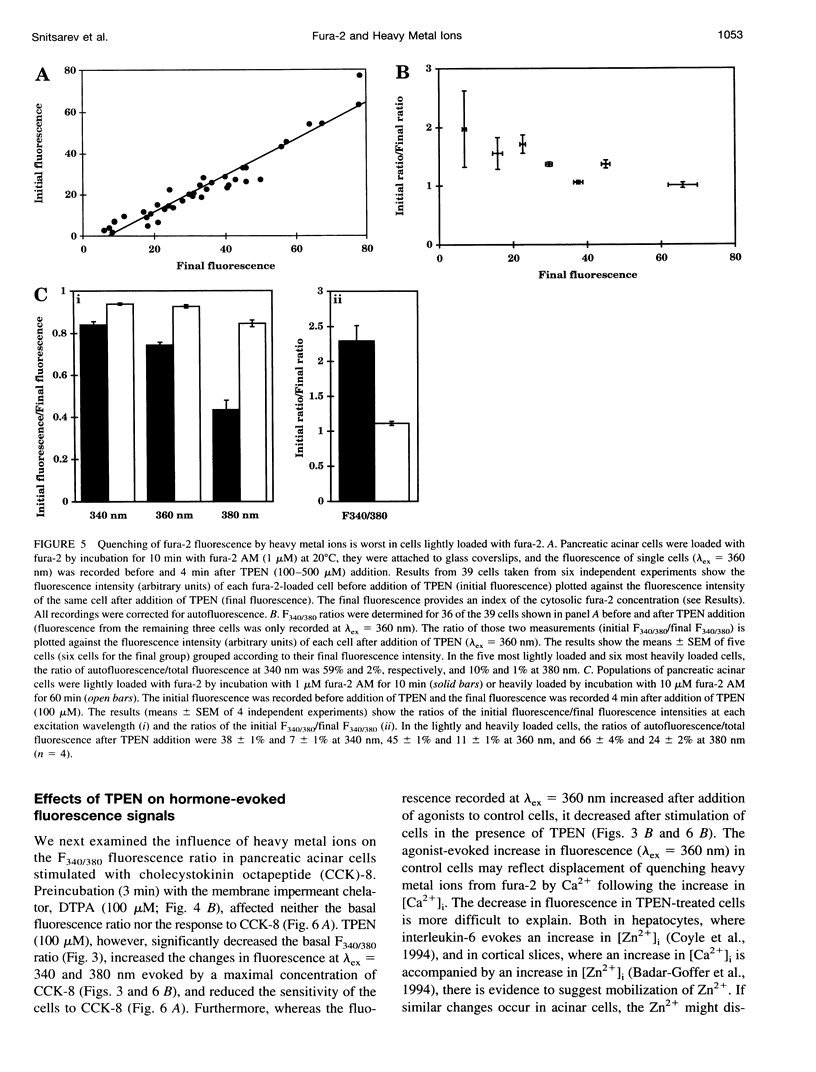
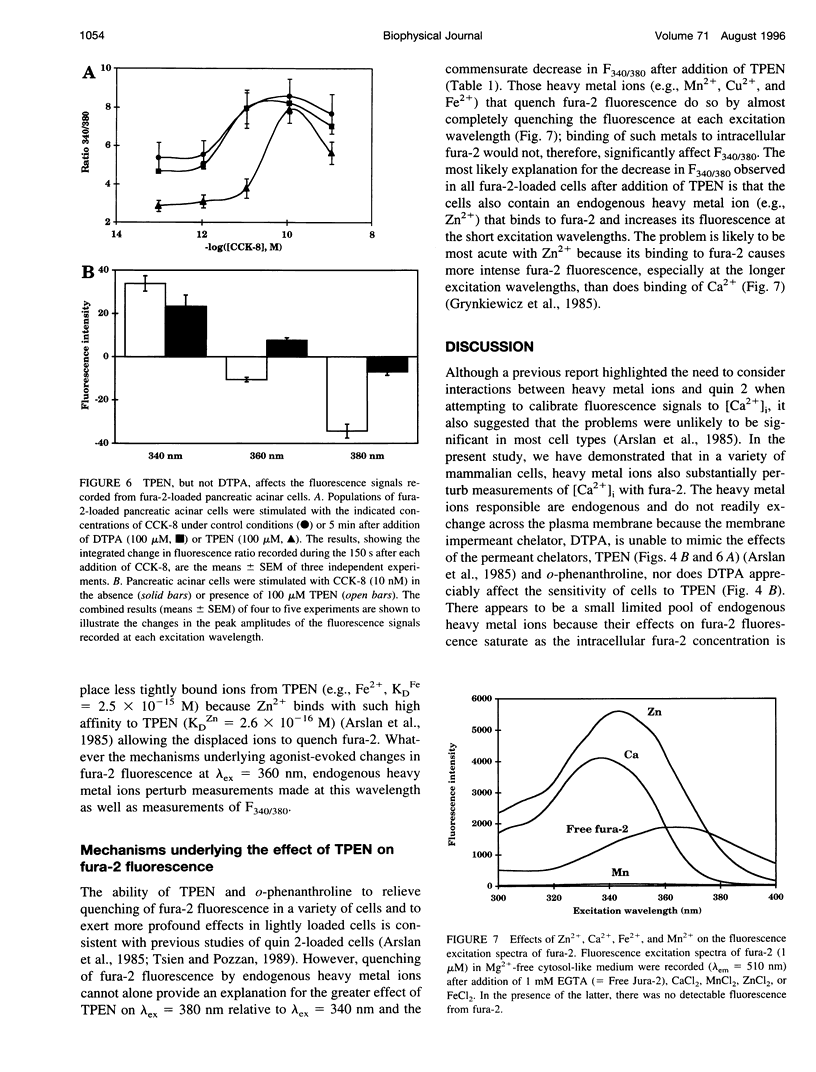
Abstract
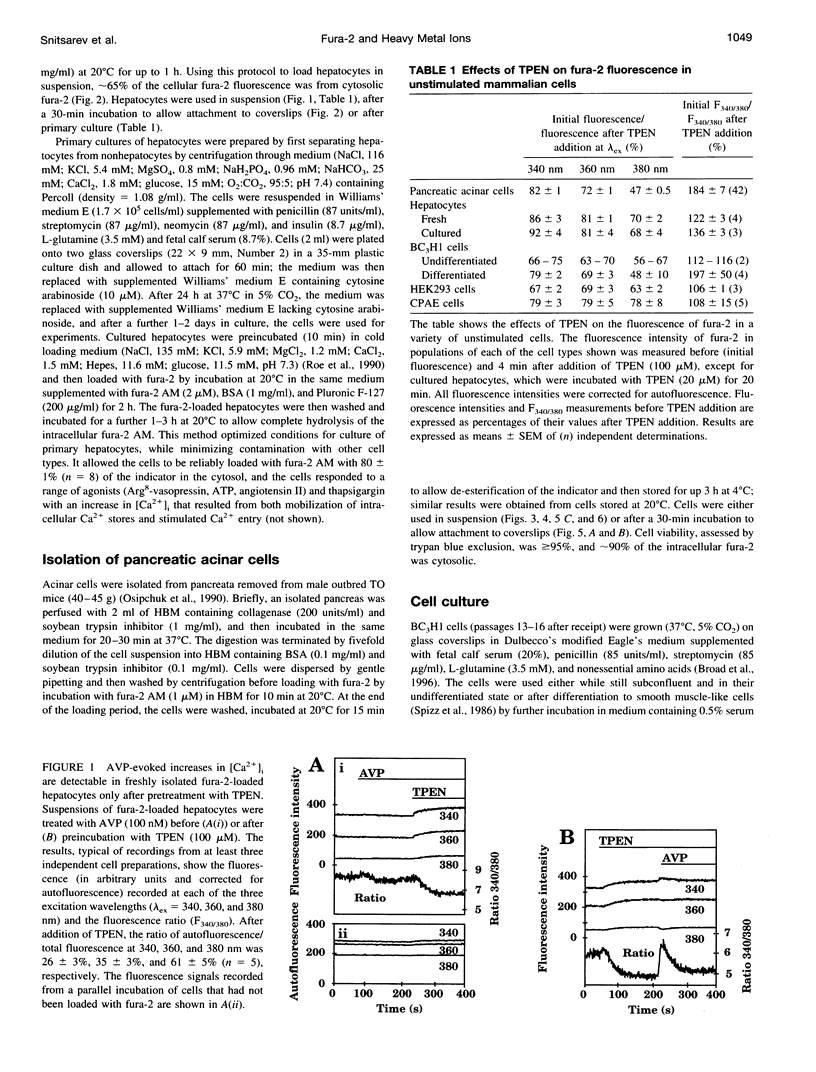
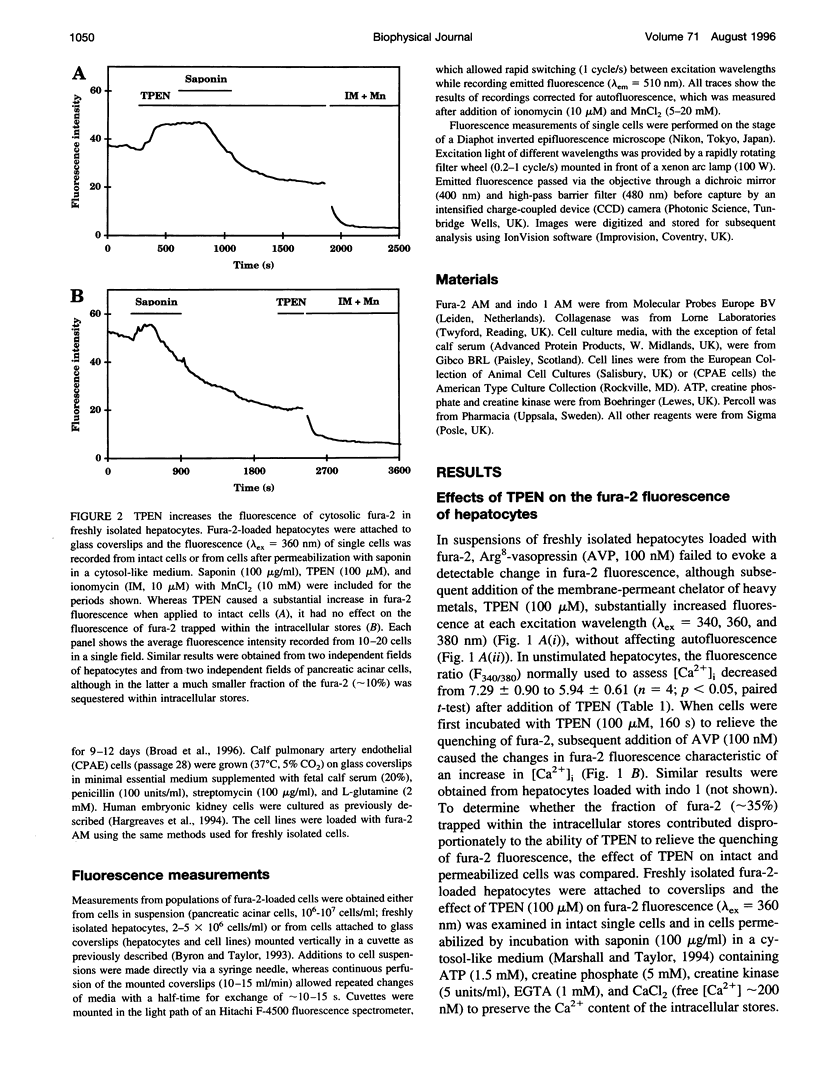
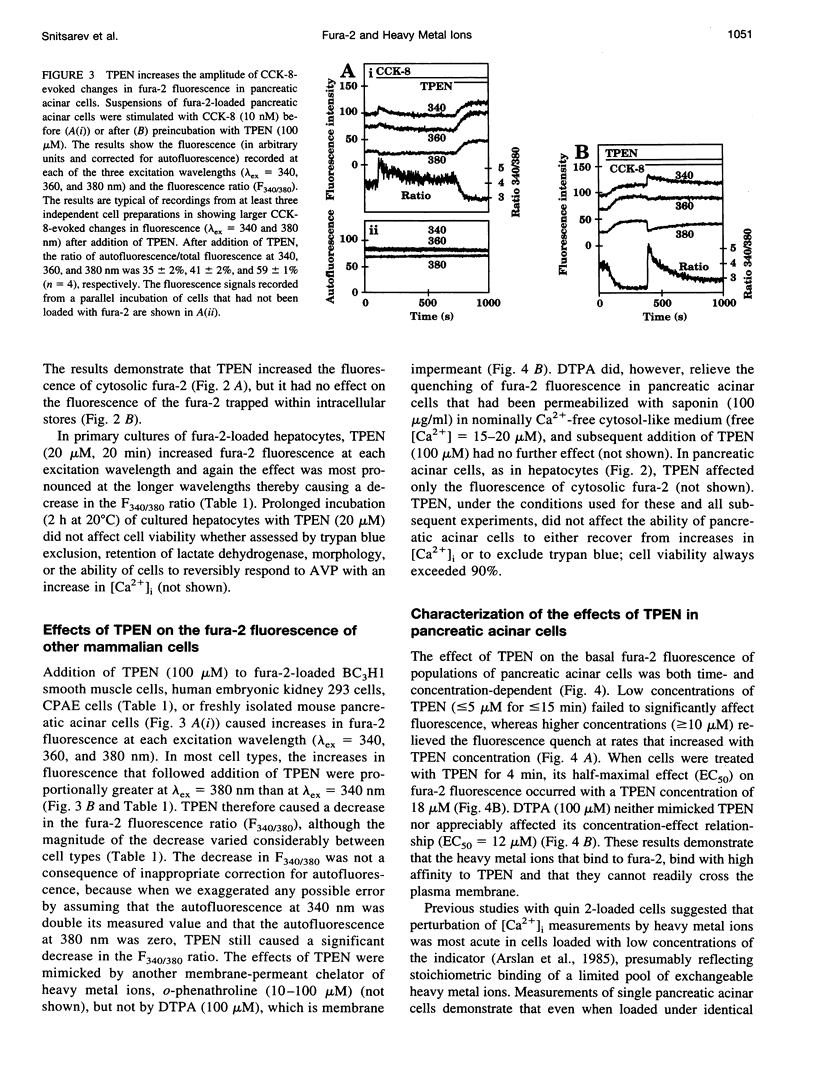
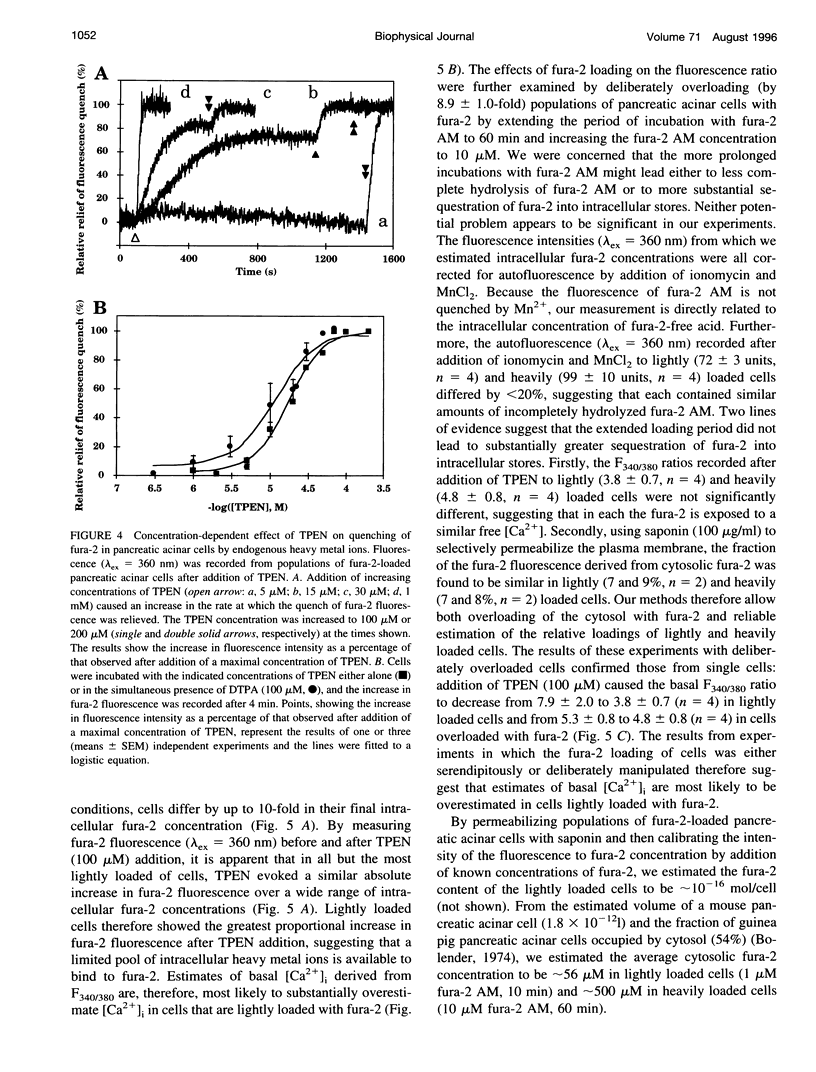
Using the membrane-permeant chelator of heavy metal ions, N,N,N',N'-tetrakis(2-pyridylmethyl)ethylene diamine (TPEN), we demonstrate that in pancreatic acinar cells, hepatocytes, and a variety of mammalian cell lines, endogenous heavy metal ions bind to cytosolic fura-2 causing basal cytosolic free [Ca2+] ([Ca2+]i) to be overestimated. TPEN had most effect in cells lightly loaded with fura-2, suggesting the presence of a limited pool of heavy metal ions (> or = 12 microM in pancreatic acinar cells) that does not rapidly exchange across the plasma membrane. In fura-2-loaded hepatocytes, vasopressin failed to evoke a detectable change in fluorescence, but after preincubation of cells with TPEN, it caused fluorescence changes characteristic of an increase in [Ca2+]i. We conclude that in many mammalian cells, a slowly exchanging mixture of cytosolic heavy metal ions binds to fura-2 both to quench its fluorescence and to mimic the effects of Ca2+ binding, thereby causing basal [Ca2+]i to be overestimated. By chelating endogenous heavy metal ions, TPEN allows basal [Ca2+]i to be accurately measured and, by preventing competition between heavy metal ions and Ca2+ for binding to fura-2, unmasks the full effect of agonists in increasing [Ca2+]i.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arslan P., Di Virgilio F., Beltrame M., Tsien R. Y., Pozzan T. Cytosolic Ca2+ homeostasis in Ehrlich and Yoshida carcinomas. A new, membrane-permeant chelator of heavy metals reveals that these ascites tumor cell lines have normal cytosolic free Ca2+. J Biol Chem. 1985 Mar 10;260(5):2719–2727. [PubMed] [Google Scholar]
- Badar-Goffer R., Morris P., Thatcher N., Bachelard H. Excitotoxic amino acids cause appearance of magnetic resonance spectroscopy-observable zinc in superfused cortical slices. J Neurochem. 1994 Jun;62(6):2488–2491. doi: 10.1046/j.1471-4159.1994.62062488.x. [DOI] [PubMed] [Google Scholar]
- Bolender R. P. Stereological analysis of the guinea pig pancreas. I. Analytical model and quantitative description of nonstimulated pancreatic exocrine cells. J Cell Biol. 1974 May;61(2):269–287. doi: 10.1083/jcb.61.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad L. M., Powis D. A., Taylor C. W. Differentiation of BC3H1 smooth muscle cells changes the bivalent cation selectivity of the capacitative Ca2+ entry pathway. Biochem J. 1996 Jun 15;316(Pt 3):759–764. doi: 10.1042/bj3160759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byron K. L., Taylor C. W. Spontaneous Ca2+ spiking in a vascular smooth muscle cell line is independent of the release of intracellular Ca2+ stores. J Biol Chem. 1993 Apr 5;268(10):6945–6952. [PubMed] [Google Scholar]
- Coyle P., Zalewski P. D., Philcox J. C., Forbes I. J., Ward A. D., Lincoln S. F., Mahadevan I., Rofe A. M. Measurement of zinc in hepatocytes by using a fluorescent probe, zinquin: relationship to metallothionein and intracellular zinc. Biochem J. 1994 Nov 1;303(Pt 3):781–786. doi: 10.1042/bj3030781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F., Steinberg T. H., Silverstein S. C. Inhibition of Fura-2 sequestration and secretion with organic anion transport blockers. Cell Calcium. 1990 Feb-Mar;11(2-3):57–62. doi: 10.1016/0143-4160(90)90059-4. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hargreaves A. C., Lummis S. C., Taylor C. W. Ca2+ permeability of cloned and native 5-hydroxytryptamine type 3 receptors. Mol Pharmacol. 1994 Dec;46(6):1120–1128. [PubMed] [Google Scholar]
- Hechtenberg S., Beyersmann D. Differential control of free calcium and free zinc levels in isolated bovine liver nuclei. Biochem J. 1993 Feb 1;289(Pt 3):757–760. doi: 10.1042/bj2890757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall I. C., Taylor C. W. Two calcium-binding sites mediate the interconversion of liver inositol 1,4,5-trisphosphate receptors between three conformational states. Biochem J. 1994 Jul 15;301(Pt 2):591–598. doi: 10.1042/bj3010591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipchuk Y. V., Wakui M., Yule D. I., Gallacher D. V., Petersen O. H. Cytoplasmic Ca2+ oscillations evoked by receptor stimulation, G-protein activation, internal application of inositol trisphosphate or Ca2+: simultaneous microfluorimetry and Ca2+ dependent Cl- current recording in single pancreatic acinar cells. EMBO J. 1990 Mar;9(3):697–704. doi: 10.1002/j.1460-2075.1990.tb08162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A., Taylor C. W. Effects of Ca2+ chelators on purified inositol 1,4,5-trisphosphate (InsP3) receptors and InsP3-stimulated Ca2+ mobilization. J Biol Chem. 1993 Jun 5;268(16):11528–11533. [PubMed] [Google Scholar]
- Roe M. W., Lemasters J. J., Herman B. Assessment of Fura-2 for measurements of cytosolic free calcium. Cell Calcium. 1990 Feb-Mar;11(2-3):63–73. doi: 10.1016/0143-4160(90)90060-8. [DOI] [PubMed] [Google Scholar]
- Spizz G., Roman D., Strauss A., Olson E. N. Serum and fibroblast growth factor inhibit myogenic differentiation through a mechanism dependent on protein synthesis and independent of cell proliferation. J Biol Chem. 1986 Jul 15;261(20):9483–9488. [PubMed] [Google Scholar]
- Taylor C. W., Berridge M. J., Cooke A. M., Potter B. V. Inositol 1,4,5-trisphosphorothioate, a stable analogue of inositol trisphosphate which mobilizes intracellular calcium. Biochem J. 1989 May 1;259(3):645–650. doi: 10.1042/bj2590645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y. Fluorescent probes of cell signaling. Annu Rev Neurosci. 1989;12:227–253. doi: 10.1146/annurev.ne.12.030189.001303. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R., Pozzan T. Measurement of cytosolic free Ca2+ with quin2. Methods Enzymol. 1989;172:230–262. doi: 10.1016/s0076-6879(89)72017-6. [DOI] [PubMed] [Google Scholar]
- Wahl M., Carpenter G. Regulation of epidermal growth factor-stimulated formation of inositol phosphates in A-431 cells by calcium and protein kinase C. J Biol Chem. 1988 Jun 5;263(16):7581–7590. [PubMed] [Google Scholar]


