Abstract
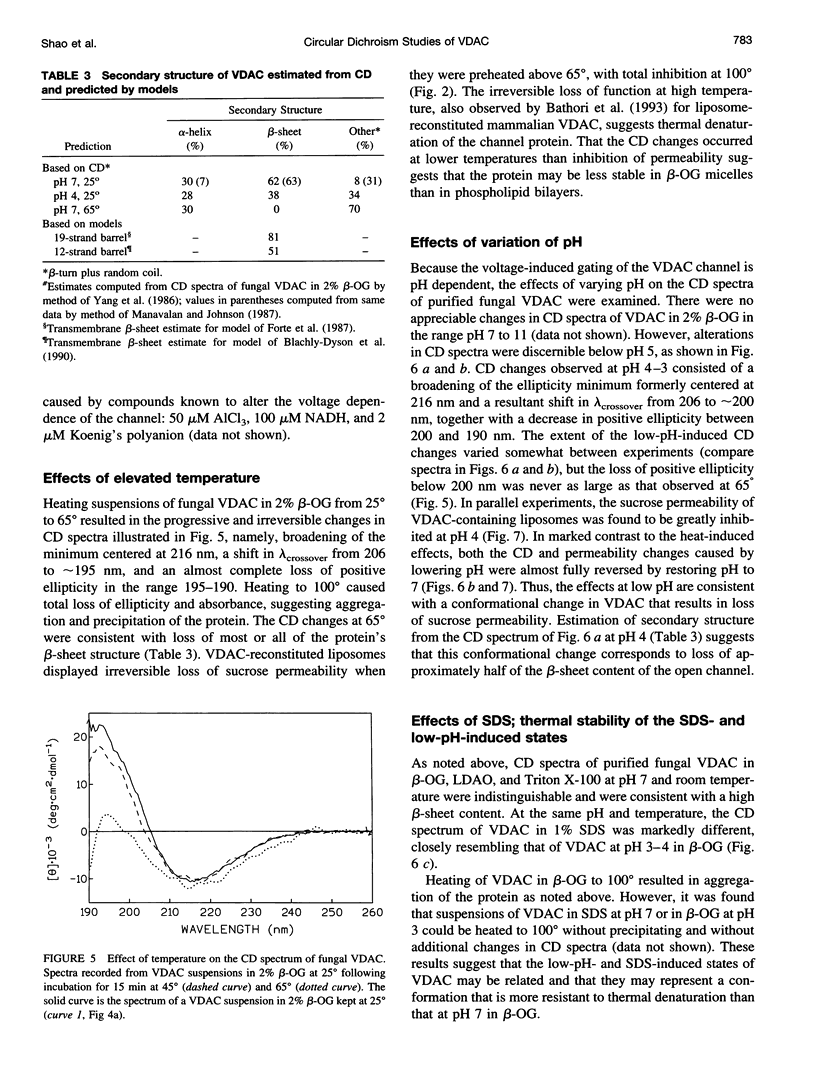
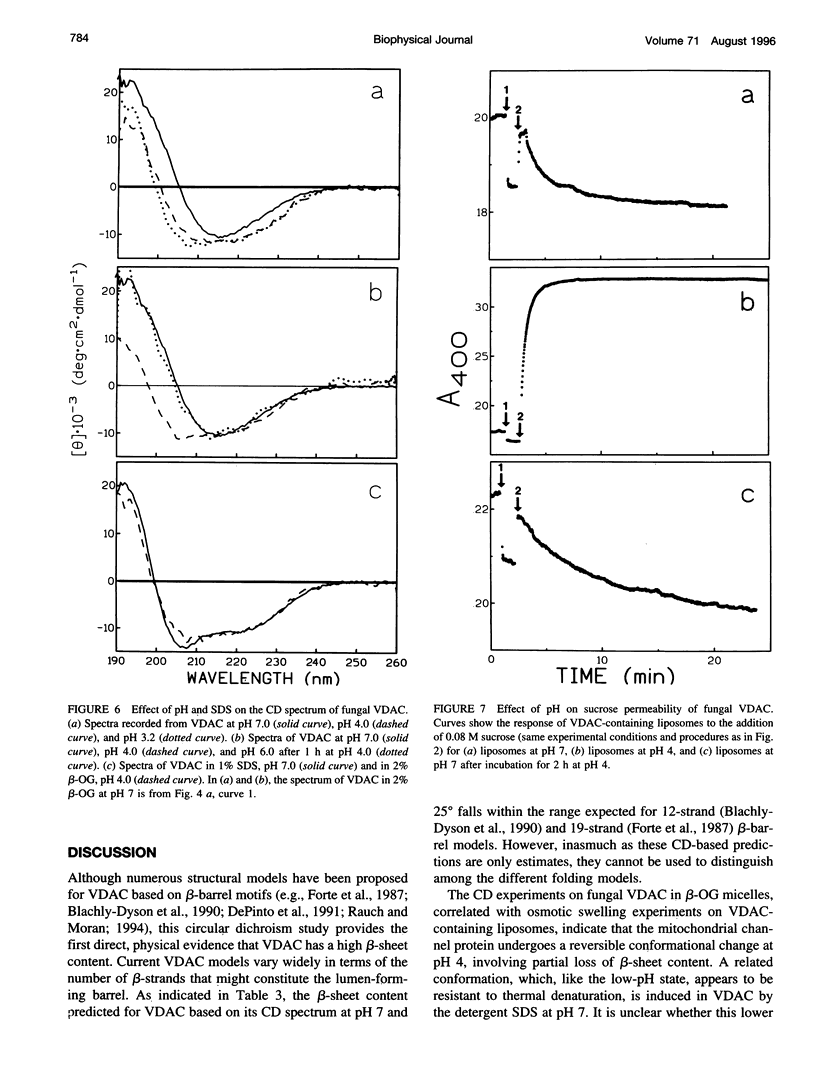
The protein that forms the voltage-gated channel VDAC (or mitochondrial porin) has been purified from Neurospora crassa. At room temperature and pH 7, the circular dichoism (CD) spectrum of VDAC suspended in octyl beta-glucoside is similar to those of bacterial porins, consistent with a high beta-sheet content. When VDAC is reconstituted into phospholipid liposomes at pH 7, a similar CD spectrum is obtained and the liposomes are rendered permeable to sucrose. Heating VDAC in octyl beta-glucoside or in liposomes results in thermal denaturation. The CD spectrum irreversibly changes to one consistent with total loss of beta-sheet content, and VDAC-containing liposomes irreversibly lose sucrose permeability. When VDAC is suspended at room temperature in octyl beta-glucoside at pH < 5 or in sodium dodecyl sulfate at pH 7, its CD spectrum is consistent with partial loss of beta-sheet content. The sucrose permeability of VDAC-containing liposomes is decreased at low pH and restored at pH 7. Similarly, the pH-dependent changes in the CD spectrum of VDAC suspended in octyl beta-glucoside also are reversible. These results suggest that VDAC undergoes a reversible conformational change at low pH involving reduced beta-sheet content and loss of pore-forming activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R., Wojtczak L., Bosch W., Brdiczka D. Inhibition of adenine nucleotide transport through the mitochondrial porin by a synthetic polyanion. FEBS Lett. 1988 Apr 11;231(1):75–80. doi: 10.1016/0014-5793(88)80706-3. [DOI] [PubMed] [Google Scholar]
- Blachly-Dyson E., Peng S., Colombini M., Forte M. Selectivity changes in site-directed mutants of the VDAC ion channel: structural implications. Science. 1990 Mar 9;247(4947):1233–1236. doi: 10.1126/science.1690454. [DOI] [PubMed] [Google Scholar]
- Bowen K. A., Tam K., Colombini M. Evidence for titratable gating charges controlling the voltage dependence of the outer mitochondrial membrane channel, VDAC. J Membr Biol. 1985;86(1):51–59. doi: 10.1007/BF01871610. [DOI] [PubMed] [Google Scholar]
- Báthori G., Sahin-Tóth M., Fonyó A., Ligeti E. Transport properties and inhibitor sensitivity of isolated and reconstituted porin differ from those of intact mitochondria. Biochim Biophys Acta. 1993 Jan 18;1145(1):168–176. doi: 10.1016/0005-2736(93)90394-f. [DOI] [PubMed] [Google Scholar]
- Chevalier G., Duclohier H., Thomas D., Shechter E., Wróblewski H. Purification and characterization of protein H, the major porin of Pasteurella multocida. J Bacteriol. 1993 Jan;175(1):266–276. doi: 10.1128/jb.175.1.266-276.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombini M. A candidate for the permeability pathway of the outer mitochondrial membrane. Nature. 1979 Jun 14;279(5714):643–645. doi: 10.1038/279643a0. [DOI] [PubMed] [Google Scholar]
- Colombini M. Structure and mode of action of a voltage dependent anion-selective channel (VDAC) located in the outer mitochondrial membrane. Ann N Y Acad Sci. 1980;341:552–563. doi: 10.1111/j.1749-6632.1980.tb47198.x. [DOI] [PubMed] [Google Scholar]
- Colombini M., Yeung C. L., Tung J., König T. The mitochondrial outer membrane channel, VDAC, is regulated by a synthetic polyanion. Biochim Biophys Acta. 1987 Dec 11;905(2):279–286. doi: 10.1016/0005-2736(87)90456-1. [DOI] [PubMed] [Google Scholar]
- Cowan S. W., Schirmer T., Rummel G., Steiert M., Ghosh R., Pauptit R. A., Jansonius J. N., Rosenbusch J. P. Crystal structures explain functional properties of two E. coli porins. Nature. 1992 Aug 27;358(6389):727–733. doi: 10.1038/358727a0. [DOI] [PubMed] [Google Scholar]
- De Pinto V., Benz R., Palmieri F. Interaction of non-classical detergents with the mitochondrial porin. A new purification procedure and characterization of the pore-forming unit. Eur J Biochem. 1989 Jul 15;183(1):179–187. doi: 10.1111/j.1432-1033.1989.tb14911.x. [DOI] [PubMed] [Google Scholar]
- De Pinto V., Prezioso G., Thinnes F., Link T. A., Palmieri F. Peptide-specific antibodies and proteases as probes of the transmembrane topology of the bovine heart mitochondrial porin. Biochemistry. 1991 Oct 22;30(42):10191–10200. doi: 10.1021/bi00106a017. [DOI] [PubMed] [Google Scholar]
- Dill E. T., Holden M. J., Colombini M. Voltage gating in VDAC is markedly inhibited by micromolar quantities of aluminum. J Membr Biol. 1987;99(3):187–196. doi: 10.1007/BF01995699. [DOI] [PubMed] [Google Scholar]
- Forte M., Guy H. R., Mannella C. A. Molecular genetics of the VDAC ion channel: structural model and sequence analysis. J Bioenerg Biomembr. 1987 Aug;19(4):341–350. doi: 10.1007/BF00768537. [DOI] [PubMed] [Google Scholar]
- Freitag H., Neupert W., Benz R. Purification and characterisation of a pore protein of the outer mitochondrial membrane from Neurospora crassa. Eur J Biochem. 1982 Apr;123(3):629–636. doi: 10.1111/j.1432-1033.1982.tb06578.x. [DOI] [PubMed] [Google Scholar]
- Guo X. W., Smith P. R., Cognon B., D'Arcangelis D., Dolginova E., Mannella C. A. Molecular design of the voltage-dependent, anion-selective channel in the mitochondrial outer membrane. J Struct Biol. 1995 Jan-Feb;114(1):41–59. doi: 10.1006/jsbi.1995.1004. [DOI] [PubMed] [Google Scholar]
- Holden M. J., Colombini M. The outer mitochondrial membrane channel, VDAC, is modulated by a protein localized in the intermembrane space. Biochim Biophys Acta. 1993 Oct 4;1144(3):396–402. doi: 10.1016/0005-2728(93)90126-z. [DOI] [PubMed] [Google Scholar]
- Holzenburg A., Engel A., Kessler R., Manz H. J., Lustig A., Aebi U. Rapid isolation of OmpF porin-LPS complexes suitable for structure-function studies. Biochemistry. 1989 May 16;28(10):4187–4193. doi: 10.1021/bi00436a010. [DOI] [PubMed] [Google Scholar]
- Kinnally K. W., Zorov D. B., Antonenko Y. N., Snyder S. H., McEnery M. W., Tedeschi H. Mitochondrial benzodiazepine receptor linked to inner membrane ion channels by nanomolar actions of ligands. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1374–1378. doi: 10.1073/pnas.90.4.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene R., Pfanner N., Pfaller R., Link T. A., Sebald W., Neupert W., Tropschug M. Mitochondrial porin of Neurospora crassa: cDNA cloning, in vitro expression and import into mitochondria. EMBO J. 1987 Sep;6(9):2627–2633. doi: 10.1002/j.1460-2075.1987.tb02553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindén M., Gellerfors P., Nelson B. D. Purification of a protein having pore forming activity from the rat liver mitochondrial outer membrane. Biochem J. 1982 Oct 15;208(1):77–82. doi: 10.1042/bj2080077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manavalan P., Johnson W. C., Jr Variable selection method improves the prediction of protein secondary structure from circular dichroism spectra. Anal Biochem. 1987 Nov 15;167(1):76–85. doi: 10.1016/0003-2697(87)90135-7. [DOI] [PubMed] [Google Scholar]
- Mannella C. A., Forte M., Colombini M. Toward the molecular structure of the mitochondrial channel, VDAC. J Bioenerg Biomembr. 1992 Feb;24(1):7–19. doi: 10.1007/BF00769525. [DOI] [PubMed] [Google Scholar]
- Mannella C. A., Neuwald A. F., Lawrence C. E. Detection of likely transmembrane beta strand regions in sequences of mitochondrial pore proteins using the Gibbs sampler. J Bioenerg Biomembr. 1996 Apr;28(2):163–169. doi: 10.1007/BF02110647. [DOI] [PubMed] [Google Scholar]
- Mannella C. A. Structure of the outer mitochondrial membrane: ordered arrays of porelike subunits in outer-membrane fractions from Neurospora crassa mitochondria. J Cell Biol. 1982 Sep;94(3):680–687. doi: 10.1083/jcb.94.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovic-Housley Z., Garavito R. M. Effect of temperature and low pH on structure and stability of matrix porin in micellar detergent solutions. Biochim Biophys Acta. 1986 Jan 30;869(2):158–170. doi: 10.1016/0167-4838(86)90290-6. [DOI] [PubMed] [Google Scholar]
- Mirzabekov T. A., Ermishkin L. N. The gate of mitochondrial porin channel is controlled by a number of negative and positive charges. FEBS Lett. 1989 Jun 5;249(2):375–378. doi: 10.1016/0014-5793(89)80662-3. [DOI] [PubMed] [Google Scholar]
- Neuwald A. F., Liu J. S., Lawrence C. E. Gibbs motif sampling: detection of bacterial outer membrane protein repeats. Protein Sci. 1995 Aug;4(8):1618–1632. doi: 10.1002/pro.5560040820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S., Blachly-Dyson E., Forte M., Colombini M. Large scale rearrangement of protein domains is associated with voltage gating of the VDAC channel. Biophys J. 1992 Apr;62(1):123–135. doi: 10.1016/S0006-3495(92)81799-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller R., Neupert W. High-affinity binding sites involved in the import of porin into mitochondria. EMBO J. 1987 Sep;6(9):2635–2642. doi: 10.1002/j.1460-2075.1987.tb02554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch G., Moran O. On the structure of mitochondrial porins and its homologies with bacterial porins. Biochem Biophys Res Commun. 1994 Apr 29;200(2):908–915. doi: 10.1006/bbrc.1994.1536. [DOI] [PubMed] [Google Scholar]
- Schindler M., Rosenbusch J. P. Structural transitions of porin, a transmembrane protein. FEBS Lett. 1984 Jul 23;173(1):85–89. doi: 10.1016/0014-5793(84)81022-4. [DOI] [PubMed] [Google Scholar]
- Stanley S., Dias J. A., D'Arcangelis D., Mannella C. A. Peptide-specific antibodies as probes of the topography of the voltage-gated channel in the mitochondrial outer membrane of Neurospora crassa. J Biol Chem. 1995 Jul 14;270(28):16694–16700. doi: 10.1074/jbc.270.28.16694. [DOI] [PubMed] [Google Scholar]
- Surrey T., Jähnig F. Kinetics of folding and membrane insertion of a beta-barrel membrane protein. J Biol Chem. 1995 Nov 24;270(47):28199–28203. doi: 10.1074/jbc.270.47.28199. [DOI] [PubMed] [Google Scholar]
- Tokunaga M., Tokunaga H., Okajima Y., Nakae T. Characterization of porins from the outer membrane of Salmonella typhimurium. 2. Physical properties of the functional oligomeric aggregates. Eur J Biochem. 1979 Apr;95(3):441–448. doi: 10.1111/j.1432-1033.1979.tb12983.x. [DOI] [PubMed] [Google Scholar]
- Weiss M. S., Wacker T., Weckesser J., Welte W., Schulz G. E. The three-dimensional structure of porin from Rhodobacter capsulatus at 3 A resolution. FEBS Lett. 1990 Jul 16;267(2):268–272. doi: 10.1016/0014-5793(90)80942-c. [DOI] [PubMed] [Google Scholar]
- Worobec E. A., Martin N. L., McCubbin W. D., Kay C. M., Brayer G. D., Hancock R. E. Large-scale purification and biochemical characterization of crystallization-grade porin protein P from Pseudomonas aeruginosa. Biochim Biophys Acta. 1988 Apr 7;939(2):366–374. doi: 10.1016/0005-2736(88)90082-x. [DOI] [PubMed] [Google Scholar]
- Wunder U. R., Colombini M. Patch clamping VDAC in liposomes containing whole mitochondrial membranes. J Membr Biol. 1991 Jul;123(1):83–91. doi: 10.1007/BF01993966. [DOI] [PubMed] [Google Scholar]
- Yang J. T., Wu C. S., Martinez H. M. Calculation of protein conformation from circular dichroism. Methods Enzymol. 1986;130:208–269. doi: 10.1016/0076-6879(86)30013-2. [DOI] [PubMed] [Google Scholar]
- Zizi M., Forte M., Blachly-Dyson E., Colombini M. NADH regulates the gating of VDAC, the mitochondrial outer membrane channel. J Biol Chem. 1994 Jan 21;269(3):1614–1616. [PubMed] [Google Scholar]