Abstract
Toward developing anticancer agents, heterocycle-based carboxymethyl cellulose conjugates have been synthesized. 2-Cyano-N′-(aryl/heteroarylethylidene)acetohydrazides and ethyl 2-cyano-3-(heteryl)acrylates were utilized as precursors for the synthesis of pyridine-based compounds. The chemical structures of the synthesized derivatives were characterized using various spectroscopic techniques, including 1H-, 13C-NMR, Fourier transform infrared spectroscopy (FTIR), as well as scanning electron microscopy (SEM).The anticancer effects of compounds on HCT-116, MCF-7, PC3 and A549 cancer cell lines were investigated and their cytotoxicity against RPE-1 normal cells was estimated to determine their safety. Compounds 4b and 7c exhibit high selectivity toward cancer cells while maintaining a strong safety margin for normal cells. The results demonstrated that the novel heterocycle-based carboxymethyl cellulose conjugates are promising and can be further evaluated as a potential therapeutic agent.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-14146-1.
Keywords: Synthesis, Pyridine, Anticancer, Carboxymethyl cellulose
Subject terms: Cancer, Chemistry, Materials science
Introduction
Cancer is a complex and heterogeneous disease characterized by abnormal, uncontrolled cell division, leading to tumor formation and the invasion of surrounding healthy tissues. It is the second leading cause of death globally, following cardiovascular diseases, accounting for one in every six deaths worldwide. This alarming prevalence highlights the urgent need to develop more effective therapeutic agents to combat the disease1.
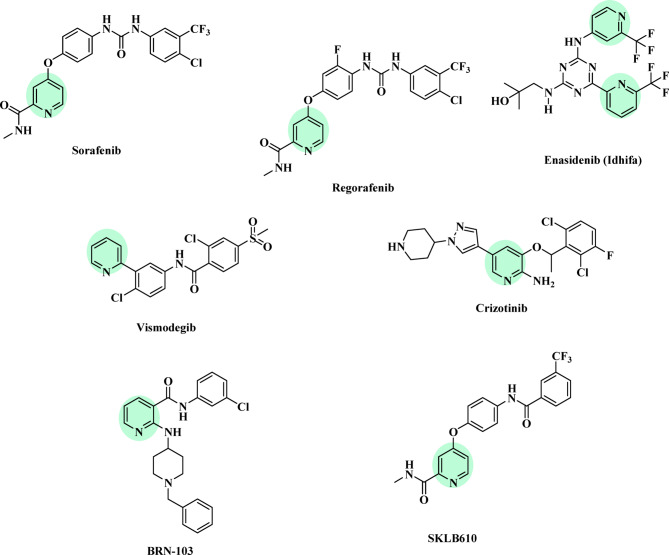
Due to its significant impact on quality of life, cancer remains a major global health concern. The rising incidence of cancer, along with the resistance of cancer cells to existing treatments, has intensified the search for new anticancer drugs with enhanced potency, selectivity, improved pharmacokinetics, and reduced toxicity2. Over decades of extensive research, many anticancer agents have been developed as structural analogs of pyrimidines, purines, hormones, vitamins, amino acids, and other essential metabolites3. Among these, oxygen- and nitrogen-containing heterocyclic compounds have demonstrated promising therapeutic potential against various diseases, including cancer1. Heterocyclic-based anticancer drugs exhibit significant antineoplastic activity. Both pyridine and pyrimidine are naturally occurring in biological systems, including genetic material. These core structures play crucial roles in various biological processes and cancer pathogenesis, making them attractive scaffolds for the development of novel therapeutic agents2. Heterocyclic compounds have a profound and wide-ranging impact on medicinal chemistry and the pharmaceutical industry. They serve as key components in the expanding field of antimetabolites. Their critical importance has led to extensive use in treating severe diseases, including cancer and malignancies4. Nitrogen-containing heterocyclic derivatives, such as azines and pyridine frameworks, are essential in both chemistry and biology (Fig. 1). They are widely utilized in pharmaceuticals, vitamins, natural products, and the synthesis of functional reagents5–8. A novel and efficient method for synthesizing various types of pyrimidines has been developed and reported9–17. Pyridines have also been explored as potential anticancer agents18,19. Heterocyclic compounds containing the pyridine nucleus exhibit a diverse range of biological activities, including anticancer, antioxidant, antimicrobial, and antiviral properties. These compounds have demonstrated significant therapeutic and biological potential across multiple applications20,21.
Fig. 1.
Targeted anticancer agents containing pyridine scaffolds.
On the other side, biopolymers are indispensable materials for the chemical industry, finding many applications due to their biocompatibility, biodegradability, and low toxicity22,23. Among these biopolymers, cellulose and its derivatives have great concern. It is a renewable, cost- effective and abundant material24.
Carboxymethyl cellulose (CMC) is a wildly used cellulose derivative; it is a water soluble polymer with carboxmethyl group that attached to some of the hydroxyl groups of the glucopyranose monomers in the cellulose backbone. CMC participate in various applications water treatment25, drug delivery26 and food packaging27,28. Herein, in this work CMC heterocyclic composite (ECFA, ABOC) has prepared and investigated via 1H-, 13C-NMR, FTIR, SEM, XRD and TGA.
Result and discussion
Synthesis
New derivatives of pyrido[2,1-b][1,3,4] oxadiazine-7-carboxylic acid (7) were synthesized as depicted in figure 2. This approach in synthesizing of those novel derivatives is a continuation of our recent program of synthesizing the pyridooxadiazine ring29. The approach to access compound 7 was achieved starting from synthesizing the ethylidene-2-cyanoacetohydrazide derivatives (3) through reacting the cyano acid hydrazide (1) with the acetophenone derivatives (2) under reflux in ethanol for one hour29. Further reacting the substituted ethylidene-2-cyanoacetohydrazides 3 with the ethyl 2-cyano-3-(pyridin-3-yl)acrylate (4) yielded the substituted pyrido[2,1-b][1,3,4] oxadiazine-7-carboxylic acid (7).
Fig. 2.
Synthetic pathway for compounds 7a-d. Reagents and conditions: i) EtOH, reflux, 1 hour. ii) AcOH/MeOH (2:1), reflux, 3h.
The proposed synthesis mechanism of compound 7 likely follows a Michael addition, where the active methylene group of compound 3 reacts with the double bond in compound 4 to form the intermediate 5. Its worthy to note that the starting compounds 3 was synthesized according to previous procedures19. The resulting intermediate 5 then undergoes cyclization to form compound 6. Subsequent hydrolysis of the latter compound in the presence of acetic acid afforded compound 7. The assignment of the chemical structures of compounds 7 was detected on the basis of its spectral data and elemental analysis. As an example, 1H NMR spectrum of the compound 7c showed two singlets at δ 4.47 & 4.96 ppm, respectively, which are corresponding to the methylene and CH protons accordingly. Signals between δ 7.36 to 8.70 ppm were observed for the eight aromatic protons on the two substituted benzene rings. Additionally, singlet signal for the amino and hydroxyl groups appeared at δ 11.00 ppm. Herein, compound 7b & 7c were synthesized under identical conditions, whereas compound 7a was not generated under the same reaction condition. The derivative 7d is previously synthesized29; in the present study its biological evaluation against HCT116, MCF7, PC3 and A549 cells is reported. Furthermore, the preparation of the pyridine-based carboxymethyl cellulose conjugates is accomplished (Fig. 3). Additionally, ethyl 2-cyano-3-(5-methylfuran-2-yl)acrylate 4b was reacted with compound 8 to yield the corresponding furan-based carboxymethyl cellulose conjugates.
Fig. 3.

Suggested modifications of CMC with the synthesized compounds 4b & 7c.
FTIR- analysis
FTIR is the most useful tool to describe the compounds function group. Figure 4 shows FTIR of CMC and CMC with heterocyclic compounds (ECFA & ABOC). CMC spectra shows different peaks at 3500 cm−1, 2900 cm−1, 1650 cm−1, and 1040 cm−1 at 550 cm−1 corresponding to OH stretching, C-H stretching, C = O of carboxylic group, ether linkage of glucose unite and C-Br stretching30,31. Spectra of CMC composite through CMC interaction with heterocyclic compounds (ECFA & ABOC show that the proceeded peaks of CMC of OH, CH, C = O, and C-O- C became more intensity and sharp. It may be returned to the homogeneity between the compounds32,33.
Fig. 4.

FTIR of CMC and composites.
Scanning electron microscope
Figure 5 illustrates the surface morphology of CMC and CMC with heterocyclic compounds (ECFA, ABOC). The surface of CMC appears as collected fibers have a large thickness. While CMC/ECFA appears a small fibers, it seems as CMC has split through the interaction. Not only CMC/ECFA appears as a splitting fiber, but also CMC/ABOC looks like rows and layers put up to each other.
Fig. 5.
SEM of CMC and composites.
Powder X-ray diffraction
XRD analysis is indispensible method that used to identify the crystalline and amorphous region of prepared compounds. Figure 6 displays XRD pattern of CMC and CMC with heterocyclic compounds (ECFA, ABOC). The XRD pattern of CMC shows characteristic peaks of amorphous region at 2Ɵ=10o and 20. While the XRD pattern of the CMC composite displays these characteristic peaks only slightly, revealing a change in crystallinity34–36.
Fig. 6.

XRD of CMC and composites.
Evaluation of anticancer activity
The cytotoxic effect of the synthesized compounds on different cell lines
The cytotoxic effect of the synthesized compounds was investigated with MTT assay on four cancer cell lines (HCT-116, MCF-7, PC3, and A549) and normal cell line RPE-1. First, we screened the new compounds at only one dose (100 µg/ml) for 48 h and the percentage of cytotoxicity was measured and compared to control untreated cells and positive control Doxorubicin. Results of this initial investigation showed that all compounds showed concentration dependent inhibitory effect against cell proliferation in all cell lines except MCF-7.
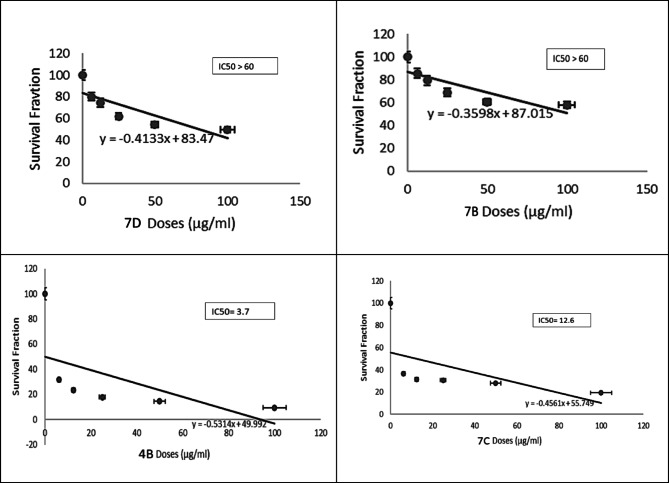
After that, we used different concentrations of all CMC/ABOC compounds and studied their cytotoxic effect against the rest of cell lines including both normal and cancerous cells. According to results shown in Table 1, all compounds has cytotoxic effect against HCT-116, PC3 and A549 cells. The IC50 values were calculated for compounds which showed high cytotoxicity on different tested cell lines. As shown in (Figs. 7, 8, 9 and 10), compound CMC/ECFA and CMC/ABOC showed the highest significant cytotoxicity while HCT-116 and A549 cells were the most sensitive cells to our compounds. IC50 values of compound CMC/ECFA were 3.7, 32.81 and 19.96 µg/ml and for compound 7c they were 12.6, 53.54 and 11.4 µg/ml against HCT-116, PC3 and A549 cells respectively. According to these results we can highlight the potent anticancer effect of compound 4b,7c against HCT-116, PC3 and A549 cancer cells by recording the least IC50 values compared to untreated cells.
Table 1.
Percentage of cytotoxicity of different concentrations of the synthesized compounds on cancer and normal cell lines.
| Cell line | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCT-116 | A549 | ||||||||||||
|
Dose (µg/ml) |
7b | 7c | 7d | 4b | 7b | 7c | 7d | 4b | |||||
| 100 | 42.09 | 80.66* | 50.36 | 90.56* | . | 77.43* | . | 80.93* | |||||
| 50 | 39.46 | 71.96* | 45.53 | 85.50* | . | 77.10* | . | 75.73* | |||||
| 25 | 31.13 | 69.26* | 38.09 | 82.12* | . | 75.61* | . | 66.61* | |||||
| 12.5 | 20.66 | 68.53* | 25.46 | 76.53* | . | 67.85* | . | 60.04* | |||||
| PC3 | RPE-1 | ||||||||||||
|
Dose (µg/ml) |
7b | 7c | 7d | 4b | 7b | 7c | 7d | 4b | |||||
| 100 | 40.98 | 62.56* | 38.16 | 95.65* | 18.06 | 52.28 | 12.14 | 58.13 | |||||
| 50 | 35.06 | 59.17* | 23.16 | 65.28* | 12.54 | 45.25 | 11.29 | 49.43 | |||||
| 25 | 25.40 | 46.43* | 4.77 | 58.47* | 8.61 | 38.66 | 10.76 | 44.43 | |||||
| 12.5 | 20.74 | 39.51* | 4.27 | 43.63* | 3.27 | 29.23 | 4.21 | 37.33 | |||||
* p < 0.01 when compared with untreated cells.
Fig. 7.
Effect of the synthesized compounds on HCT-116 Cell viability. The survival fraction of HCT-116 cells was assessed using the MTT assay after 48 h of treatment with varying concentrations of the synthesized compounds (6.25–100 µg/mL). Data are presented as mean ± SD from three independent experiments. *p < 0.01 compared to untreated cells.
Fig. 8.
Effect of synthesized compounds on PC3 cells viability. Survival fraction evaluated using the MTT assay after 48 h of treatment with varying concentrations of compounds (6.25–100) µg/ml. Data are expressed as mean ± SD from three independent experiments. * p˂0.01 compared to untreated cells.
Fig. 9.
Effect of synthesized compounds 4b and 7c on A549 cells viability. Survival fraction was evaluated using the MTT assay after 48 h of treatment with varying concentrations of compounds 4b and 7c (6.25–100 µg/mL). Data are expressed as mean ± SD from three independent experiments. * p˂0.01 compared to untreated cells.
Fig. 10.
Effect of synthesized compounds on RPE-1 cells viability. The viability of RPE-1 cells was assessed using the MTT assay after 48 h of treatment with varying concentrations of the synthesized compounds (6.25–100 µg/mL). Data are expressed as mean ± SD from three independent experiments. *p < 0.01 compared to untreated cells.
After investigating the significant anticancer effect of compound 4b, 7c we investigated their cytotoxicity against RPE-1 normal cells to determine their safety. It is known that the therapeutic safety of anticancer drugs is a crucial consideration for treating cancer patients. It is important factor for using these drugs clinically to avoid serious side effects. IC50 is commonly used to indicate the potency and sensitivity of an anticancer drug against cancer cells. The therapeutic safety of the drug can be evaluated by comparing the IC50 values and Hill slope of the anticancer drug in cancer cells relative to normal cells37,38. Fortunately, compounds 4b and 7c showed significant low toxicity against RPE-1 normal cells by recording very low IC50 values compared to studied cancer cells HCT-116, PC3 and A549.
According to our results, we can conclude that compounds 4b and 7c have high selectivity towards cancer cells and possess a strong safety margin for normal cells. This underscores their potential as potent and safe anticancer candidates for further investigation.
The cytotoxic effect of the synthesized compounds on different cell lines
The cytotoxic effect of thesynthesized compounds was investigated with MTT assay on four cancer cell lines (HCT-116, MCF-7, PC3, and A549) and normal cell line RPE-1. First, we screened the new compounds at only one dose (100 µg/ml) for 48 h and the percentage of cytotoxicity was measured and compared to control untreated cells and positive control Doxorubicin. Results of this initial investigation showed that all compounds showed concentration dependent inhibitory effect against cell proliferation in all cell lines except MCF-7.
After that, we used different concentrations of all the targeted compounds and studied their cytotoxic effect against the rest of cell lines including both normal and cancerous cells. According to results shown at (Table 1), all compounds has cytotoxic effect against HCT-116, PC3 and A549 cells. The IC50 values were calculated for compounds which showed high cytotoxicity on different tested cell lines. As shown in (Figs. 6, 7 and 8), compound 4b and 7c showed the highest significant cytotoxicity while HCT-116 and A549 cells were the most sensitive cells to our compounds. IC50 values of compound 4b were 3.7, 32.81 and 19.96 µg/ml and for compound 7c they were 12.6, 53.54 and 11.4 µg/ml against HCT-116, PC3 and A549 cells respectively. According to these results we can highlight the potent anticancer effect of compound 4b, 7c against HCT-116, PC3 and A549 cancer cells by recording the least IC50 values compared to untreated cells.
After investigating the significant anticancer effect of compound 4b, 7c we investigated their cytotoxicity against RPE-1 normal cells to determine their safety. It is known that the therapeutic safety of anticancer drugs is one of the most important concerns of the physician treating the cancer patient. It is important factor for using these drugs clinically to avoid serious side effects. IC50 is usually used to represent the strength and sensitivity of an anticancer drug on cancer cells. The therapeutic safety of the anticancer drug can be assessed by comparing the IC50 and hillslope of anticancer drugs on cancer cells relative to normal cells37,38. Fortunately, compounds 4b and 7c showed significant low toxicity against RPE-1 normal cells by recording very low IC50 values compared to studied cancer cells HCT-116, PC3 and A549. According to our results, we can conclude that compounds 4b and 7c have high selectivity against cancer cells and high safety margins to normal cells, thereby highlighting their utilities as potent and safe anticancer drugs for further studies.
Effect of the synthesized compounds on nitric oxide levels in LPS-stimulated RAW 264.7 macrophages
The discovery of the potent activity of the synthesized compounds motivated us to further investigate the cellular mechanisms responsible for the potent cytotoxic effects observed against the cancer cells used in this study.
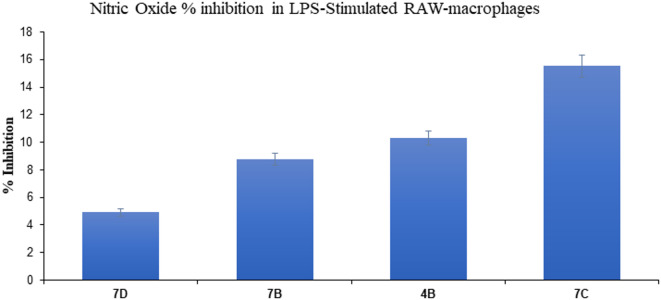
To achieve this goal, we studied the anti-inflammatory effect of these compounds. It is well documented that the inflammation is one of the cellular mechanisms involved in carcinogenesis37‚39.Accumulating evidence suggests that nitric oxide (NO) is a key mediator of inflammation. While NO plays a crucial role in various physiological functions, its excessive production, particularly in macrophages, can contribute to cytotoxicity, inflammation, and autoimmune disorders. The impact of different compounds on NO levels in LPS-stimulated RAW 264.7 cells was evaluated (Fig. 8). Cells were treated with various compounds in the presence of LPS or with LPS alone for 24 h. NO production was assessed by measuring nitrite levels in the culture medium utilizing the Griess reagent. The results showed that LPS alone significantly increased NO production compared to the control. However, pretreatment with the tested compounds did not inhibit NO release in LPS-stimulated RAW 264.7 cells (Fig. 11). These findings suggest that the anticancer effects of these compounds are independent of inflammation. Further studies are required to explore their precise mechanisms of action.
Fig. 11.
Effect of different compounds on the inhibition of nitric oxide production in LPS-stimulated RAW264.7 macrophages. RAW 264.7 macrophages were exposed to different compounds (100 µg/mL) along with LPS (1 µg/mL) or LPS alone for 24 h. Nitric oxide (NO) production was measured using the Griess reagent, and the results are expressed as mean ± SD (n = 3).
Experimental
Synthesis of Ethyl 2-cyano-3-(5-methylfuran-2-yl)acrylate (4b)
A mixture of 3-methylfurfural (0.01 mol) and ethyl cyanoacetate (0.01 mol) was reacted at room temperature in ethanol in the presence of drops of piperidine. The precipitated solid was filtered off and re-crystallized from ethanol. Compound 4b was afforded as a brown solid (90%); 1 H NMR (500 MHz, DMSO-d6): 1.24 (t, J = 7.5 Hz, 3 H, CH3), 2.40 (s, 3 H, CH3), 4.22–4.25 (m, 2 H, CH2), 6.52 (s, 1 H, CH), 7.43 (s, 1H, CH), 7.98 (s, 1H, CH). 13C NMR (125 MHz, DMSO-d6): 14.24, 62.58, 95.00, 99.98, 112.17, 116.21, 127.50, 139.07, 147.47, 161.40, 163.18. Elemental analysis calculated for C11H11NO3 (205.21): C, 64.38; H, 5.40; N, 6.83. Found: C, 64.35; H, 5.35; N, 6.80.
General procedure of synthesizing compounds 7
A mixture of 2-Cyano-N′-(aryl/heteroarylethylidene)aceto hydrazides (0.01 mol) and ethyl 2-cyano-3-(heteryl)acrylates was refluxed for three hours in methanol/acetic acid (1:2). The precipitated solid was filtered off and re-crystallized from methyl alcohol.
Synthesis of 6-amino-9-cyano-2,9a-dihydro-3-phenyl-8-(pyridin-3-yl)pyrido[2,1-b][1,3,4] oxadiazine-7-carboxylic acid (7b)
Compound 7b was afforded as a yellow solid (74%); m.p.:308–310 °C, 1 H NMR (500 MHz, DMSO-d6): 4.46 (s, 2 H, CH2), 4.95 (s, 1 H, CH), 7.35–7.42 (m, 3 H, CH), 7.64 (s, 1 H, CH), 8.37–8.46 (m, 5 H, CH), 10.97 (br s, 3 H, COOH, NH2). 13C NMR (125 MHz, DMSO-d6): 35.00, 37.00, 42.10, 43.00, 83.15, 83.90, 117.13, 117.73, 124.19, 124.35, 135.46, 135.67, 136.15, 146.67, 149.21, 149.37, 149.83, 162.89, 163.01. Elemental analysis calculated for C20H15N5O3 (373.36): C, 64.34; H, 4.05; N, 18.76. Found: C, 64.32; H, 4.00; N, 18.70.
Synthesis of 6-amino-3-(4-bromophenyl)−9-cyano-2,9a-dihydro-8-(pyridin-3-yl)pyrido[2,1-b][1,3,4] oxadiazine-7-carboxylic acid (7c)
Compound 7c was afforded as a yellow solid (83%), 1 H NMR (500 MHz, DMSO-d6): 4.47 (s, 2 H, CH2), 4.96 (s, 1 H, CH), 7.36–7.42 (m, 3 H, CH), 7.65 (s, 1 H, CH), 8.37–8.70 (m, 4 H, CH), 11.00 (br s, 3 H, COOH, NH2). 13C NMR (125 MHz, DMSO-d6): 35.00, 38.00, 41.10, 42.00, 83.13, 83.88, 117.13, 117.72, 124.22, 124.37, 135.73, 136.17, 136.38, 146.67, 149.02, 149.17, 149.33, 162.87, 163.00. Elemental analysis calculated for C20H14BrN5O3 (452.26): C, 53.11; H, 3.12; Br, 17.67; N, 15.49. Found: C, 53.09; H, 3.08; Br, 17.63; N, 15.44.
Synthesis of 6-amino-3-(3-aminophenyl)−9-cyano-2,9a-dihydro-8-(pyridin-3-yl)pyrido[2,1-b][1,3,4] oxadiazine-7-carboxylic acid (7d)
Compound 7d was obtained as a yellow solid with an 81% yield and a melting point of 300–303 °C29.
Preparation of CMC composite
A homogeneous CMC solution was prepared by dissolving 0.5 g of CMC in 10 mL of distilled water under continuous stirring until fully dissolved. Then 0.15 g of heterocyclic compounds (AMOC, ABOC) was added with continues stirring to prevent agglomeration for 30 min. Afterward, the precursor was poured into in petri dish and frozen at − 20 °C, followed by a lyophilization process.
Characterizations
The characterization of the synthesized compounds was performed using various analytical techniques. FT-IR spectra were recorded in the range of 400–4000 cm − 1 using a Shimadzu 8400 S FT-IR spectrophotometer. The surface morphology was examined via scanning electron microscopy (SEM) using an FEI IN SPECTS electron microscope (Philips, Poland) in an environmental scanning mode without coating, along with a JEOL JEM-2100 electron microscope at 100k× magnification and an acceleration voltage of 120 kV.
X-ray diffraction (XRD) patterns were analyzed using a Diano X-ray diffractometer with a CuKα radiation source, operating at 45 kV. Additionally, a Philips X-ray diffractometer (PW 1930 generator, PW 1820 goniometer) was used, employing a CuK radiation source (λ = 0.15418\lambda = 0.15418λ = 0.15418 nm) within a diffraction angle range of 2θ from 10° to 80° in reflection mode.
Reaction progress was monitored using thin-layer chromatography (TLC) on pre-coated silica gel 60 F254 aluminum plates, with visualization under UV light. The melting points of the compounds were determined utilized open capillary tubes on a Stuart SMP30 melting point apparatus and remained uncorrected.
The spectral analysis of the compounds was conducted at the Micro-analytical Labs of the National Research Centre in Cairo, Egypt. The 1H NMR spectrum was recorded using a Bruker Fourier 400 spectrometer at 400 MHz and 75 MHz, respectively, at 300 K.
In-Vitro study
Anticancer activity
Cell culture
The cytotoxic potential of the synthesized compounds was assessed against human carcinoma cell lines, including HCT-116 (colon), MCF-7 (breast), PC3 (prostate), and A549 (lung), along with the normal hTERT-RPE-1 (human immortalized epithelial) cell line. These cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/mL), and streptomycin (2 mg/mL). The cultures were maintained at 37 °C in a humidified atmosphere containing 5% CO₂ and 95% humidity. Cells were routinely passaged utilizing a 0.15% trypsin-versene solution.
The cell lines used in this study were generously provided by Professor Stig Linder from the Oncology and Pathology Department at the Karolinska Institute, Stockholm, Sweden, and were originally sourced from the American Type Culture Collection (ATCC).
Cytotoxicity assessment on cancer monolayers
Cells were seeded in a 96-well plate at specific densities: 20,000 cells/well for HCT-116 and PC3 cell lines, 10,000 cells/well for A549 and MCF-7 cell lines, and 20,000 cells/well for the normal human immortalized epithelial cell line (hTERT-RPE1). After 24 h of incubation, the culture medium was aspirated and replaced with serum-free medium containing the test extracts (100 µg/mL). Cells were then treated for 48 h in triplicate.
Doxorubicin (100 µg/mL) served as the positive control, while 0.5% dimethyl sulfoxide (DMSO) was used as the negative control. Cell viability was determined using the MTT assay [3-(4,5-dimethylthiazol-2-yl)−2,5-diphenyltetrazolium bromide] following a previously established protocol38.
Cytotoxicity was calculated using the following equation:
% cytotoxicity = [1-(AVx/AVNC)]x100.
where AVx represents the average absorbance of the treated sample well, and AVNC corresponds to the average absorbance of the negative control well, measured at 595 nm with a reference wavelength of 690 nm.
Determination of IC50 values
Compounds exhibiting cytotoxicity of ≥ 60% against various cancer cell lines were selected for dose-response studies at various concentrations. The final tested concentrations ranged from 100, 50, 25, 12.5, and 6.25 µg/mL down to 0.78 µg/mL, with all experiments performed in triplicate. IC50 values were determined by fitting the concentration-response curve to a non-linear regression model utilizing GraphPad Prism® v6.0 (GraphPad Software Inc., San Diego, CA, USA).
Anti‑inflammatory activity
Cell culture (Seeding and Treatment)
The RAW 264.7 macrophage cell line was obtained from the American Type Culture Collection (ATCC). The cells were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum and 1% penicillin-streptomycin. Prior to the experiment, the cells were subcultured twice and incubated in a humidified incubator at 37 °C with 5% CO₂.
Procedure
All experiments were conducted in a clean environment using a biosafety class II laminar flow cabinet (Baker, SG403INT, Sanford, ME, USA). RAW 264.7 cells were suspended in RPMI medium and seeded at a density of 1 × 1051 \times 10^51 × 105 cells per well in 96-well plates. After 24 h of incubation, the cells were treated with test samples at concentrations of 100, 50, 25, and 12.5 µg/mL for 1 h. Following this, they were stimulated with 10 µg/mL lipopolysaccharide (LPS) for an additional 24 h.
After incubation, the supernatant was carefully transferred to a new 96-well plate for nitric oxide (NO) measurement. The remaining cells in the original plate were used for cell viability assessment utilizing the MTT assay. The test samples were prepared in RPMI medium after dissolving the stock solutions in DMSO.
Cell viability was determined based on the mitochondrial-dependent reduction of yellow MTT [3-(4,5-dimethylthiazol-2-yl)−2,5-diphenyl tetrazolium bromide] to purple formazan40. The percentage change in viability was calculated using the following formula:
((Reading of extract/Reading of negative control) −1) x 10031.
Nitric oxide assay
The production of nitric oxide (NO) was assessed in the supernatants of cultured RAW 264.7 cells, following a previously described method with slight modifications41. Nitrite, a stable NO metabolite, was used as an indicator of NO production in the culture medium. After pre-incubating RAW 264.7 cells (1 × 1051 \times 10^51 × 105 cells/mL) with LPS (10 µg/mL) for 24 h, nitrite levels were measured using the Griess reagent. The reagent consisted of 1% sulfanilamide and 0.1% naphthyl ethylenediamine dihydrochloride in 2.5% phosphoric acid.
For the assay, 50 µL of cell culture supernatant was mixed with 50 µL of Griess reagent and incubated at room temperature for 15 min. The absorbance was then measured at 540 nm using a microplate reader. A fresh culture medium blank was included in each experiment. Nitrite concentration was determined using a sodium nitrite standard curve, as calculated by the following equation:
Conclusion
Heterocycle-based compounds have been synthesized utilizing the 2-cyano-N′-(aryl/heteroarylethylidene)acetohydrazides and ethyl 2-cyano-3-(heteryl)acrylates. Based on the synthesized heterocycle-based compounds, the CMC-heterocycle composites were prepared. The structures of synthesized derivatives were confirmed using spectroscopic techniques such as NMR, fourier transform infrared spectroscopy (FTIR) as well as scanning electron microscopy (SEM). The investigations of anticancer effects of compounds on HCT-116, MCF-7, PC3 and A549 cancer cell lines were evaluated and their cytotoxicity against RPE-1 normal cells was estimated to determine their safety. Compounds 4b and 7c have high selectivity towards cancer cells and high safety margins to normal cells. The study demonstrated that the new heterocycle-based carboxymethyl cellulose conjugates are promising and could act as potential therapeutic agents.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors gratefully acknowledge the support for the research presented in this article from the National Research Centre, project ID:13010104.
Author contributions
“Conceptualization: Reham A. Mohamed-Ezzat and Sawsan Dacrory; Methodology: Reham A. Mohamed-Ezzat, Zeinab A. Elshahid, Shaimaa A. Gouhar and Sawsan Dacrory; Writing-original draft preparation: Reham A. Mohamed-Ezzat, Zeinab A. Elshahid, Shaimaa A. Gouhar and Sawsan Dacrory; Writing-review and editing: Reham A. Mohamed-Ezzat, Zeinab A. Elshahid, Shaimaa A. Gouhar and Sawsan Dacrory.”
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
Data is provided within the manuscript.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was approved by the Medical Research Ethics Committee (MERC) fedral (accurance no. : FWA 00014747).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Manaithiya, A. et al. Current status of novel pyridine fused derivatives as anticancer agents: An insight into future perspectives and structure activity relationship (SAR). Curr. Top. Med. Chem.21 (25), 2292–2349 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Prachayasittikul, S. et al. Roles of pyridine and pyrimidine derivatives as privileged scaffolds in anticancer agents. Mini Rev. Med. Chem.17 (10), 869–901 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Mohamed-Ezzat, R. A., Kariuki, B. & Elgemeie, G. Synthesis and crystal structure of N-phenyl-2-(phenylsulfanyl) acetamide. Acta Crystallogr. Sect. E: Crystallographic Commun., 80 (4), 392–395 (2024). [DOI] [PMC free article] [PubMed]
- 4.Mohamed-Ezzat, R. A., Kariuki, B., Al-Ashmawy, A. K. & Srour, A. M. Synthesis and crystal structure of 5-{(E)-[(1H-indol-3-ylformamido)imino]methyl}-2-methoxyphenyl propane-1-sulfonate. Acta Crystallogr. Sect. E: Crystallographic Commun.81 (4), 310–313 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henry, G. D. De Novo synthesis of substituted pyridines. Tetrahedron29 (60), 6043–6061 (2004). [Google Scholar]
- 6.Jones, G. Pyridines and their benzo Derivatives:(V) synthesis. Compr. Heterocycl. Chem.2, 395 (1984). [Google Scholar]
- 7.Michael, J. P. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep.22 (5), 627–646 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Elgemeie, G. H. & Mohamed, R. A. Recent trends in synthesis of five- and six-membered heterocycles using dimethyl N-cyanodithioiminocarbonate. Heterocycl. Commun.20 (5), 257–269. 10.1515/hc-2014-0090 (2014). [Google Scholar]
- 9.Mohamed-Ezzat, R. A. & Elgemeie, G. H. Discovery and synthesis of novel Bio-Isostere of purine analogues inhibiting SARS-CoV-2. Egypt. J. Chem.66 (13), 167–185 (2023). [Google Scholar]
- 10.Mohamed-Ezzat, R. A. & Elgemeie, G. H. Novel synthesis of the first new class of triazine sulfonamide thioglycosides and the evaluation of their anti-tumor and anti-viral activities against human coronavirus. Nucleosides Nucleotides Nucleic Acids. 43 (12), 1511–1528 (2024). [DOI] [PubMed] [Google Scholar]
- 11.Mohamed-Ezzat, R. A. & Elgemeie, G. H. Novel synthesis of new triazine sulfonamides with antitumor, anti-microbial and anti-SARS-CoV-2 activities. BMC Chem., 18, 58. (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohamed-Ezzat, R. A., Elgemeie, G. H. & Jones, P. G. An unexpected tautomer: Synthesis and crystal structure of N-[6-amino-4-(methylsulfanyl)-1, 2-dihydro-1, 3, 5-triazin-2-ylidene] benzenesulfonamide. Acta Crystallographica Section E: Crystallographic Communications, 80 (2). 120–124 (2024). [DOI] [PMC free article] [PubMed]
- 13.Mohamed-Ezzat, R. A., Kariuki, B. M. & Azzam, R. A. Synthesis and crystal structure of N-(5-acetyl-4-methylpyrimidin-2-yl) benzenesulfonamide. Acta Crystallogr. Sect. E: Crystallographic Commun., 79 (4), 331–334 (2023). [DOI] [PMC free article] [PubMed]
- 14.Mohamed-Ezzat, R.A., Kariuki, B. M. & Azzam R. A. Morpholin-4-ium [5-cyano-6-(4-methylphenyl)-4-(morpholin-4-yl) pyrimidin-2-yl](phenylsulfonyl) amide. IUCrData, 7 (11), x221033 (2022).
- 15.Mohamed-Ezzat, R.A., Elgemeie, G. H. & Jones P. G. Crystal structures of (E)-2-amino-4-methylsulfanyl-6-oxo-1-(1-phenylethylideneamino)-1, 6-dihydropyrimidine-5-carbonitrile and (E)-2-amino-4-methylsulfanyl-6-oxo-1-[1-(pyridin-2-yl) ethylideneamino]-1, 6-dihydropyrimidine-5-carbonitrile. Acta Crystallographica Section E: Crystallographic Communications,77(5), 547–550 (2021). [DOI] [PMC free article] [PubMed]
- 16.Mohamed-Ezzat, R. A., Kariuki, B. M. & Azzam, R. A. Discovery of promising sulfadiazine derivatives with anti-proliferative activity against tumor cell lines. Egypt. J. Chem.61 (12), 1980–1998 (2024). [Google Scholar]
- 17.Elgemeie, G. H. & Mohamed, R. A. Application of dimethyl N-cyanodithioiminocarbonate in synthesis of fused heterocycles and in biological chemistry. Heterocycl. Commun.20 (6), 313–331 (2014). [Google Scholar]
- 18.Mohamed-Ezzat, R. A. & Srour, A. M. Design and synthesis of Aspirin-chalcone mimic conjugates as potential anticancer agents. Anticancer Agents Med. Chem.24 (7), 544–557 (2024). [DOI] [PubMed] [Google Scholar]
- 19.Mohamed-Ezzat, R. A., Kariuki, B. M. & Elgemeie, G. H. Unexpected products of the reaction of cyanoacetylhydrazones of aryl/heteryl ketones with hydrazine: a new route to aryl/heteryl hydrazones, x-ray structure, and in vitro anti-proliferative activity against Nci 60-cell line panel. Egypt. J. Chem.66 (13), 225–239 (2023). [Google Scholar]
- 20.Hassan, A. Y. et al. Synthesis of pyrido-annelated [1, 2, 4, 5] tetrazines,[1, 2, 4] triazepine, and [1, 2, 4, 5] tetrazepines for anticancer, DFT, and molecular docking studies. Sci. Rep.13 (1), 5585 (2023). [DOI] [PMC free article] [PubMed]
- 21.Elgemeie, G. H. & Mohamed-Ezzat, R. A. New Strategies Targeting Cancer Metabolism. 1–619. Amsterdam: Elsevier. ISBN: 978-0-12-821783-2 (2022).
- 22.Baseer, R. A. et al. A biodegradable film based on cellulose and Thiazolidine bearing UV shielding property. Sci. Rep.12 (1), 7887 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dacrory, S., Hashem, A. H. & Kamel, S. Antimicrobial and antiviral activities with molecular Docking study of chitosan/carrageenan@clove oil beads. Biotechnol. J.17 (2), 2100298 (2022). [DOI] [PubMed] [Google Scholar]
- 24.D’Amora, U. et al. Advances in the Physico-Chemical, antimicrobial and angiogenic properties of Graphene-Oxide/Cellulose nanocomposites for wound healing. Pharmaceutics15 (2), 338 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abouzeid, R. E., Owda, M. E., Dacrory, S. Effective adsorption of cationic methylene blue dye on cellulose nanofiber/graphene oxide/silica nanocomposite: kinetics and equilibrium. J. Appl. Polym. Sci.139 (25), e52377 ,1–10 (2022).
- 26.Mohamed-Ezzat, R. A. et al. Synthesis and crystal structure of piperidinyl propanenitrile towards the Preparation of piperidine based bioactive films for drug delivery applications. Sci. Rep.15, 705. 10.1038/s41598-024-81996-6 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang, F. et al. Preparation and characterization of carboxymethyl cellulose containing quaternized chitosan for potential drug carrier. Int. J. Biol. Macromol.154, 1392–1399 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Salem, S. S. et al. Synthesis of silver nanocomposite based on carboxymethyl cellulose: antibacterial, antifungal and anticancer activities. Polymers14 (16), 3352 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohamed-Ezzat, R. A., Hashem, A. H. & Dacrory, S. Synthetic strategy towards novel composite based on substituted pyrido [2, 1-b][1, 3, 4] oxadiazine-dialdehyde chitosan conjugate with antimicrobial and anticancer activities. BMC chem. 17 (1), 88 (2023). [DOI] [PMC free article] [PubMed]
- 30.El-Shall, F. N., Fahim, A. M. & Dacrory, S. Making a new bromo-containing cellulosic dye with antibacterial properties for use on various fabrics using computational research. Sci. Rep.13 (1), 10066 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akl, E. M., Hasanin, M. S. & Dacrory, S. Skin mask hydrogel-based natural sources: characterization and biological properties evaluations. Bioactive Carbohydr. Diet. Fibre. 29, 100355 (2023). [Google Scholar]
- 32.Fahim, A. M., Dacrory, S. & Elsayed, G. H. Anti-proliferative activity, molecular genetics, Docking analysis, and computational calculations of uracil cellulosic aldehyde derivatives. Sci. Rep.13 (1), 14563 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elsayed, G. H., Dacrory, S. & Fahim, A. M. Anti-proliferative action, molecular investigation and computational studies of novel fused heterocyclic cellulosic compounds on human cancer cells. Int. J. Biol. Macromol.222 (Pt B), 3077–3099 (2022). [DOI] [PubMed] [Google Scholar]
- 34.Fahim, A. M. et al. Development of semiconductive foams based on cellulose-benzenesulfonate/CuFe2O4-nanoparticles and theoretical studies with DFT/B3PW91/LANDZ2 basis set. J. Mol. Struct.1247, 131390 (2022). [Google Scholar]
- 35.Dacrory, S. et al. Cyanoethyl cellulose/BaTiO3/GO flexible films with electroconductive properties. ECS J. Solid State Sci. Technol.10 (8), 083004 (2021). [Google Scholar]
- 36.Al Kiey, S. A., Mohamed-Ezzat, R. A. & Dacrory, S. Eco-friendly anti-corrosion performance of chitosan modified with fused heterocyclic compound on mild steel in acidic medium. Int. J. Biol. Macromol.263 (Pt 1), 130133 (2024). [DOI] [PubMed] [Google Scholar]
- 37.Chang, M. C. et al. Comparative assessment of therapeutic safety of norcantharidin, N-farnesyloxy-norcantharimide, and N-farnesyl-norcantharimide against Jurkat T cells relative to human normal lymphoblast: A quantitative pilot study. Medicine95 (31), e4467 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Daly, S. M. et al. Synergistic effect of α-solanine and cisplatin induces apoptosis and enhances cell cycle arrest in human hepatocellular carcinoma cells. Anti-Cancer Agents Med. Chem. (Formerly Curr. Med. Chemistry-Anti-Cancer Agents) 19 (18), 2197–2210 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Elshahid, Z.A., Salama, A. & Gouhar, S.A. Assessment of the synergistic anti-inflammatory effect of naringin/sulindac for the treatment of osteoarthritis: in vitro and in vivo. ADV TRADIT MED (ADTM) 24, 265–283 (2024). [Google Scholar]
- 40.EL-Shahid, Z. A. et al. Antimicrobial, cytotoxic, and α-glucosidase inhibitory potentials using the one strain many compounds technique for red sea soft corals associated fungi’secondary metabolites and chemical composition correlations. J. Biologically Act. Prod. Nat.11 (5–6), 467–489 (2021). [Google Scholar]
- 41.Eid, M. et al. Plasmonic superparamagnetic SPION@ ag@ Chitosan Core-shell: uptake and nitric oxide Inhibition by colorectal cell lines. J. Inorg. Organomet. Polym Mater.32 (3), 931–940 (2022). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript.