Modulation. ACS Nano 19(19) (2025) 10433-10451. https://doi.org/10.1021/ACSNANO.5C00136 10.1021/ACSNANO.5C00136 [DOI] [PubMed] [Google Scholar]
- 150.Hu P., Su L., Wang Y., Chen Y., Tian X., Qian Y.. Targeting Liver Fibrosis with Nanoparticle Technology: The Dual-Drug Strategy for Hepatic Stellate Cell Activation Inhibition. ACS APplied Materials and Interfaces 17(17) (2025) 25071-25082. https://doi.org/10.1021/acsami.5c02796 10.1021/acsami.5c02796 [DOI] [PubMed] [Google Scholar]
- 151.Su X., Zhong H., Zeng Y., Zhang Y., Zhang B., Guo W., Huang Q., Ye Y.. Dual-ligand-functionalized nanostructured lipid carriers as a novel dehydrocavidine delivery system for liver fibrosis therapy. Colloids and Surfaces B 246 (2025) 114376. https://doi.org/10.1016/j.colsurfb.2024.114376 10.1016/j.colsurfb.2024.114376 [DOI] [PubMed] [Google Scholar]
- 152.Liu H., Qi Y., Wang L., Zhang Y., Chen H., Zhao Y., Min H., Nie G., Liang H.. Lysosome-Targeting Nanochimeras Attenuating Liver Fibrosis by Interconnected Transforming Growth Factor-β Reduction and Activin Receptor-Like Kinase 5 Degradation. ACS Nano 19 (2025) 25645-25661. https://doi.org/10.1021/acsnano.5c00985 10.1021/acsnano.5c00985 [DOI] [PubMed] [Google Scholar]
- 153.Togami K., Kanehira Y., Nakamura Y., Ishii H., Abe R., Yamamoto A., Takehara K., Yasuda M., Tada H., Chono S.. Pirfenidone encapsulated in succinylated gelatin-coated liposomes exhibits sustained antifibrotic effects in vitro models of renal, pulmonary, and hepatic fibrosis. Journal of Pharmacetical Sciences 114 (2025) 103819. https://doi.org/10.1016/j.xphs.2025.103819 10.1016/j.xphs.2025.103819 [DOI] [PubMed] [Google Scholar]
- 154.Liu X., Fang C., Yu H., Huang L., Feng J., Luo S., Song L., Wu M., Tan Y., Dong J., Gong T., Xiao P.. Chondroitin Sulfate-Based Imatinib Nanoparticles Targeting Activated Hepatic Stellate Cells Against Hepatic Fibrosis. Pharmaceutics 17 (2025) 351. https://doi.org/10.3390/pharmaceutics17030351 10.3390/pharmaceutics17030351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lu M., Dai Z., Lin Y., Sun C., Li S., Guo Z., Liu Y., Liu X., Li S., Liu R., Xu B., Lei H.. Co-Assembled Nanoparticles Comprising Sorafenib and Hederagenin Derivative for Enhanced Anti-Hepatic Fibrosis Activity. International Journal of Nanomedicine 20 (2025) 9135-9153. https://doi.org/10.2147/IJN.S512005 10.2147/IJN.S512005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yang R., Yang L., Zhang N., Wan Y., Chen S., Xiao Y., Liang X., Yang S., Zhong Y., Huang D., Chen W., Zhao B.. Targeted delivery of polymeric NO-donor micelles to hepatic stellate cells for restoration of liver function and inhibition of hepatic fibrosis. Journal of Controlled Release 379 (2025) 466-477. https://doi.org/10.1016/j.jconrel.2025.01.036 10.1016/j.jconrel.2025.01.036 [DOI] [PubMed] [Google Scholar]
- 157.Mohsen M.K., azm Diab S.A.E., Shafik A.N., Osman A.H., Naguib M.J., Kamel A.M., Mehesen M.N.. Sofosbuvir’s hepatoprotective efficacy in rats is enhanced by encapsulating in taurocholate-stabilized galactose-anchored bilosomes. Future Journal of Pharmaceutical Sciences 11 (2025) 21. https://doi.org/10.1186/s43094-025-00775-w 10.1186/s43094-025-00775-w [DOI] [Google Scholar]
- 158.Mokhtariye A., Varshosaz J., Mohammadalipour A., Hashemnia M., Mofid M.R.. Xanthosomes loaded with recombinant TMEM219 for targeting IGFBP-3: A novel approach for targeted drug delivery to ameliorate liver fibrosis. Life Sciences 377 (2025) 123748. https://doi.org/10.1016/j.lfs.2025.123748 10.1016/j.lfs.2025.123748 [DOI] [PubMed] [Google Scholar]
- 159.Li R., Tai Y., Zhang X., Liu Z., Si H., Kong D., Zhao L., Li J., Midgley A.C.. Tissue-Microenvironment-Responsive Self-Assembling Peptide Nanoshells Boost Pirfenidone Efficacy in the Treatment of Liver Fibrosis. Advanced Healthcare Materials 14 (2025) 2500101. https://doi.org/10.1002/adhm.202500101 10.1002/adhm.202500101 [DOI] [PubMed] [Google Scholar]
- 160.Xiang X., Shao Y., Xiang L., Jiao Q., Zhang W., Qin Y., Chen Y.. Suppression of Liver Fibrogenesis with Photothermal Sorafenib Nanovesicles via Selectively Inhibiting Glycolysis and Amplification of Active HSCs. Molecular Pharmaceutics 22 (2025) 1939-1957. https://doi.org/10.1021/acs.molpharmaceut.4c01135 10.1021/acs.molpharmaceut.4c01135 [DOI] [PubMed] [Google Scholar]
- 161.Liu X., Mu X., Wang Y., Liu Z., Li Y., Lan J., Feng S., Wang S., Zhao Q.. Metal-based mesoporous polydopamine with dual enzyme-like activity as biomimetic nanodrug for alleviating liver fibrosis. Journal of Colloid and Interface Science 684 (2025) 586-599. https://doi.org/10.1016/j.jcis.2025.01.081 10.1016/j.jcis.2025.01.081 [DOI] [PubMed] [Google Scholar]


