Abstract
This study explores how topological indices (TIs), which are mathematical descriptors of a drug’s molecular structure, can support to predict vital properties and biological activities. This understanding is a key for more effective drug design. We focused on drugs used to treat several arrhythmia conditions, including tachycardias, bradycardias, and premature beats. Our approach combines molecular modeling with decision-making techniques to offer a cost-effective way to understand how these drug molecules behave. Our procedure started with calculating topological indices for the chemical structures of these medications to extract information about their features. We then established quantitative structure-property relationship (QSPR) models using quadratic regression, training and validating them. We concentrated on TIs that showed a strong correlation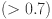 with physicochemical properties. Each property was also weighted, based on its correlation with the topological indices. As a final point, to aid in informed decision-making, we employed multiple-criteria decision-making approaches Technique for Order Preference by Similarity to Ideal Solution TOPSIS and Simple Additive Weighting SAW to rank the anti- arrhythmia medications. Drug Amiodarone ranked highest due to strong correlation with boiling point and polarizability. The study also highlights the potential of machine learning to analyze large datasets, allowing for accurate predictions of chemical behavior. This comprehensive method can facilitate the detection of new drugs with valuable qualities and improve our understanding of how chemical structures affect drug effectiveness.
with physicochemical properties. Each property was also weighted, based on its correlation with the topological indices. As a final point, to aid in informed decision-making, we employed multiple-criteria decision-making approaches Technique for Order Preference by Similarity to Ideal Solution TOPSIS and Simple Additive Weighting SAW to rank the anti- arrhythmia medications. Drug Amiodarone ranked highest due to strong correlation with boiling point and polarizability. The study also highlights the potential of machine learning to analyze large datasets, allowing for accurate predictions of chemical behavior. This comprehensive method can facilitate the detection of new drugs with valuable qualities and improve our understanding of how chemical structures affect drug effectiveness.
Keywords: Correlation coefficient, Heatmap, QSPR analysis, Topological indices, MCDM, TOPSIS, SAW
Subject terms: Chemistry, Mathematics and computing
Introduction
An arrhythmia is a condition characterized by an irregular heartbeat, meaning the heartbeats either occur too quickly (tachycardia), too slowly (bradycardia), or unpredictably. This abnormality can delay normal blood flow, possibly leading to severe health problems such as stroke, heart failure, or unexpected cardiac arrest. Arrhythmias can stem from several factors, including structural heart abnormalities, electrolyte imbalances, and underlying medical conditions. A systematic understanding of how arrhythmias develop and their possible repercussions is vital for developing effective cures1,2.
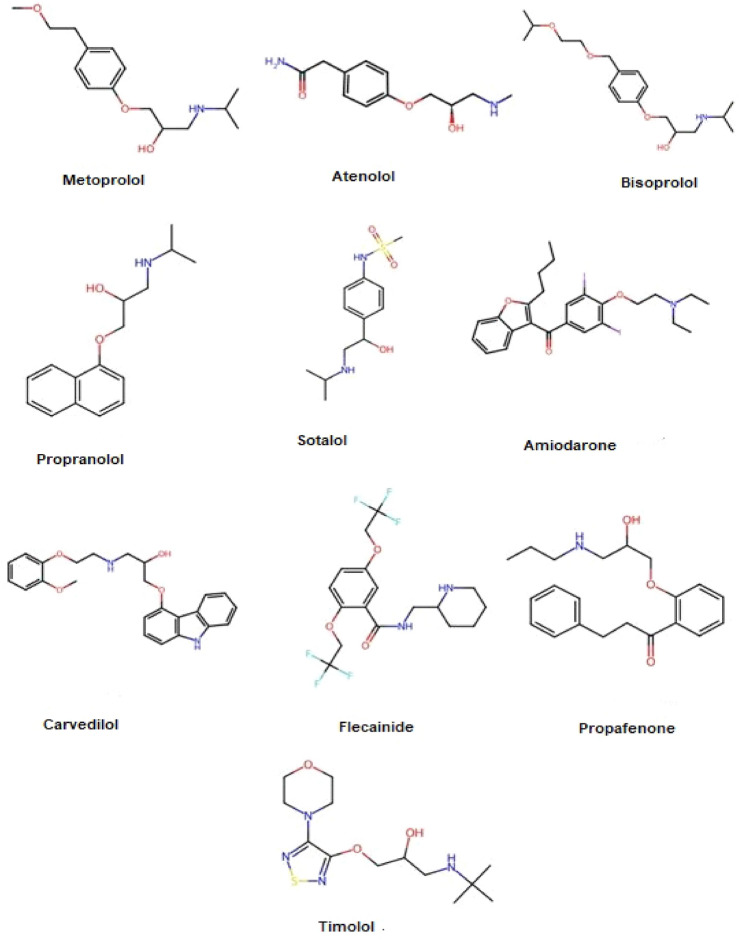
Our research included ten effective medications for treating arrhythmias, as illustrated in Fig. 1 and sourced from existing literature. These include Beta-blockers such as Metoprolol3, Atenolol4, Bisoprolol5, and Propranolol6 work by slowing heart rate and reducing myocardial oxygen demand. Timolol7 is another beta-blocker used for heart rate control in specific arrhythmias. Zhang et al.13 presented that exosomal derived from M2 macrophages inhibits CVB3-induced viral myocarditis by regulating PKM2/HIF-1a, which results in macrophages metabolism, and immunoreaction. Dual-action agents such as Sotalol8 acts as both a beta-blocker and an anti arrhythmic, helping to maintain sinus rhythm.
Fig. 1.
Structures of anti-arrhythmia drugs.
Li et al.14 developed an upper-tyramine (TY) signal amplification probe based on nanozyme for myocarditis-related miRNAs with high sensitivity in heart without pre-amplification, and established a nanospring TMB microplate method for a rapid clinical diagnostic of myocarditis. Broad-spectrum anti arrhythmics are Amiodarone9 and Carvedilol10 are commonly prescribed for more severe arrhythmias due to their ability to control heart rate. Pei et al.15 designed extracellular vesicles for multitargeted immunomodulatory therapy, which could provide a novel approach to treat viral myocarditis based on enhancing host immune regulation.
Flecainide11, and Propafenone12 are Sodium channel blockers that stabilize electrical signals of heart. These drugs were chosen due to their well-known mechanisms of action that effectively target various types of arrhythmias. While generally effective, some have minor side effects, such as fatigue and dizziness with metoprolol and atenolol. More severe adverse effects associated with amiodarone are thyroid dysfunction, pulmonary toxicity, and sotalol has the risk of inducing arrhythmias by prolonging the QT interval. Zhang et al.16 published the first case of subcutaneous ICD placement in a child with Timothy syndrome demonstrating a new technique for pediatric patient management of cardiac risk. Wei et al.17 performed a systematic review and meta-analysis that indicated that Shenfu injection was effective and safe in the treatment of bradyarrhythmia, supporting that it can be applied as a complementary therapy. Liu et al.18 in mice, they had different effects on cardiac function and thus can be new targets for heart failure therapies. To accelerate the development of more effective drugs, in our research, by applying topological indices, entropy, and concepts from chemical graph theory, we were able to forecast the physicochemical properties of these substances.
In mathematics, a graph is a fundamental structure consisting of a set of vertices (or nodes) and a set of edges (or links) that connect pairs of these vertices. Formally, a graph G is an ordered pair (V, E), where V is the set of vertices and E is the set of edges. Graph theory is the branch of mathematics that studies graphs. It explores the properties, structures, and algorithms related to graphs19. Early contributions to graph theory can be traced back to Leonhard Euler’s work on the Koinigsberg bridge problem in 1736, which is often considered the birth of the field20. Since then, graph theory has grown into a vast and vigorous area of research with many uses.
Chemical graph theory is a particular area that applies graph theory to model and recognize chemical structures. In this context, atoms are classically symbolized as vertices, and the chemical bonds between them are represented as edges. This permits chemists to define molecular structures using graph-theoretical conceptions21. By converting molecules into graphs, various topological indices can be calculated to predict physicochemical properties, biological activities, and reactivity of chemical compounds. Innovative work in chemical graph theory by researchers like Wiener and Hosoya laid the basis for its improvement22,23. It has turned into an essential tool in cheminformatics, drug innovation, and materials science.
In QSPR analysis, graph-based structures play a crucial role in developing innovative drugs24. We have established relationships between molecular structure and physicochemical properties by calculating six specific indices. The findings contribute to the growing literature on topological indices and their applications in pharmaceutical research.
Huang et al.25 employed XGBoost and regressive models in the QSPR studies of glaucoma drugs, in successful relation of topological descriptors and pharmacological activities for the predictive purposes. Qin et al.26, mined Python based topological modeling to straw test pulmonary cancer drugs and accomplished 821 reliable QPSR predictions via integration of graph-theoretic indices and computation. Qin et al.27 investigated anti-arrhythmic drug features utilizing Python and topological indices, an indicative high accuracy of QSPR-based physicochemical property predicition has been demonstrated. Wei et al.28 for several drugs by the linear regression models, which proved that QSPR could be a good alternative to estimate physical properties of compounds from mathematical descriptors.
Researchers have created various graph polynomials to make calculation of topological indices easier. For example, the Hosoya polynomial29 simplifies indices based on distance, the M-polynomial helps with calculations based on vertex degrees30, and the neighborhood M-polynomial handle indices related to the sum of degrees of neighboring vertices. These methods have been widely applied to many different molecules, graph networks, and structures in various studies. In 2021, Mondal et al.31 introduced the neighborhood M-polynomial. This tool streamlines the calculation of neighborhood degree sum-based indices, much like how the M-polynomial simplifies degree-based index computations. Essentially, it allows the direct and easy computation of these indices. The neighborhood M-polynomial of a graph  , denoted by
, denoted by  , is defined as:
, is defined as:
| 1 |
where  , is the number of edges uv in
, is the number of edges uv in  such that
such that  and
and  , where
, where  and
and 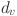 are the degrees of vertices u and v, respectively. Table 1 summarizes the relationships between neighborhood degree sum-based topological indices, and their corresponding NM-polynomial.
are the degrees of vertices u and v, respectively. Table 1 summarizes the relationships between neighborhood degree sum-based topological indices, and their corresponding NM-polynomial.
Table 1.
Degree-based topological indices derivations.
| Topological indices | Derivation fromNM(s, t) |
|---|---|
 |
 |
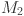 |
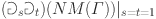 |
 |
 |
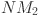 |
 |
| NH |  |
| NSS | 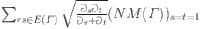 |
Where, 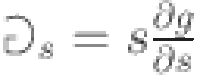 ,
, 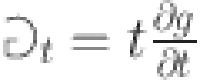 ,
,  ,
, 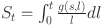 ,
,  ,
,
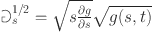 ,
, 
 ,
,  are operators.
are operators.
This motivation behind this study is to introduce a novel, cost-effective method to predict vital properties and biological activities of anti-arrhythmia drugs by combining molecular modeling with decision-making techniques and utilizing topological indices. The study claims that this model is better than the existing ones by offering a rapid approach to predict drug properties and rank alternatives, which complements traditional time-consuming and expensive clinical trials.
Methodology
This study performed a QSPR (Quantitative Structure-Property Relationship) analysis to rank medicines for arrhythmia treatment Fig. 1. We investigated how topological indices relate to key drug properties that we obtained from Camspider like boiling point (BP), molar volume (MV), density (D), polarizability (P), BFC, KOC and flash point (FP). As shown in Fig. 2, our methodology involved quadratic regression. This analysis revealed a strong correlation among the physicochemical properties of effective drugs and specific qualities derived from related neighborhood topological indices.
The first step involved identifying the top arrhythmia medications from existing studies and converting their molecular structures into graphs using chemical graph theory.
We analyzed the structures by classifying their edges according to the number of bonds on their starting and ending atoms, and then tallied the frequency of each category.
To save time on manual computations, a Python program was developed to automate the calculation of topological indices and entropies.
For more precise predictions of physicochemical properties, regression models were constructed using the indices. We used an Anaconda environment to guarantee consistent results and enhance computational speed.
We visualize the models on the basis of R,
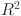 and P values and drew the scatter plots.
and P values and drew the scatter plots.By using MCDM (TOPSIS and SAW) we ranked anti arrhythmia drugs.
Fig. 2.
Methodology.
Results and discussion
In this section, we will correlate neighborhood degree sum-based topological indices with physicochemical properties of ten anti-arrhythmia drugs to demonstrate the potential of these indices in predicting drug behavior and aiding in drug design. We will apply TOPSIS and SAW multi-criteria decision-making approaches, combined with the entropy method for weight allocation, to rank the drugs, highlighting their potential effectiveness based on the analyzed properties.
Let  is the chemical graph of Metoprolol, so the neighborhood M-polynomial is:
is the chemical graph of Metoprolol, so the neighborhood M-polynomial is:
| 2 |
Let  is the chemical graph of Propranolol, so the neighborhood M-polynomial is:
is the chemical graph of Propranolol, so the neighborhood M-polynomial is:
| 3 |
Let  is the chemical graph of Atenolol, then the neighborhood M-polynomial is:
is the chemical graph of Atenolol, then the neighborhood M-polynomial is:
| 4 |
Let  is the chemical graph of Bisoprolol, so the neighborhood M-polynomial is:
is the chemical graph of Bisoprolol, so the neighborhood M-polynomial is:
| 5 |
Let  is the chemical graph of Sotalol, so the neighborhood M-polynomial is:
is the chemical graph of Sotalol, so the neighborhood M-polynomial is:
| 6 |
Let  is the chemical graph of Amiodarone, so the neighborhood M-polynomial is:
is the chemical graph of Amiodarone, so the neighborhood M-polynomial is:
| 7 |
Let  is the chemical graph of Carvedilol, so the neighborhood M-polynomial is;
is the chemical graph of Carvedilol, so the neighborhood M-polynomial is;
| 8 |
Let  is the chemical graph of Flecainide, so the neighborhood M-polynomial is:
is the chemical graph of Flecainide, so the neighborhood M-polynomial is:
| 9 |
Let  is the chemical graph of Timolol, so the neighborhood M-polynomial is:
is the chemical graph of Timolol, so the neighborhood M-polynomial is:
| 10 |
Let  is the chemical graph of Propafenone, so the neighborhood M-polynomial is:
is the chemical graph of Propafenone, so the neighborhood M-polynomial is:
| 11 |
By using Eqs. (2)–(11) and Table 1 we get Table 2. Where as, Table 3 shows the physicochemical properties of anti arrhythmia drugs sourced from Chemspider.
Table 2.
Topological indices of anti-arrhythmia drugs.
| Drugs | 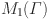 |
 |
 |
 |
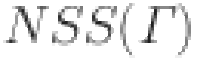 |
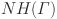 |
|---|---|---|---|---|---|---|
| Metoprolol | 84 | 89 | 178 | 427 | 28.77 | 4.24 |
| Atenolol | 86 | 91 | 162 | 395 | 25.9 | 3.75 |
| Bisoprolol | 102 | 107 | 214 | 505 | 34.79 | 5.07 |
| Propranolol | 92 | 103 | 206 | 547 | 31.63 | 4.07 |
| Sotalol | 86 | 92 | 181 | 461 | 28.21 | 3.69 |
| Amiodarone | 156 | 184 | 368 | 1074 | 53.72 | 6.46 |
| Carvedilol | 154 | 180 | 360 | 1029 | 53.58 | 6.41 |
| Flecainide | 140 | 153 | 303 | 803 | 46.42 | 5.69 |
| Propafenone | 116 | 128 | 256 | 649 | 40.18 | 5.56 |
| Timolol | 104 | 115 | 230 | 614 | 35.13 | 4.36 |
Table 3.
Physicochemical properties of anti-arrhythmia drugs.
| Drugs | D | BP | FP | BCF | KOC | P | MV |
|---|---|---|---|---|---|---|---|
| Metoprolol | 1.00 | 398.60 | 194.90 | 1.00 | 1.00 | 30.60 | 258.70 |
| Atenolol | 1.10 | 508.00 | 261.10 | 1.00 | 1.00 | 29.40 | 236.70 |
| Bisoprolol | 1.00 | 445.00 | 222.90 | 1.00 | 1.00 | 36.70 | 315.00 |
| Propranolol | 1.10 | 434.90 | 216.80 | 1.25 | 1.00 | 31.30 | 237.20 |
| Sotalol | 1.20 | 443.30 | 221.90 | 1.00 | 1.00 | 28.60 | 219.70 |
| Amiodarone | 1.60 | 635.10 | 337.90 | 214.87 | 223.42 | 57.20 | 408.20 |
| Carvedilol | 1.30 | 655.20 | 350.10 | 1.51 | 8.15 | 47.40 | 325.10 |
| Flecainide | 1.30 | 434.90 | 216.80 | 1.00 | 1.07 | 34.80 | 322.10 |
| Propafenone | 1.10 | 519.60 | 268.00 | 1.00 | 1.61 | 39.80 | 311.40 |
| Timolol | 1.20 | 487.20 | 248.50 | 1.00 | 1.00 | 32.60 | 258.50 |
QSPR analysis of anti-arrhythmia drugs
In this study, we apply quadratic QSPR (Quantitative Structure-Property Relationship) models to understand the connections between these measurements, aiming to identify the most effective treatments for arrhythmia by studying their molecular properties. Table 3 lists the physicochemical properties of the arrhythmia drugs under investigation sourced from Chemspider. This work draws inspiration from prior studies on anti-cancer drugs, and QSPR analysis of various topological indices across different molecular architectures. This research aims to explore the effectiveness of topological indices (TIs) in replicating QSPR properties and their potential in managing arrhythmia treatment. A quadratic regression model is a statistical method used to define the relationship between a dependent variable and one or more independent variables. The general form of a quadratic regression equation is typically expressed as:
where  are constants. We are using a quadratic regression model because it offers greater flexibility in linking topological indices (independent variables), to chemical properties P (response variables). This allows us to thoroughly examine the relationships between these indices and the compounds’ characteristics. Our aim is to pinpoint the strongest correlations, uncovering patterns that help to anticipate or interpret chemical behaviors. Statistical details for the quadratic QSPR model, including various topological indices, are presented in Tables 4 through 9. Additionally, Table 10 illustrates the correlation coefficients between these indices and different properties. All computations were performed using (SPSS Statistics 27.0.1.0, (https://www.ibm.com/support/pages/downloading-ibm-spss-statistics-27010)). We specifically selected the topological indices
are constants. We are using a quadratic regression model because it offers greater flexibility in linking topological indices (independent variables), to chemical properties P (response variables). This allows us to thoroughly examine the relationships between these indices and the compounds’ characteristics. Our aim is to pinpoint the strongest correlations, uncovering patterns that help to anticipate or interpret chemical behaviors. Statistical details for the quadratic QSPR model, including various topological indices, are presented in Tables 4 through 9. Additionally, Table 10 illustrates the correlation coefficients between these indices and different properties. All computations were performed using (SPSS Statistics 27.0.1.0, (https://www.ibm.com/support/pages/downloading-ibm-spss-statistics-27010)). We specifically selected the topological indices 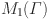 ,
,  ,
,  ,
,  ,
, 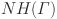 ,
, 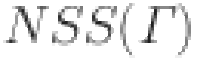 because of their strong correlations with the physicochemical properties of potential arrhythmia drugs. We confirmed these correlations using a quadratic regression model in SPSS, which showed these indices were not chosen arbitrarily but for their significant relationship with drug properties. This deliberate selection enabled more effective ranking of the drugs and informed decision-making regarding their use in arrhythmia treatment.
because of their strong correlations with the physicochemical properties of potential arrhythmia drugs. We confirmed these correlations using a quadratic regression model in SPSS, which showed these indices were not chosen arbitrarily but for their significant relationship with drug properties. This deliberate selection enabled more effective ranking of the drugs and informed decision-making regarding their use in arrhythmia treatment.
Table 4.
Regression results for 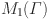 .
.
| Property | N | A | B | C | R | R
|
F | RMSE | P |
|---|---|---|---|---|---|---|---|---|---|
| D | 10 | 2.0622 | −0.0208 | 0.0001 | 0.8539 | 0.7292 | 9.423 | 0.0925 | 0.01 |
| BP | 10 | 603.7442 | −3.7566 | 0.0229 | 0.6792 | 0.4612 | 2.9978 | 51.48 | 0.11 |
| FP | 10 | 479.0312 | −5.3411 | 0.0281 | 0.8008 | 0.6412 | 6.256 | 29.86 | 0.02 |
| BCF | 10 | 568.9568 | −10.9117 | 0.0509 | 0.6609 | 0.4367 | 2.713 | 0.275 | 0.13 |
| KOC | 10 | 600.5683 | −11.5314 | 0.0539 | 0.6798 | 0.4621 | 3.0006 | 48.77 | 0.11 |
| P | 10 | 34.9526 | −0.2498 | 0.0023 | 0.9175 | 0.8419 | 18.63 | 3.45 | 0.001 |
| MV | 10 | 7.7104 | 3.2116 | −0.0058 | 0.8794 | 0.7733 | 10.9397 | 26.35 | 0.005 |
Table 9.
Regression results for 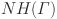 .
.
| Property | N | A | B | C | R | R
|
F | RMSE | P |
|---|---|---|---|---|---|---|---|---|---|
| D | 10 | 3.24 | −0.9249 | 0.1032 | 0.8266 | 0.6823 | 7.55 | 0.10 | 0.02 |
| BP | 10 | 1395.99 | −410.67 | 44.28 | 0.7422 | 0.5509 | 4.29 | 47.01 | 0.06 |
| FP | 10 | 1020.79 | −345.68 | 37.22 | 0.8703 | 0.7574 | 10.93 | 24.56 | 0.007 |
| BCF | 10 | 789.88 | −345.42 | 37.15 | 0.6708 | 0.4499 | 2.86 | 47.57 | 0.12 |
| KOC | 10 | 837.32 | −366.47 | 39.45 | 0.6908 | 0.4772 | 3.19 | 48.08 | 0.10 |
| P | 10 | 52.54 | −14.62 | 2.25 | 0.9441 | 0.8913 | 28.69 | 2.86 | 0.0004 |
| MV | 10 | −121.42 | 112.44 | −5.64 | 0.9240 | 0.8537 | 20.42 | 0.81 | 0.001 |
Table 10.
Correlation coefficients between topological indices and physicochemical properties.
| Topological indices | D | BP | FP | BCF | KOC | P | MV |
|---|---|---|---|---|---|---|---|
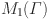 |
0.8539 | 0.6792 | 0.8008 | 0.6609 | 0.6798 | 0.9175 | 0.8794 |
 |
 |
0.7137 | 0.8462 | 0.7028 | 0.7222 | 0.9342 | 0.8657 |
 |
0.8545 | 0.7257 | 0.8622 | 0.6976 | 0.7170 | 0.9391 | 0.8681 |
 |
0.8615 | 0.7210 | 0.8695 |  |
 |
0.9367 | 0.8373 |
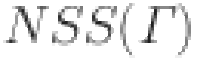 |
0.8032 | 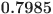 |
 |
0.6198 | 0.6422 | 0.9404 |  |
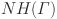 |
0.8266 | 0.7422 | 0.8703 | 0.6708 | 0.6908 | 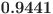 |
0.9240 |
Siginificance values are in bold.
The following sections will detail the quadratic regression model’s findings, discussing the various physicochemical properties of these potential drugs in relation to the topological indices for arrhythmia disease, all computed by using SPSS software.
Regression models for 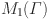
 |
Regression models for 
 |
Regression models for 
 |
Regression models for 
 |
Regression models for 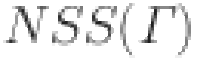
 |
Regression models for 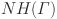
 |
We use things like 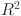 , and Adjusted
, and Adjusted 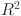 to see how reliable our prediction formulas are, and they are defined as:
to see how reliable our prediction formulas are, and they are defined as: 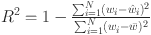 ,
,
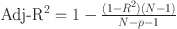 .
.
Where 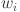 is the actual value of the dependent variable,
is the actual value of the dependent variable,  is predicted value of the dependent variable,
is predicted value of the dependent variable, 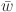 is mean of the actual values of dependent variable and p represents several predictors used in the regression model. N represents the number of data points we used to create the prediction formulas. changeR-squared
is mean of the actual values of dependent variable and p represents several predictors used in the regression model. N represents the number of data points we used to create the prediction formulas. changeR-squared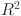 tells us how well the formulas fit the data.
tells us how well the formulas fit the data. 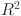 and Adj-
and Adj-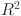 close to 1 indicate a good regression model. To get more information we refer to32.
close to 1 indicate a good regression model. To get more information we refer to32.
As shown in Table 4, the 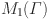 index’s regression models and statistical parameters highlight its strong predictive capability for Density, Molar Volume, and Flash Point, with high R values (0.8539, 0.8794, and 0.8008). However, its predictive power for Boiling Point, BCF, and KOC is only average, given their moderate R values and high P-values. Models for Polarizability demonstrate weak prediction with lower R values. From Table 5, it is clear that regression models for density, flash point, polarizability and molar volume have high correlation coefficient values (
index’s regression models and statistical parameters highlight its strong predictive capability for Density, Molar Volume, and Flash Point, with high R values (0.8539, 0.8794, and 0.8008). However, its predictive power for Boiling Point, BCF, and KOC is only average, given their moderate R values and high P-values. Models for Polarizability demonstrate weak prediction with lower R values. From Table 5, it is clear that regression models for density, flash point, polarizability and molar volume have high correlation coefficient values ( ), low (
), low (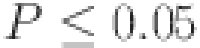 ) and all these models have high F-statistics values which show models are statistically efficient to predict values of physicochemical properties of anti arrhythmia drugs. Table 6, shows that polarizability has high R-value (0.9391) that is
) and all these models have high F-statistics values which show models are statistically efficient to predict values of physicochemical properties of anti arrhythmia drugs. Table 6, shows that polarizability has high R-value (0.9391) that is  is strong predictor for polarizability.
is strong predictor for polarizability.  show moderate correlation for density, flash point, and molar volume with R-values (0.8545, 0.8622, 0.8681). Table 7, shows that the regression models for polarizability, density, flash point and molar volume are strong predictors, evidenced by their high R-values (0.9367, 0.8615, 0.8695 and0.8373 respectively) and low P-values
show moderate correlation for density, flash point, and molar volume with R-values (0.8545, 0.8622, 0.8681). Table 7, shows that the regression models for polarizability, density, flash point and molar volume are strong predictors, evidenced by their high R-values (0.9367, 0.8615, 0.8695 and0.8373 respectively) and low P-values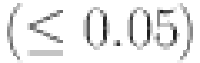 . This suggests that models like BCF, KOC and BP, with their lower values are good predictors for these properties.
. This suggests that models like BCF, KOC and BP, with their lower values are good predictors for these properties.
Table 5.
Regression results for  .
.
| Property | N | A | B | C | R | R
|
F | RMSE | P |
|---|---|---|---|---|---|---|---|---|---|
| D | 10 | 1.54 | −0.009 | 0.0001 | 0.8554 | 0.7316 | 9.54 | 0.921 | 0.01 |
| BP | 10 | 565.01 | −2.608 | 0.0148 | 0.7137 | 0.5093 | 3.63 | 49.73 | 0.08 |
| FP | 10 | 413.46 | −3.637 | 0.0176 | 0.8462 | 0.7161 | 8.82 | 26.56 | 0.01 |
| BCF | 10 | 416.88 | −7.284 | 0.0307 | 0.7028 | 0.4940 | 3.42 | 45.62 | 0.09 |
| KOC | 10 | 438.55 | −7.675 | 0.0325 | 0.7220 | 0.5213 | 3.81 | 72.20 | 0.07 |
| P | 10 | 32.10 | −0.147 | 0.0014 | 0.9342 | 0.8727 | 23.99 | 3.81 | 0.007 |
| MV | 10 | 77.63 | 2.101 | −0.0023 | 0.8657 | 0.7495 | 10.47 | 23.99 | 0.007 |
Table 6.
Regression results for  .
.
| Property | N | A | B | C | R | R
|
F | RMSE | P |
|---|---|---|---|---|---|---|---|---|---|
| D | 10 | 1.5641 | −0.0051 | 0.0000 | 0.8545 | 0.7302 | 9.47 | 0.0923 | 0.01 |
| BP | 10 | 669.01 | −2.128 | 0.0052 | 0.7257 | 0.5266 | 3.89 | 48.26 | 0.07 |
| FP | 10 | 451.80 | −2.13 | 0.0050 | 0.8622 | 0.7434 | 10.13 | 25.25 | 0.08 |
| BCF | 10 | 330.52 | −3.00 | 0.0066 | 0.6976 | 0.4876 | 3.32 | 45.95 | 0.09 |
| KOC | 10 | 348.18 | −3.17 | 0.0070 | 0.7170 | 0.5141 | 3.70 | 46.35 | 0.08 |
| P | 10 | 32.48 | −0.075 | 0.0004 | 0.9391 | 0.8820 | 25.66 | 3.012 | 0.0006 |
| MV | 10 | 112.31 | −0.766 | −0.0002 | 0.8681 | 0.7535 | 10.70 | 27.47 | 0.07 |
Table 7.
Regression results for  .
.
| Property | N | A | B | C | R | R
|
F | RMSE | P |
|---|---|---|---|---|---|---|---|---|---|
| D | 10 | 1.8 | −0.0007 | 0.0000 | 0.8615 | 0.7421 | 10.07 | 0.0903 | 0.0008 |
| BP | 10 | 523.01 | −0.343 | 0.0004 | 0.7210 | 0.5199 | 3.78 | 48.60 | 0.07 |
| FP | 10 | 339.3 | −0.430 | 0.0004 | 0.8695 | 0.7561 | 10.84 | 24.62 | 0.007 |
| BCF | 10 | 225.19 | −0.784 | 0.0006 | 0.7398 | 0.5473 | 4.23 | 43.15 | 0.06 |
| KOC | 10 | 235.32 | −0.821 | 0.0007 | 0.7583 | 0.575 | 4.73 | 43.35 | 0.05 |
| P | 10 | 30.95 | −0.015 | 0.0000 | 0.9367 | 0.8773 | 25.03 | 3.04 | 0.0006 |
| MV | 10 | 177.97 | 0.1439 | 0.0000 | 0.8373 | 0.7010 | 8.21 | 30.26 | 0.01 |
Tables 8 and 9, show that the regression models for polarizability, density, flash point and molar volume are strong predictors, evidenced by their high R-values (0.9404, 0.8032, 0.9001 and 0.8767), (0.9441, 0.8266, 0.8703 and 0.9240) respectively, and low P-values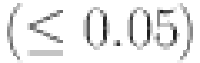 . This suggests that models like BP, with their lower values, are moderate predictors for boiling point. For BCF and KOC, the model shows a much lower predictive capability, which is reflected in their respective R-values, indicating that
. This suggests that models like BP, with their lower values, are moderate predictors for boiling point. For BCF and KOC, the model shows a much lower predictive capability, which is reflected in their respective R-values, indicating that 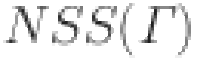 and
and 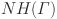 cannot predict these properties with high accuracy. Table 10, illustrates the correlation coefficients between topological indices and physical and chemical properties of anti arrhythmia drugs.
cannot predict these properties with high accuracy. Table 10, illustrates the correlation coefficients between topological indices and physical and chemical properties of anti arrhythmia drugs.
Table 8.
Regression results for 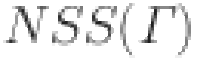 .
.
| Property | N | A | B | C | R | R
|
F | RMSE | P |
|---|---|---|---|---|---|---|---|---|---|
| D | 10 | 1.50 | −0.0306 | 0.0005 | 0.8032 | 0.6451 | 6.36 | 0.1059 | 0.02 |
| BP | 10 | 826.21 | −22.73 | 0.3414 | 0.7985 | 0.6377 | 6.15 | 42.22 | 0.02 |
| FP | 10 | 524.28 | −18.12 | 0.2725 | 0.9001 | 0.8102 | 14.94 | 21.72 | 0.003 |
| BCF | 10 | 247.53 | −15.32 | 0.232 | 0.6198 | 0.3841 | 2.18 | 50.33 | 0.18 |
| KOC | 10 | 267.17 | −16.53 | 0.251 | 0.6422 | 0.4124 | 2.46 | 50.97 | 0.15 |
| P | 10 | 24.22 | −0.142 | 0.012 | 0.9404 | 0.88844 | 26.77 | 2.97 | 0.0005 |
| MV | 10 | −10.7559 | 10.8872 | −0.0724 | 0.8767 | 0.7686 | 11.62 | 26.62 | 0.006 |
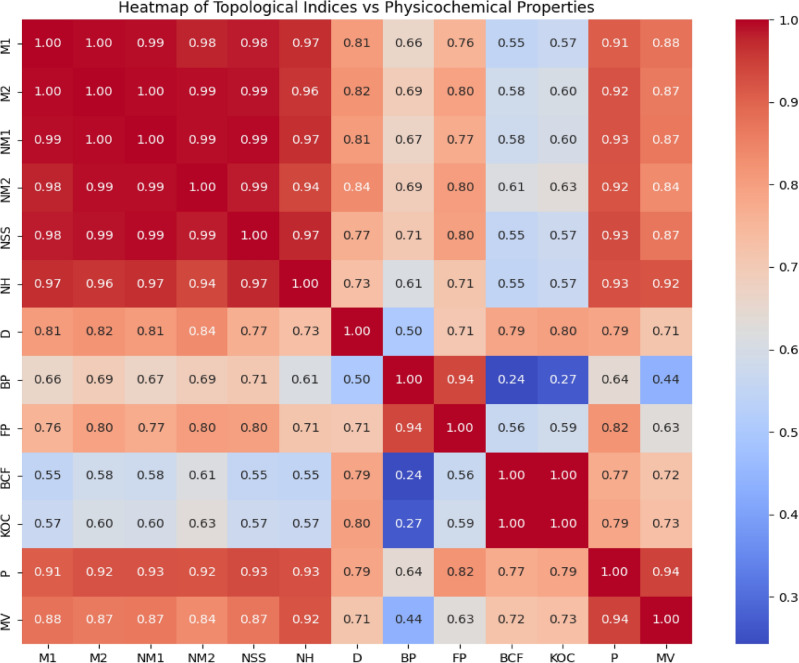
Figure 3, visualize the correlation between topological indices and physicochemical properties which offers a powerful graphical representation for understanding intricate relationships in chemical systems. Each cell within the heatmap displays the correlation coefficient between a specific topological index derived from molecular structures of anti arrhythmia drugs and a particular physicochemical property (like boiling point, density). This visualization allows researchers to quickly identify which structural descriptors are most strongly correlated, positively or negatively, with various molecular characteristics, aiding in property prediction and rational drug design.
Fig. 3.
Heatmap of topological indices vs physicochemical properties.
The Figure 4, presents the optimal quadratic fits for D by  , BP by
, BP by 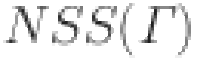 , FP by
, FP by 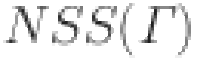 , BCF by
, BCF by  , KOC by
, KOC by  , P by
, P by 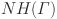 , and MV by
, and MV by 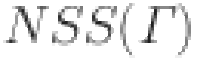 . The graphs have a quadratic fit with high
. The graphs have a quadratic fit with high 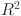 values that represents the data fairly accurate. The high
values that represents the data fairly accurate. The high 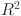 value (0.8913) indicate that the quadratically related relationship between
value (0.8913) indicate that the quadratically related relationship between 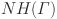 and P is very exact in predicting the data.
and P is very exact in predicting the data.
Fig. 4.
Plots of quadratic regression models that predict optimal physicochemical properties using degree-based topological indices.
Application of multi-criteria decision making to drug analysis
Multi-Criteria Decision Making (MCDM) helps choose the best option when several good choices exist instead of one perfect answer. This research uses specific types of mathematical descriptors, called degree based and neighborhood degree-based topological indices, to analyze drugs that treat arrhythmia. By performing QSPR (Quantitative Structure-Property Relationship) analysis, the study reveals a strong link between these indices and the physical and chemical characteristics of arrhythmia medications like Metoprolol, Atenolol, Amiodarone, Bisoprolol, Propranolol, Sotalol, Carvedilol, Flecainide, Propafenone and Timolol.
This research investigates the chemical properties of substances by employing various topological indices, including the Zagreb, neighborhood harmonic, neighborhood Zagreb, and neighborhood Shilpa-Shanmukha indices. To efficiently pinpoint both the best and worst possible outcomes, a weighted evaluation of these indices will be performed using a decision-making technique like TOPSIS and SAW.
This study also assesses how accurately molecular compounds are described using mathematical techniques. It combines Multi-Criteria Decision Making (MCDM), a method dating back to the 1980s33, with the entropy method to assign weights to various topological indices. The entropy method ensures an impartial assessment of each index’s relevance in predicting drug properties by assigning weights according to data variability. This means that structural features of a drug that show more diversity across compounds will be given greater importance. This systematic approach helps eliminate personal biases, leading to more precise molecular evaluations. The weights are allocated using the formula:
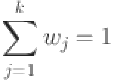 |
Effectiveness of a drug hinges on its physicochemical properties. To identify the most and least desirable characteristics, it’s crucial to examine factors like density, molar volume, and boiling point, flash point, KOC and BFC. For example, a lower density generally leads to better solubility, while a lower melting point makes a drug easier to use. Molar volume plays a role in how a drug crystallizes, with most effective medications typically weighing less than 1000 g/mol. A higher boiling point is beneficial for storage, and the complexity of a drug can impact both how it is administered and its cost.
In Table 13, using the entropy method, we objectively assigned weights to each drug property. This data-driven approach accurately assesses drug characteristics by determining each property’s weight based on its ability to differentiate between drugs, effectively removing any subjective bias.
Table 13.
Weight allocation using entropy method.
| Topological Index | 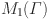 |
 |
 |
 |
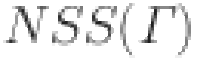 |
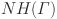 |
|---|---|---|---|---|---|---|
| Weights | 0.170372 | 0.166171 | 0.164530 | 0.155294 | 0.168411 | 0.175222 |
Ranking of drugs using TOPSIS
We look at each property on its own, then figure out the best choices by seeing how closely they match what we ideally want. Table 11 presents n criteria such as Zagreb indices and harmonic index, and m alternatives i.e, drug structures. To determine the best option, appropriate attribute weights are assigned.
Table 11.
Topological indices(attributes) and drugs(alternatives).
| Drugs | 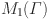 |
 |
 |
 |
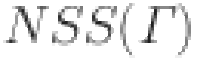 |
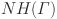 |
|---|---|---|---|---|---|---|
| Metoprolol | 84 | 89 | 178 | 427 | 28.77 | 4.24 |
| Atenolol | 86 | 91 | 162 | 395 | 25.9 | 3.75 |
| Bisoprolol | 102 | 107 | 214 | 505 | 34.79 | 5.07 |
| Propranolol | 92 | 103 | 206 | 547 | 31.63 | 4.07 |
| Sotalol | 86 | 92 | 181 | 461 | 28.21 | 3.69 |
| Amiodarone | 156 | 184 | 368 | 1074 | 53.72 | 6.46 |
| Carvedilol | 154 | 180 | 360 | 1029 | 53.58 | 6.41 |
| Flecainide | 140 | 153 | 303 | 803 | 46.42 | 5.69 |
| Propafenone | 116 | 128 | 256 | 649 | 40.18 | 5.56 |
| Timolol | 104 | 115 | 230 | 614 | 35.13 | 4.36 |
Step 1 Construct an evaluation matrix 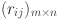 representing where each metric connects with each available choice.
representing where each metric connects with each available choice.
Step 2 Systematize this matrix to get a normalized version 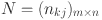 , Table 12.
, Table 12.
Table 12.
Decision matrix.
| Drugs | 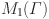 |
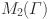 |
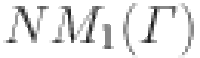 |
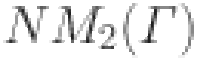 |
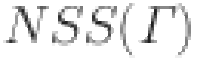 |
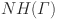 |
|---|---|---|---|---|---|---|
| Metoprolol | 0.230644 | 0.218443 | 0.220074 | 0.195706 | 0.233787 | 0.267839 |
| Atenolol | 0.236135 | 0.223352 | 0.200292 | 0.181040 | 0.210465 | 0.236886 |
| Bisoprolol | 0.280068 | 0.262623 | 0.264583 | 0.231456 | 0.282706 | 0.320269 |
| Propranolol | 0.252610 | 0.252805 | 0.254692 | 0.250706 | 0.257028 | 0.257100 |
| Sotalol | 0.236135 | 0.225806 | 0.223783 | 0.211289 | 0.232487 | 0.256468 |
| Amiodarone | 0.428339 | 0.451613 | 0.454984 | 0.492245 | 0.436533 | 0.408075 |
| Carvedilol | 0.422847 | 0.441795 | 0.445093 | 0.471620 | 0.443521 | 0.404916 |
| Flecainide | 0.384407 | 0.375526 | 0.374620 | 0.368038 | 0.354459 | 0.359434 |
| Propafenone | 0.318508 | 0.314166 | 0.316510 | 0.297455 | 0.326506 | 0.319638 |
| Timolol | 0.285559 | 0.282258 | 0.284365 | 0.281414 | 0.285469 | 0.275419 |
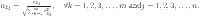
Step 3 Let’s break down how we get to our weighted normalized decision matrix, which we are calling  and you can find in Table 14. We created it by applying the weights that were given to us in a separate Table 13. We will do this by using a specific formula
and you can find in Table 14. We created it by applying the weights that were given to us in a separate Table 13. We will do this by using a specific formula
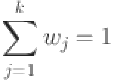 |
The normalized value is 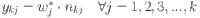 , where
, where 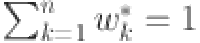
Table 14.
Weighted decision matrix.
| Drugs |
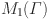
|

|

|

|
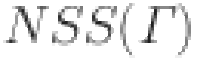
|
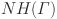
|
|---|---|---|---|---|---|---|
| Metoprolol | 0.039295 | 0.036299 | 0.036209 | 0.030392 | 0.039372 | 0.046931 |
| Atenolol | 0.040231 | 0.037115 | 0.032954 | 0.028114 | 0.035444 | 0.041508 |
| Bisoprolol | 0.047716 | 0.043640 | 0.043532 | 0.035944 | 0.047611 | 0.056118 |
| Propranolol | 0.043038 | 0.042009 | 0.041904 | 0.038933 | 0.043286 | 0.045050 |
| Sotalol | 0.040231 | 0.037522 | 0.036819 | 0.032812 | 0.039153 | 0.044939 |
| Amiodarone | 0.072977 | 0.075045 | 0.074858 | 0.076443 | 0.073517 | 0.071504 |
| Carvedilol | 0.072041 | 0.073414 | 0.073231 | 0.073240 | 0.074693 | 0.070950 |
| Flecainide | 0.065492 | 0.062402 | 0.061636 | 0.057154 | 0.059694 | 0.062981 |
| Propafenone | 0.054265 | 0.052205 | 0.052075 | 0.046193 | 0.054987 | 0.056008 |
| Timolol | 0.048651 | 0.046903 | 0.046787 | 0.043702 | 0.048076 | 0.048259 |
Step 4 We are going to determine the best-case scenario ( positive ideal) and the worst-case scenario (negative ideal) for each of our evaluation categories, Table 15. We are going to compare each option to the absolute best possible option (the “ideal” one) and see how much they differ, which is described as, 
Table 15.
Calculation of ideal best and ideal worst.
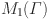
|

|

|

|
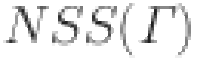
|
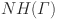
|
|
|---|---|---|---|---|---|---|
Ideal Best ( ) ) |
0.072977 | 0.075045 | 0.074858 | 0.076443 | 0.074693 | 0.071504 |
Ideal Worst (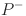 ) ) |
0.039295 | 0.036299 | 0.032954 | 0.028114 | 0.035444 | 0.041508 |
Step 5 We measure this difference using a method called Euclidean distance, which calculates the “straight-line” distance in multiple dimensions. This gives us a clear number showing how far apart they are. The optimal solution stands apart from the other options because. 
Figure 5, depicts the weights allocated to the topological indices. The Table 12 shows a decision matrix contrasting different drugs according to the value of different topological indices, such as 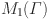 ,
,  ,
,  ,
,  ,
, 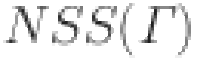 ,
, 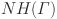 . Each drug corresponds to particular numerical scores for these indices. For example, Bisoprolol has scores of 0.280068, 0.262623, and 0.264583 for
. Each drug corresponds to particular numerical scores for these indices. For example, Bisoprolol has scores of 0.280068, 0.262623, and 0.264583 for 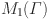 ,
,  , and
, and  , respectively, but Atenolol displays lower scores, such as 0.236135 for
, respectively, but Atenolol displays lower scores, such as 0.236135 for 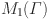 . This matrix can not predict if a drug will work, but it does assess how well drugs perform based on their topological characteristics. These characteristics might be related to a drug’s effectiveness in treating conditions like arrhythmia. The scores in the matrix show quantitative differences, which could help in making decisions since specific index scores should correlate with therapeutic success.
. This matrix can not predict if a drug will work, but it does assess how well drugs perform based on their topological characteristics. These characteristics might be related to a drug’s effectiveness in treating conditions like arrhythmia. The scores in the matrix show quantitative differences, which could help in making decisions since specific index scores should correlate with therapeutic success.
Fig. 5.
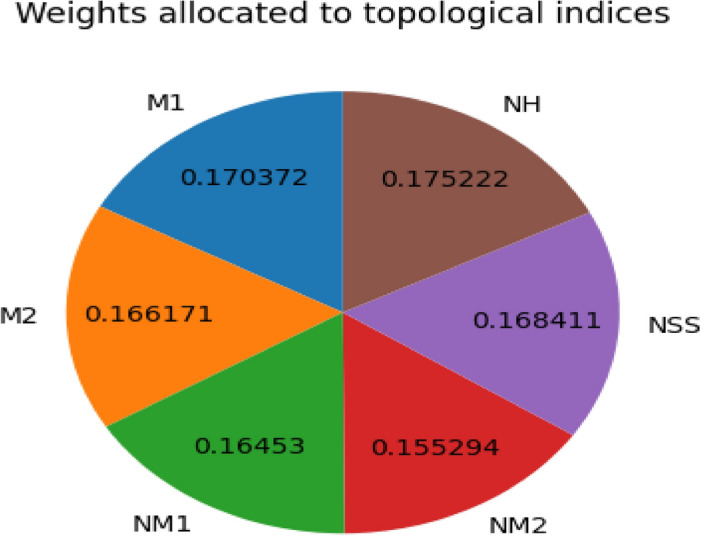
Weights allocated to topological indices.
Now we compute weights for each neighborhood degree based on topological indices in Table 13, and the weighted decision matrix Table 14.
Step 6 Determine the relative proximity 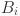 of each alternative answer to the ideal answer Table 15.
of each alternative answer to the ideal answer Table 15.

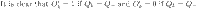
Step 7 This step involves ordering the reference answers from most similar to the ideal answer to least similar, based on the calculated proximity values 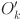 . The references are ranked in descending order of
. The references are ranked in descending order of 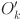 Table16.
Table16.
Table 16.
Drug ranking using TOPSIS.
| Drugs |

|
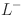
|

|
Rank |
|---|---|---|---|---|
| Amiodarone | 0.001176 | 0.095272 | 0.987807 | 1 |
| Carvedilol | 0.004093 | 0.092281 | 0.957534 | 2 |
| Flecainide | 0.032563 | 0.063897 | 0.662416 | 3 |
| Propafenone | 0.054170 | 0.041973 | 0.436569 | 4 |
| Timolol | 0.067026 | 0.028973 | 0.301809 | 5 |
| Bisoprolol | 0.072222 | 0.025681 | 0.262307 | 6 |
| Propranolol | 0.078530 | 0.017827 | 0.185013 | 7 |
| Sotalol | 0.088316 | 0.008057 | 0.083599 | 8 |
| Metoprolol | 0.090009 | 0.007786 | 0.079616 | 9 |
| Atenolol | 0.095094 | 0.001242 | 0.012890 | 10 |
Our study establishes what constitutes the best and worst choices by examining specific properties that influence how effective medicine is. The ideal best picks comprise high boiling point, polarizability, BCF and KOC. High boiling point can improve drug stability. This means the drug is less likely to break down or degrade at typical storage, A stable drug guarantees that patients get the correct amount of the active ingredient, and the medicine remains effective for its entire shelf life. A drug’s polarizability helps it interact well with different biological molecules and environments, which directly affects its ADME profile (how it’s absorbed, distributed, metabolized, and excreted). Higher value of BCF could reduce the required dosage frequency and potentially enhance therapeutic effect. A medicine with a high value of KOC means that the drug is less likely to leach into water sources, which is a significant environmental and public health benefit.
On the other hand, the ideal worst picks comprise lower values of boiling point, density, and molar volume. Lower boiling point make the compound unstable. A drug which has a very small molecular volume, it might be processed and eliminated from the body quickly. This shortens the time the drug stays active, meaning patients would need to take it more often. Such frequent dosing can make it harder for patients to stick to their medication schedule, potentially leading to inconsistent drug levels in their system which make it worst. When deciding on a drug for arrhythmia, we prioritize characteristics that improve how well it works, giving them more importance in our evaluation. On the other hand, we give less weight to properties that might reduce its effectiveness. This organized method helps us more effectively evaluate and select the best drug candidates for disease treatment.
SAW-based drug ranking
The Simple Additive Weighting (SAW) method, sometimes called the weighted scoring method, is a multi-criteria decision-making tool. It operates by computing a weighted average for each available option. In essence, SAW calculates an overall score for each alternative by multiplying its performance on various criteria by the corresponding importance (weight) of those criteria, and then summing these products. The steps involved in the SAW method compromise ranking procedure are:
Step 1 Create a conclusion matrix Table 11, list the m alternatives and n attributes, identifying the best and worst values for each attribute.
Step 2 Determine the weights by using previously established weighted norms. Subsequently, construct a normalized decision matrix Table 17, using the following formulas:
Table 17.
Normalized decision matrix.
| Drugs | 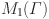 |
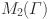 |
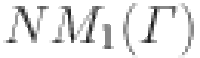 |
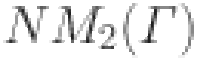 |
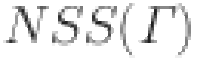 |
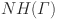 |
|---|---|---|---|---|---|---|
| Metoprolol | 0.538462 | 0.483696 | 0.483696 | 0.397579 | 0.527116 | 0.656347 |
| Atenolol | 0.551282 | 0.494565 | 0.440217 | 0.367784 | 0.474533 | 0.580495 |
| Bisoprolol | 0.653846 | 0.581522 | 0.581522 | 0.470205 | 0.637413 | 0.784830 |
| Propranolol | 0.589744 | 0.559783 | 0.559783 | 0.509311 | 0.579516 | 0.630031 |
| Sotalol | 0.551282 | 0.500000 | 0.491848 | 0.429236 | 0.524185 | 0.628483 |
| Amiodarone | 1.000000 | 1.000000 | 1.000000 | 1.000000 | 0.984243 | 1.000000 |
| Carvedilol | 0.987179 | 0.978261 | 0.978261 | 0.958101 | 1.000000 | 0.992260 |
| Flecainide | 0.897436 | 0.831522 | 0.823370 | 0.747672 | 0.799194 | 0.880805 |
| Propafenone | 0.743590 | 0.695652 | 0.695652 | 0.604283 | 0.736167 | 0.783282 |
| Timolol | 0.666667 | 0.625000 | 0.625000 | 0.571695 | 0.643642 | 0.674923 |

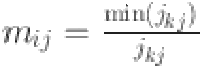
where 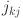 is the original value,
is the original value, 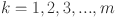 and
and  .
.
Step 3 Find the value of each replacement by applying the formula. The formula to calculate each substitute’s score is:

where  is the weighted sum for substitute i,
is the weighted sum for substitute i,  is the weight of attribute j.
is the weight of attribute j.  is the normalized value of substitute i for attribute j, and n is the total number of attributes Table 18.
is the normalized value of substitute i for attribute j, and n is the total number of attributes Table 18.
Table 18.
Weighted normalized decision matrix.
| Drugs | 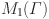 |
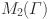 |
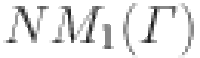 |
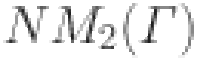 |
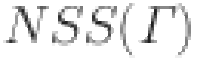 |
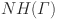 |
|---|---|---|---|---|---|---|
| Metoprolol | 0.091739 | 0.080376 | 0.079582 | 0.061742 | 0.088772 | 0.115006 |
| Atenolol | 0.093923 | 0.082182 | 0.072429 | 0.057115 | 0.079917 | 0.101716 |
| Bisoprolol | 0.111397 | 0.096632 | 0.095678 | 0.073020 | 0.107347 | 0.137519 |
| Propranolol | 0.100476 | 0.093020 | 0.092101 | 0.079093 | 0.097597 | 0.110395 |
| Sotalol | 0.093923 | 0.083086 | 0.080924 | 0.066658 | 0.088278 | 0.110124 |
| Amiodarone | 0.170372 | 0.166171 | 0.164530 | 0.155294 | 0.165757 | 0.175222 |
| Carvedilol | 0.168188 | 0.162559 | 0.160953 | 0.148787 | 0.168411 | 0.173866 |
| Flecainide | 0.152898 | 0.138175 | 0.135469 | 0.116109 | 0.134593 | 0.154336 |
| Propafenone | 0.126687 | 0.115597 | 0.114456 | 0.093842 | 0.123979 | 0.137248 |
| Timolol | 0.113581 | 0.103857 | 0.102831 | 0.088781 | 0.108396 | 0.118261 |
Our research is really useful for making new medicines, especially for arrhythmia problems. We use special math tools and computer models to figure out how well different drugs might work, Table 19. This approach can help drug companies identify the most promising drug candidates for early testing, which saves them both time and money. Instead of lab-testing every compound, they can use our method to predict which ones are most likely to be effective. This technique is not just for drugs; it can also be useful for environmental chemical analysis or developing new materials.
Table 19.
Final ranking using SAW.
| Drugs | SAW score | Rank |
|---|---|---|
| Amiodarone | 0.997346 | 1 |
| Carvedilol | 0.982764 | 2 |
| Flecainide | 0.831580 | 3 |
| Propafenone | 0.711808 | 4 |
| Timolol | 0.635708 | 5 |
| Bisoprolol | 0.621594 | 6 |
| Propranolol | 0.572682 | 7 |
| Sotalol | 0.522993 | 8 |
| Metoprolol | 0.517218 | 9 |
| Atenolol | 0.487281 | 10 |
The normalized decision matrix of different drugs according to topological indices in Table 17 represents the standardized value of the different indices (i.e., 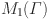 ,
,  ,
,  ,
,  ,
, 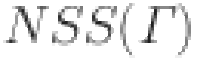 , and
, and 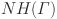 ) of each drug. The normalization process allows the different indices of all the drugs to be on the same scale, so any comparison of the drugs becomes possible on the same level. For instance, “Amiodarone” has the highest normalized value of 1.000000 in the case of
) of each drug. The normalization process allows the different indices of all the drugs to be on the same scale, so any comparison of the drugs becomes possible on the same level. For instance, “Amiodarone” has the highest normalized value of 1.000000 in the case of 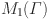 ,
,  ,
,  ,
,  , and
, and 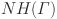 . Conversely, “Atenolol” possesses comparatively lower normalized value across all the indices; it may thus not be able to perform better in accordance with these topological characteristics. Normalization is a crucial step in preparing data because it puts all the drugs on an equal footing. This means we can compare them fairly, no matter how large or small their original data values were.
. Conversely, “Atenolol” possesses comparatively lower normalized value across all the indices; it may thus not be able to perform better in accordance with these topological characteristics. Normalization is a crucial step in preparing data because it puts all the drugs on an equal footing. This means we can compare them fairly, no matter how large or small their original data values were.
Table 18, presents the weighted normalized decision matrix. This updated matrix improves upon the previous one by adjusting the importance of each topological index based on its significance for evaluating the drugs, and then applying these adjusted weights to the normalized values. For example, “Amiodarone” consistently ranks highest across most performance indicators, showing its overall strong effectiveness. This is clear from its high SAW (Simple Additive Weighting) score of 0.997346, confirming it as the top performer, just as expected. “Atenolol” however, ranks last with a comparatively lower SAW value of 0.487281, indicating that it has lower preference when the weighted significance of the indices is used. This weighted system highlights the drugs that perform best on the most important criteria, making it a more precise tool for decision-making.
The final ranking of the drugs according to their SAW scores from the preceding table is presented in Table 19. From the table, it is clear that ”Amiodarone” with a score of 0.997346 rank highest, followed by Carvedilol, and Flecainide with scores of 0.982764 and 0.831580, respectively. This ranking results from the addition of the normalized and weighted scores of each drug, thereby providing a clear comparison. Other drugs like, Metoprolol and Atenolol rank lower, with the last position held by Atenolol. This ranking aids in the informed decision-making process of the most likely best drug.
Conclusions
This research investigated the molecular structures and physicochemical properties of ten anti arrhythmia drugs. We used various topological indices, such as Shilpa-Shanmukha Index , harmonic, and Zagreb indices, which are based on the neighborhood degree of a molecule’s atoms. Our findings show strong relationships between these indices and the drugs’ properties. Notably, the neighborhood harmonic index was the most effective in predicting polarizability when using quadratic regression models. Additionally, we employed multi-criteria decision-making methods. The entropy method was applied to allocate weights to each topological index, confirming that criteria with higher information content received greater importance during the MCDM ranking with SAW and TOPSIS. This helped us pinpoint the most promising drug candidates. This study provided a cost effective, rapid method for predicting drugs properties and ranking, which can complement time consuming and costly clinical trials.
Future research could explore distance-based topological indices and more complex polynomial regression models to further improve the accuracy of these predictions.
Author contributions
Shereen Iqbal: Data Collection, Computations, Investigation, Analysis, Writing, Editing. Hifza Iqbal: Conceptualization, Methodology, Supervision, Formal Analysis, Validation, Editing, Writing. Muhammad Akhtar Tarar: Supervision, Format, Review, Software Tools. Muhammad Farhan Hanif: Validation, Technical Assistance, Analysis Tools, Osman Abubakar Fiidow : Proof Read, Drafting, Technical Assistance.
Funding
funding to support this article.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zipes, D. P. & Wellens, H. J. Sudden cardiac death. Circulation98(21), 2334–2351 (1998). [DOI] [PubMed] [Google Scholar]
- 2.Camm, A. J. et al. Guidelines for the management of atrial fibrillation. Eur. Heart J.31(19), 2369–2429 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Dan, G. A. et al. ESC Scientific Document Group Sticherling Christian (Reviewer Coordinator) Ehrlich Joachim R Schilling Richard Pavlovic Nikola De Potter Tom Lubinski Andrzej Svendsen Jesper Hastrup Ching Keong Sapp John Lewis Chen-Scarabelli Carol Martinez Felipe. Antiarrhythmic drugs-clinical use and clinical decision making: A consensus document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology (ESC) working group on cardiovascular pharmacology, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS) and international society of cardiovascular pharmacotherapy (ISCP). Ep Europace20(5), 731–732 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Karunarathna, I. et al. Atenolol in special populations: Considerations for renal impairment and diabetes. Uva Clin. Anaesth. Intens. Care1(1), 1–10 (2024). [Google Scholar]
- 5.Maysitha, B. K. Comparison of the use of bisoprolol and metoprolol in heart failure patients systematic review study. J. Pharm. Care Anwar Med.5(2), 31–39 (2024). [Google Scholar]
- 6.Arslan, A. et al. Oral amiodarone and propranolol in maintenance therapy of postimplantation tachycardia: An observational study. Medicine103(28), e38839 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rochelson, E. et al. Safety and efficacy of intravenous sotalol following congenital heart surgery. Clin. Electrophysiol.10(1), 135–136 (2024). [DOI] [PubMed] [Google Scholar]
- 8.Yarlagadda, C. et al. Navigating the incidence of postoperative arrhythmia and hospitalization length: The role of amiodarone and other antiarrhythmics in prophylaxis. Cureus16(4), 5–10 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee, W. C. et al. Ivabradine could not decrease mitral regurgitation triggered atrial fibrosis and fibrillation compared with carvedilol. ESC Heart Fail.11(1), 251–260 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hauguel-Moreau, M. et al. Flecainide to prevent atrial arrhythmia after patent foramen ovale closure, the AFLOAT study: A randomized clinical trial. Circulation10(3), 521–530 (2024). [DOI] [PubMed] [Google Scholar]
- 11.Springer, J. et al. Effectiveness of antazoline versus amiodarone, flecainide and propafenone in restoring sinus rhythm at the emergency department. Adv. Med. Sci.69(2), 248–255 (2024). [DOI] [PubMed] [Google Scholar]
- 12.Aftab, O. M., Khan, H., Sangani, R. & Khouri, A. S. A national analysis of systemic adverse events of beta-blockers used for glaucoma therapy. Cutaneous Ocular Toxicol.50(1), 1–6 (2024). [DOI] [PubMed] [Google Scholar]
- 13.Zhang, Y. et al. M2 macrophage exosome-derived lncRNA AK083884 protects mice from CVB3-induced viral myocarditis through regulating PKM2/HIF-1a axis mediated metabolic reprogramming of macrophages. Redox Biol.69, 103016 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li, L. et al. Nanozyme-enhanced tyramine signal amplification probe for preamplification-free myocarditis-related miRNAs detection. Chem. Eng. J.503, 158093 (2025). [Google Scholar]
- 15.Pei, W. et al. Multitargeted immunomodulatory therapy for viral myocarditis by engineered extracellular vesicles. ACS Nano18(4), 2782–2799 (2024). [DOI] [PubMed] [Google Scholar]
- 16.Zhang, Z. et al. A case of pioneering subcutaneous implantable cardioverter defibrillator intervention in Timothy syndrome. BMC Pediatr.24(1), 729 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei, Y. et al. Efficacy and safety of Shenfu injection on bradyarrhythmia: A systematic review and meta-analysis. Medicine104(18), (2025). [DOI] [PMC free article] [PubMed]
- 18.Liu, X. et al. Comparison of the effects of metformin and empagliflozin on cardiac function in heart failure with preserved ejection fraction mice. Front. Cardiovasc. Med.12, 1533820 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West, D. B. Introduction to Graph Theory 2nd edn. (Prentice Hall, 2001). [Google Scholar]
- 20.Euler, L. Solutio problematis ad geometriam situs pertinentis. Commentarii Academiae Scientiarum Petropolitanae8, 128–140 (1736). [Google Scholar]
- 21.Gutman, I. & Furtula, B. (Eds.). Recent Results in Chemical Graph Theory. (University of Kragujevac, 2010).
- 22.Wiener, H. Correlation of heats of isomerization and differences in heats of vaporization of isomers with the number of branches in the molecule. J. Am. Chem. Soc.69(1), 17–20 (1947).20291038 [Google Scholar]
- 23.Hosoya, H. Topological index. A new tool for “counting’’ the structural isomers of a molecule. J. Chem. Phys.55(3), 1318–1325 (1971). [Google Scholar]
- 24.Trinajstić, N. Chemical Graph Theory 2nd edn, 50–58 (CRC Press, 1992). [Google Scholar]
- 25.Huang, L., Alhulwah, K. H., Hanif, M. F., Siddiqui, M. K. & Ikram, A. S. On QSPR analysis of glaucoma drugs using machine learning with XGBoost and regression models. Comput. Biol. Med.187, 109731 (2025). [DOI] [PubMed] [Google Scholar]
- 26.Qin, H. et al. On QSPR analysis of pulmonary cancer drugs using python-driven topological modeling. Sci. Rep.15(1), 3965 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin, H. et al. A python approach for prediction of physicochemical properties of anti-arrhythmia drugs using topological descriptors. Sci. Rep.15(1), 1742 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei, J., Hanif, M. F., Mahmood, H., Siddiqui, M. K. & Hussain, M. QSPR analysis of diverse drugs using linear regression for predicting physical properties. Polycyclic Aromat. Compd.44(7), 4850–4870 (2024). [Google Scholar]
- 29.Hosoya, H. On some counting polynomials in chemistry. Discret. Appl. Math.19(1–3), 239–257 (1988). [Google Scholar]
- 30.Deutsch, E. & Klavžar, S. M-polynomial and degree-based topological indices. arXiv preprint arXiv:1407.1592 (2014).
- 31.Mondal, S., Siddiqui, M. K., De, N. & Pal, A. Neighborhood M-polynomial of crystallographic structures. Biointerface Res. Appl. Chem.11(2), 9372–9381 (2021). [Google Scholar]
-
32.Consonni, V., Ballabio, D. & Todeschini, R. Comments on the definition of the Q
 parameter for QSAR validation. J. Chem. Inf. Model.49(7), 1669–1678 (2009).
[DOI] [PubMed] [Google Scholar]
parameter for QSAR validation. J. Chem. Inf. Model.49(7), 1669–1678 (2009).
[DOI] [PubMed] [Google Scholar] - 33.Massam, B. H. Multi-criteria decision making (MCDM) techniques in planning. Prog. Plan.30, 1–84 (1988). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.