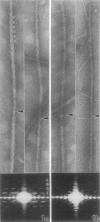
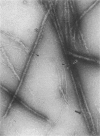
Abstract
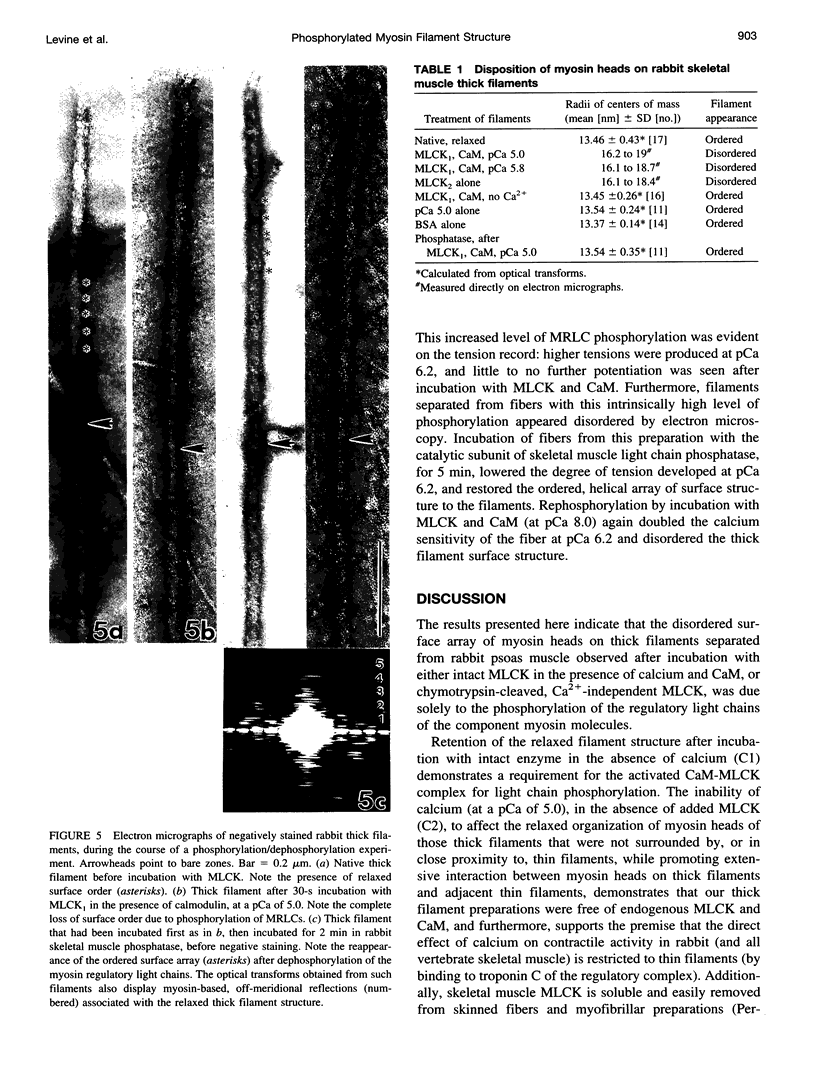
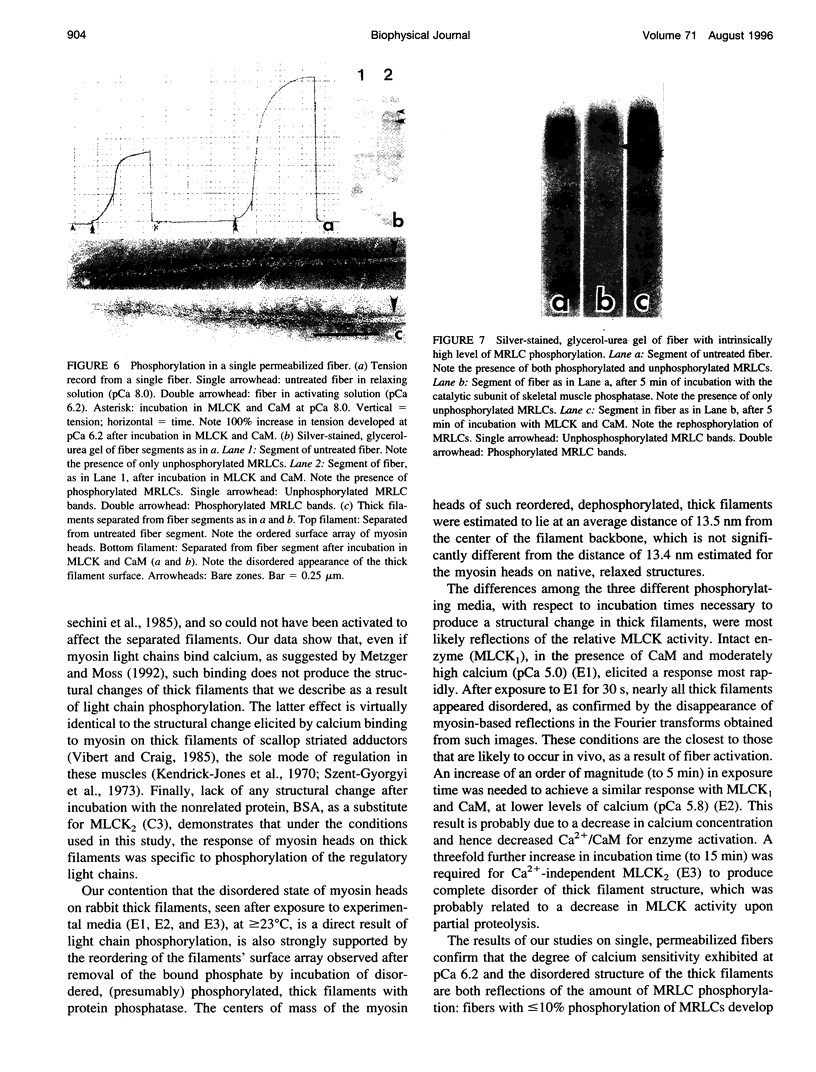
To identify the structural basis for the observed physiological effects of myosin regulatory light chain phosphorylation in skinned rabbit skeletal muscle fibers (potentiation of force development at low calcium), thick filaments separated from the muscle in the relaxed state, with unphoshorylated light chains, were incubated with specific, intact, myosin light chain kinase at moderate (pCa 5.0) and low (pCa 5.8) calcium and with calcium-independent enzyme in the absence of calcium, then examined as negatively stained preparations, by electron microscopy and optical diffraction. All such experimental filaments became disordered (lost the near-helical array of surface myosin heads typical of the relaxed state). Filaments incubated in control media, including intact enzyme in the absence of calcium, moderate calcium (pCa 5.0) without enzyme, and bovine serum albumin substituting for calcium-independent myosin light chain kinase, all retained their relaxed structure. Finally, filaments disordered by phosphorylation regained their relaxed structure after incubation with a protein phosphatase catalytic subunit. We suggest that the observed disorder is due to phosphorylation-induced increased mobility and/or changed conformation of myosin heads, which places an increased population of them close to thin filaments, thereby potentiating actin-myosin interaction at low calcium levels.
Full text
PDF

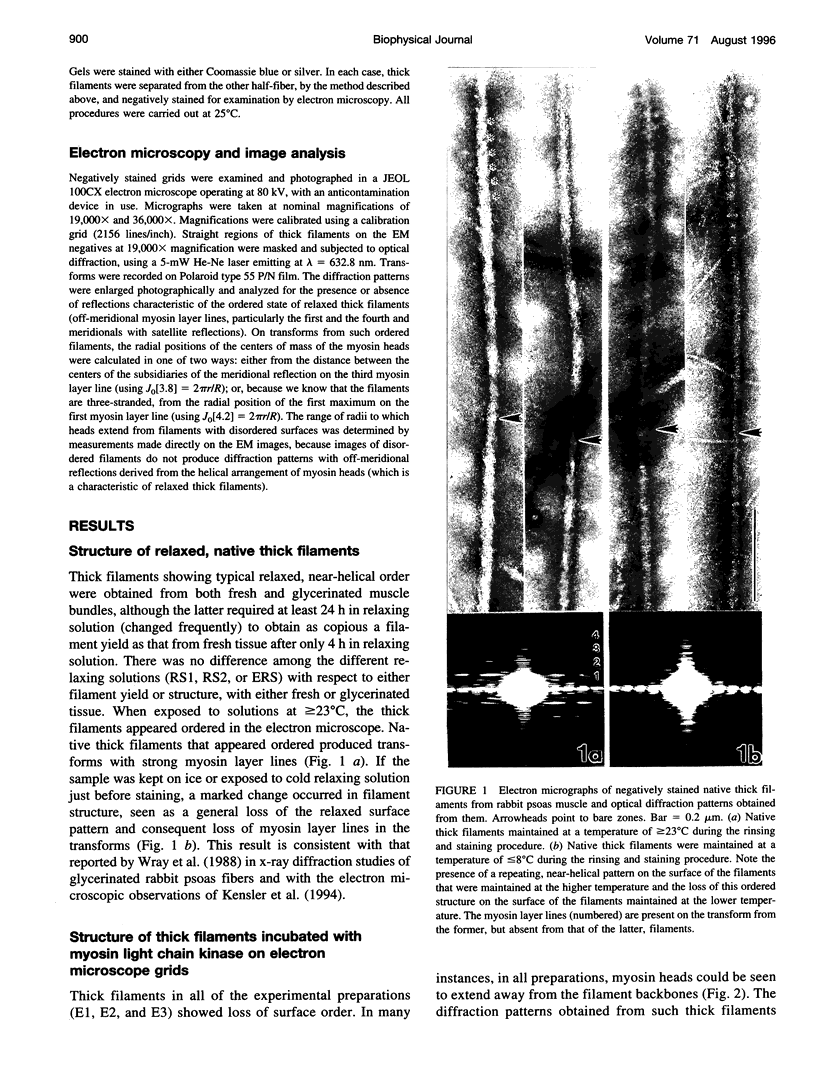
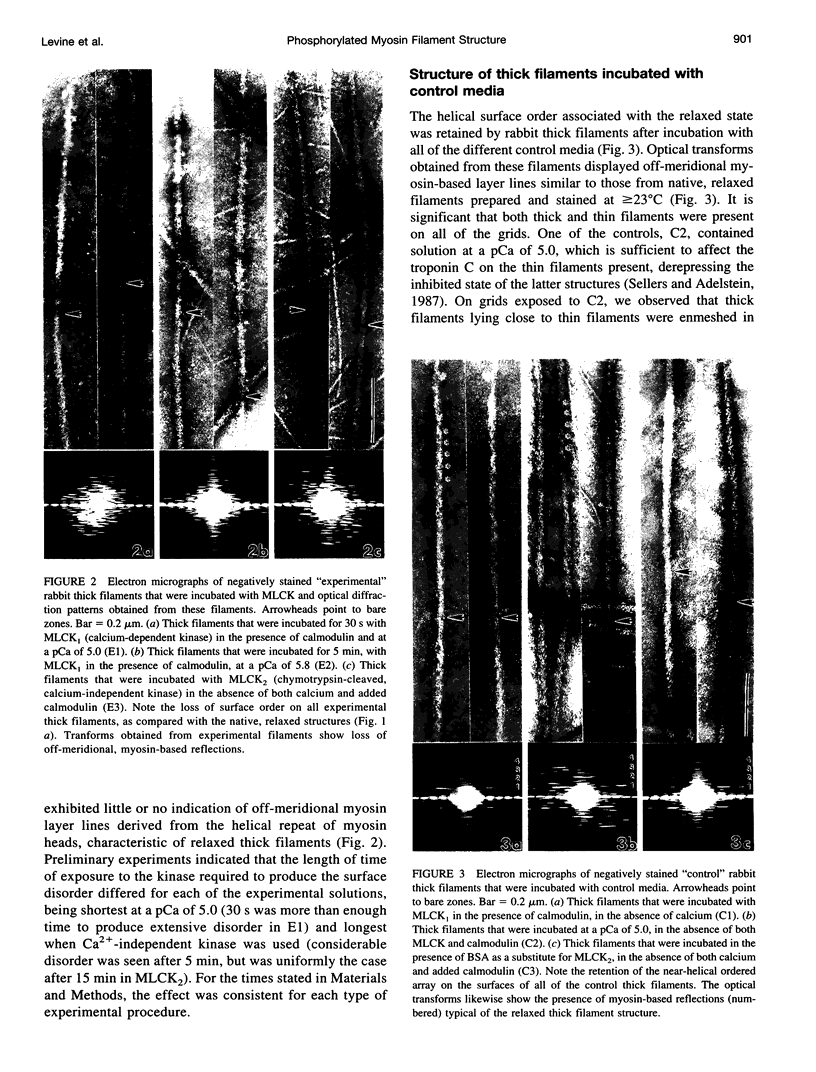




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bárány K., Bárány M., Gillis J. M., Kushmerick M. J. Phosphorylation-dephosphorylation of the 18,000-dalton light chain of myosin during the contraction-relaxation cycle of frog muscle. J Biol Chem. 1979 May 10;254(9):3617–3623. [PubMed] [Google Scholar]
- Craig R., Padrón R., Kendrick-Jones J. Structural changes accompanying phosphorylation of tarantula muscle myosin filaments. J Cell Biol. 1987 Sep;105(3):1319–1327. doi: 10.1083/jcb.105.3.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther R. A., Padrón R., Craig R. Arrangement of the heads of myosin in relaxed thick filaments from tarantula muscle. J Mol Biol. 1985 Aug 5;184(3):429–439. doi: 10.1016/0022-2836(85)90292-x. [DOI] [PubMed] [Google Scholar]
- Eastwood A. B., Wood D. S., Bock K. L., Sorenson M. M. Chemically skinned mammalian skeletal muscle. I. The structure of skinned rabbit psoas. Tissue Cell. 1979;11(3):553–566. doi: 10.1016/0040-8166(79)90062-4. [DOI] [PubMed] [Google Scholar]
- Fisher A. J., Smith C. A., Thoden J., Smith R., Sutoh K., Holden H. M., Rayment I. Structural studies of myosin:nucleotide complexes: a revised model for the molecular basis of muscle contraction. Biophys J. 1995 Apr;68(4 Suppl):19S–28S. [PMC free article] [PubMed] [Google Scholar]
- Goodno C. C. Inhibition of myosin ATPase by vanadate ion. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2620–2624. doi: 10.1073/pnas.76.6.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler R. W., Levine R. J. An electron microscopic and optical diffraction analysis of the structure of Limulus telson muscle thick filaments. J Cell Biol. 1982 Feb;92(2):443–451. doi: 10.1083/jcb.92.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler R. W., Peterson S., Norberg M. The effects of changes in temperature or ionic strength on isolated rabbit and fish skeletal muscle thick filaments. J Muscle Res Cell Motil. 1994 Feb;15(1):69–79. doi: 10.1007/BF00123834. [DOI] [PubMed] [Google Scholar]
- Kensler R. W., Stewart M. AN ultrastructural study of cross-bridge arrangement in the frog thigh muscle thick filament. Biophys J. 1986 Jan;49(1):343–351. doi: 10.1016/S0006-3495(86)83647-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler R. W., Stewart M. An ultrastructural study of crossbridge arrangement in the fish skeletal muscle thick filament. J Cell Sci. 1989 Nov;94(Pt 3):391–401. doi: 10.1242/jcs.94.3.391. [DOI] [PubMed] [Google Scholar]
- Kensler R. W., Stewart M. The relaxed crossbridge pattern in isolated rabbit psoas muscle thick filaments. J Cell Sci. 1993 Jul;105(Pt 3):841–848. doi: 10.1242/jcs.105.3.841. [DOI] [PubMed] [Google Scholar]
- Kerrick W. G., Bolles L. L. Regulation of Ca2+-activated tension in limulus striated muscle. Pflugers Arch. 1981 Dec;392(2):121–124. doi: 10.1007/BF00581259. [DOI] [PubMed] [Google Scholar]
- Levine R. J., Chantler P. D., Kensler R. W., Woodhead J. L. Effects of phosphorylation by myosin light chain kinase on the structure of Limulus thick filaments. J Cell Biol. 1991 May;113(3):563–572. doi: 10.1083/jcb.113.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R. J., Kensler R. W., Reedy M. C., Hofmann W., King H. A. Structure and paramyosin content of tarantula thick filaments. J Cell Biol. 1983 Jul;97(1):186–195. doi: 10.1083/jcb.97.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R. J., Kensler R. W., Yang Z., Sweeney H. L. Myosin regulatory light chain phosphorylation and the production of functionally significant changes in myosin head arrangement on striated muscle thick filaments. Biophys J. 1995 Apr;68(4 Suppl):224S–224S. [PMC free article] [PubMed] [Google Scholar]
- Magid A., Ting-Beall H. P., Carvell M., Kontis T., Lucaveche C. Connecting filaments, core filaments, and side-struts: a proposal to add three new load-bearing structures to the sliding filament model. Adv Exp Med Biol. 1984;170:307–328. doi: 10.1007/978-1-4684-4703-3_26. [DOI] [PubMed] [Google Scholar]
- Manning D. R., Stull J. T. Myosin light chain phosphorylation and phosphorylase A activity in rat extensor digitorum longus muscle. Biochem Biophys Res Commun. 1979 Sep 12;90(1):164–170. doi: 10.1016/0006-291x(79)91604-8. [DOI] [PubMed] [Google Scholar]
- Manning D. R., Stull J. T. Myosin light chain phosphorylation-dephosphorylation in mammalian skeletal muscle. Am J Physiol. 1982 Mar;242(3):C234–C241. doi: 10.1152/ajpcell.1982.242.3.C234. [DOI] [PubMed] [Google Scholar]
- Metzger J. M., Moss R. L. Myosin light chain 2 modulates calcium-sensitive cross-bridge transitions in vertebrate skeletal muscle. Biophys J. 1992 Aug;63(2):460–468. doi: 10.1016/S0006-3495(92)81614-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. L., Stull J. T. Myosin light chain phosphorylation in fast and slow skeletal muscles in situ. Am J Physiol. 1984 Nov;247(5 Pt 1):C462–C471. doi: 10.1152/ajpcell.1984.247.5.C462. [DOI] [PubMed] [Google Scholar]
- Perrie W. T., Smillie L. B., Perry S. B. A phosphorylated light-chain component of myosin from skeletal muscle. Biochem J. 1973 Sep;135(1):151–164. doi: 10.1042/bj1350151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persechini A., Stull J. T., Cooke R. The effect of myosin phosphorylation on the contractile properties of skinned rabbit skeletal muscle fibers. J Biol Chem. 1985 Jul 5;260(13):7951–7954. [PubMed] [Google Scholar]
- Rayment I., Holden H. M., Whittaker M., Yohn C. B., Lorenz M., Holmes K. C., Milligan R. A. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993 Jul 2;261(5117):58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- Rayment I., Rypniewski W. R., Schmidt-Bäse K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Saraswat L. D., Lowey S. Engineered cysteine mutants of myosin light chain 2. Fluorescent analogues for structural studies. J Biol Chem. 1991 Oct 15;266(29):19777–19785. [PubMed] [Google Scholar]
- Schoenberg M. The kinetics of weakly- and strongly-binding crossbridges: implications for contraction and relaxation. Adv Exp Med Biol. 1988;226:189–202. [PubMed] [Google Scholar]
- Sellers J. R. Phosphorylation-dependent regulation of Limulus myosin. J Biol Chem. 1981 Sep 10;256(17):9274–9278. [PubMed] [Google Scholar]
- Sellers J. R. Regulation of cytoplasmic and smooth muscle myosin. Curr Opin Cell Biol. 1991 Feb;3(1):98–104. doi: 10.1016/0955-0674(91)90171-t. [DOI] [PubMed] [Google Scholar]
- Stewart M., Kensler R. W., Levine R. J. Structure of Limulus telson muscle thick filaments. J Mol Biol. 1981 Dec 15;153(3):781–790. doi: 10.1016/0022-2836(81)90418-6. [DOI] [PubMed] [Google Scholar]
- Stewart M., Kensler R. W., Levine R. J. Three-dimensional reconstruction of thick filaments from Limulus and scorpion muscle. J Cell Biol. 1985 Aug;101(2):402–411. doi: 10.1083/jcb.101.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull J. T., High C. W. Phosphorylation of skeletal muscle contractile proteins in vivo. Biochem Biophys Res Commun. 1977 Aug 8;77(3):1078–1083. doi: 10.1016/s0006-291x(77)80088-0. [DOI] [PubMed] [Google Scholar]
- Sweeney H. L., Bowman B. F., Stull J. T. Myosin light chain phosphorylation in vertebrate striated muscle: regulation and function. Am J Physiol. 1993 May;264(5 Pt 1):C1085–C1095. doi: 10.1152/ajpcell.1993.264.5.C1085. [DOI] [PubMed] [Google Scholar]
- Sweeney H. L., Stull J. T. Alteration of cross-bridge kinetics by myosin light chain phosphorylation in rabbit skeletal muscle: implications for regulation of actin-myosin interaction. Proc Natl Acad Sci U S A. 1990 Jan;87(1):414–418. doi: 10.1073/pnas.87.1.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney H. L., Yang Z., Zhi G., Stull J. T., Trybus K. M. Charge replacement near the phosphorylatable serine of the myosin regulatory light chain mimics aspects of phosphorylation. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1490–1494. doi: 10.1073/pnas.91.4.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szent-Györgyi A. G., Szentkiralyi E. M., Kendrick-Jonas J. The light chains of scallop myosin as regulatory subunits. J Mol Biol. 1973 Feb 25;74(2):179–203. doi: 10.1016/0022-2836(73)90106-x. [DOI] [PubMed] [Google Scholar]
- Vibert P., Craig R. Structural changes that occur in scallop myosin filaments upon activation. J Cell Biol. 1985 Sep;101(3):830–837. doi: 10.1083/jcb.101.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh M. P., Dabrowska R., Hinkins S., Hartshorne D. J. Calcium-independent myosin light chain kinase of smooth muscle. Preparation by limited chymotryptic digestion of the calcium ion dependent enzyme, purification, and characterization. Biochemistry. 1982 Apr 13;21(8):1919–1925. doi: 10.1021/bi00537a034. [DOI] [PubMed] [Google Scholar]
- Wang F., Martin B. M., Sellers J. R. Regulation of actomyosin interactions in Limulus muscle proteins. J Biol Chem. 1993 Feb 15;268(5):3776–3780. [PubMed] [Google Scholar]
- Whittaker M., Wilson-Kubalek E. M., Smith J. E., Faust L., Milligan R. A., Sweeney H. L. A 35-A movement of smooth muscle myosin on ADP release. Nature. 1995 Dec 14;378(6558):748–751. doi: 10.1038/378748a0. [DOI] [PubMed] [Google Scholar]
- Wray J. S., Goody R. S., Holmes K. C. Towards a molecular mechanism for the crossbridge cycle. Adv Exp Med Biol. 1988;226:49–59. [PubMed] [Google Scholar]