Abstract
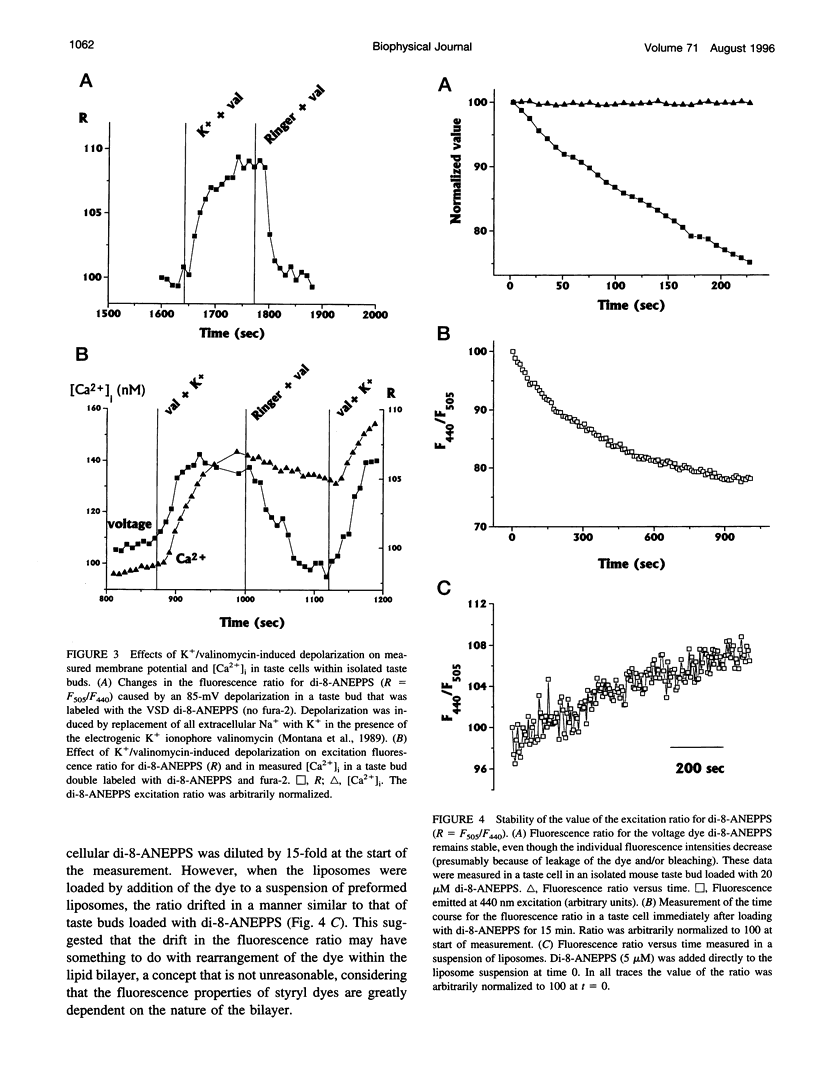
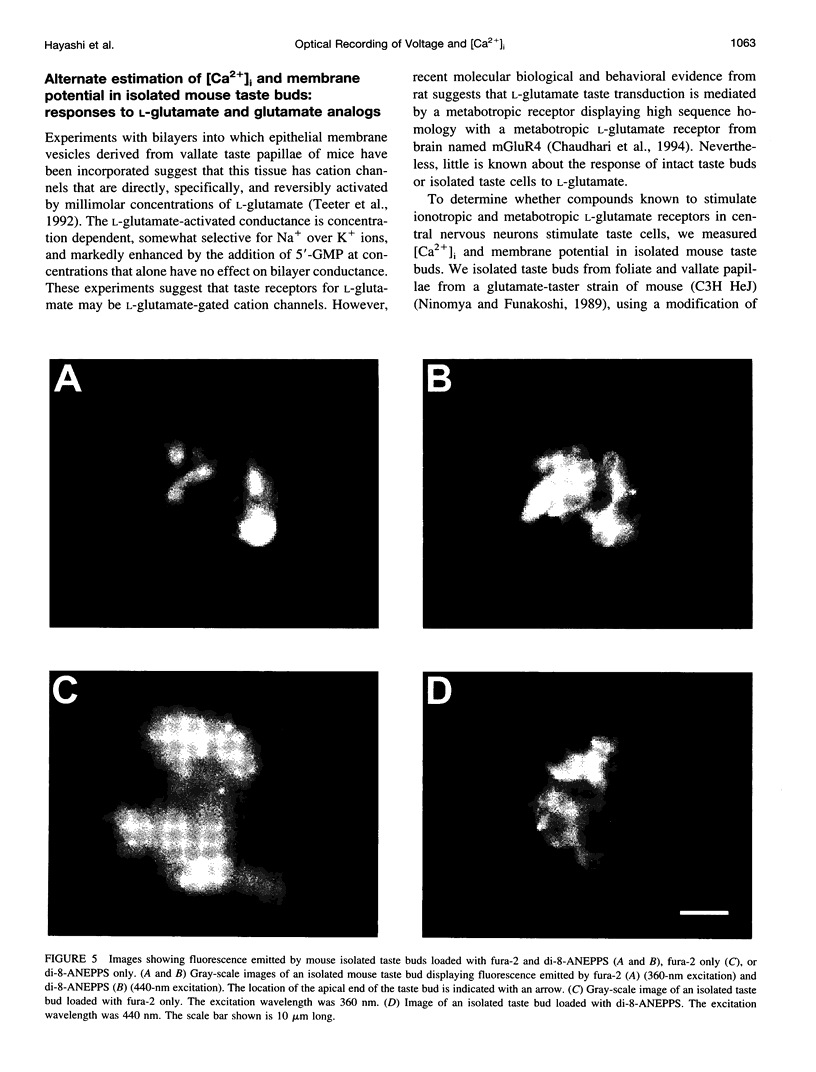
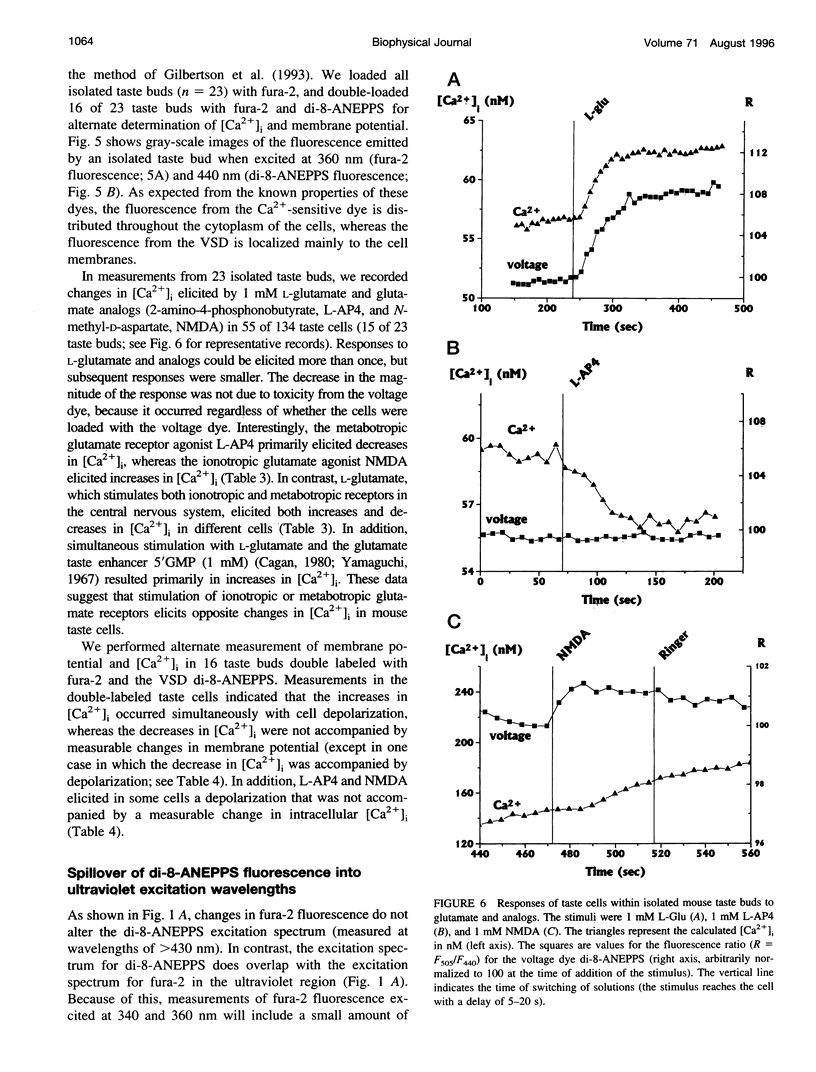
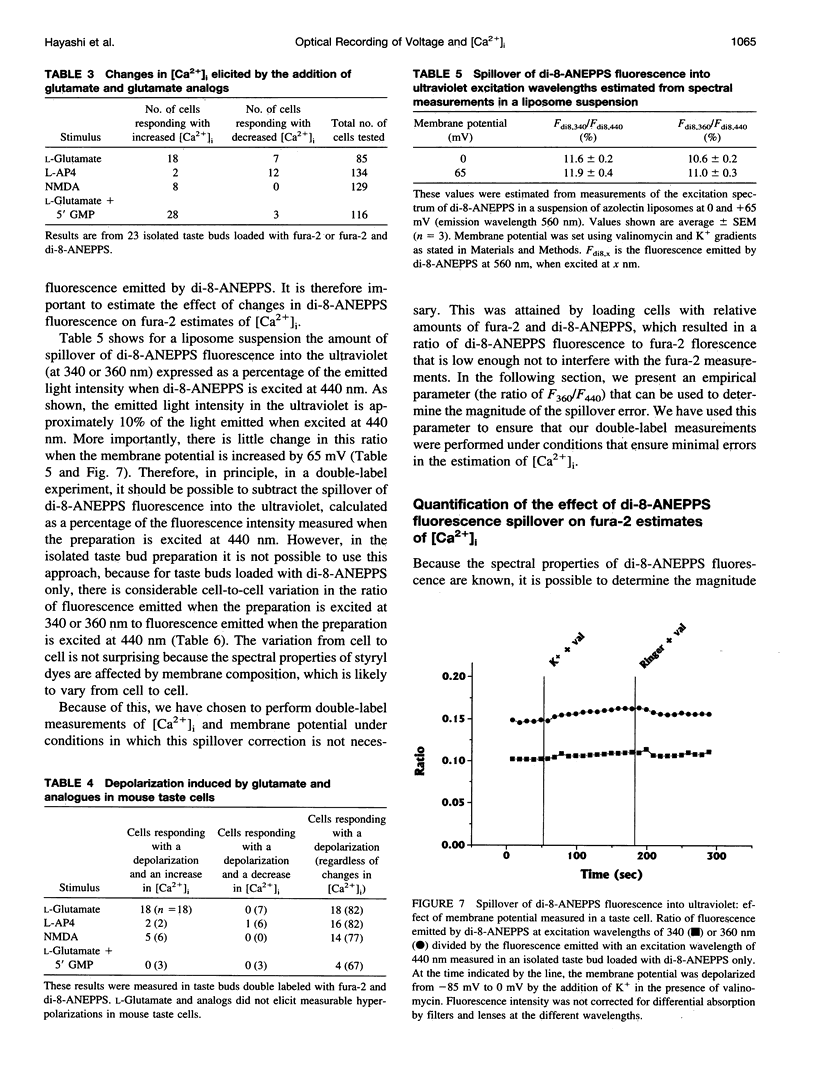
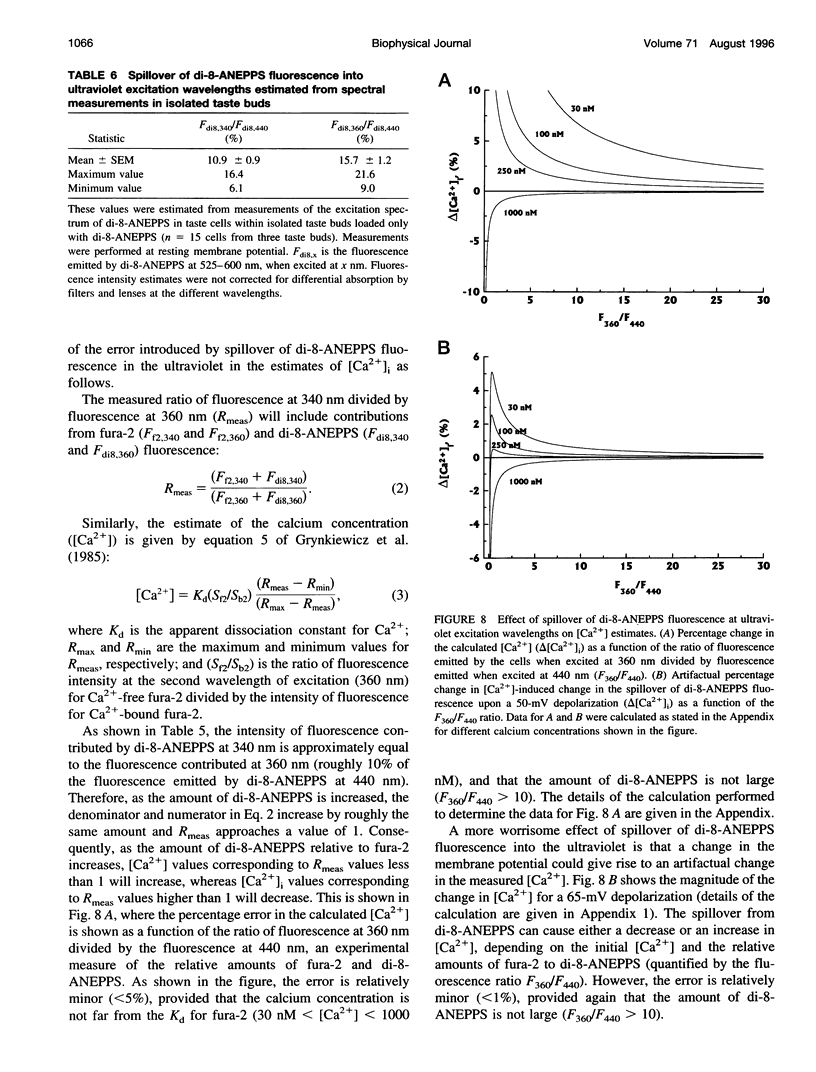
We have studied the spectral properties of the voltage-sensitive dye, 1-(3-sulfonatopropyl)-4-[beta [2-(di-n-octylamino)-6-naphtyl]vinyl] pyridinium betaine (di-8-ANEPPS), and the Ca(2+)-sensitive dye, fura-2, in azolectin liposomes and in isolated taste buds from mouse. We find that the fluorescence excitation spectra of di-8-ANEPPS and fura-2 are largely nonoverlapping, allowing alternate ratio measurements of membrane potential and intracellular calcium ([Ca2+]i). There is a small spillover of di-8-ANEPPS fluorescence at the excitation wavelengths used for fura-2 (340 and 360 nm). However, voltage-induced changes in the fluorescence of di-8-ANEPPS, excited at the fura-2 wavelengths, are small. In addition, di-8-ANEPPS fluorescence is localized to the membrane, whereas fura-2 fluorescence is distributed throughout the cytoplasm. Because of this, the effect of spillover of di-8-ANEPPS fluorescence in the [Ca2+]i estimate is < 1%, under the appropriate conditions. We have applied this method to study of the responses of multiple taste cells within isolated taste buds. We show that membrane potential and [Ca2+]i can be measured alternately in isolated taste buds from mouse. Stimulation with glutamate and glutamate analogs indicates that taste cells express both metabotropic and ionotropic receptors. The data suggest that the receptors responding to 2-amino-4-phosphonobutyrate (L-AP4), presumably metabotropic L-glutamate receptors, do not mediate excitatory glutamate taste responses.
Full text
PDF

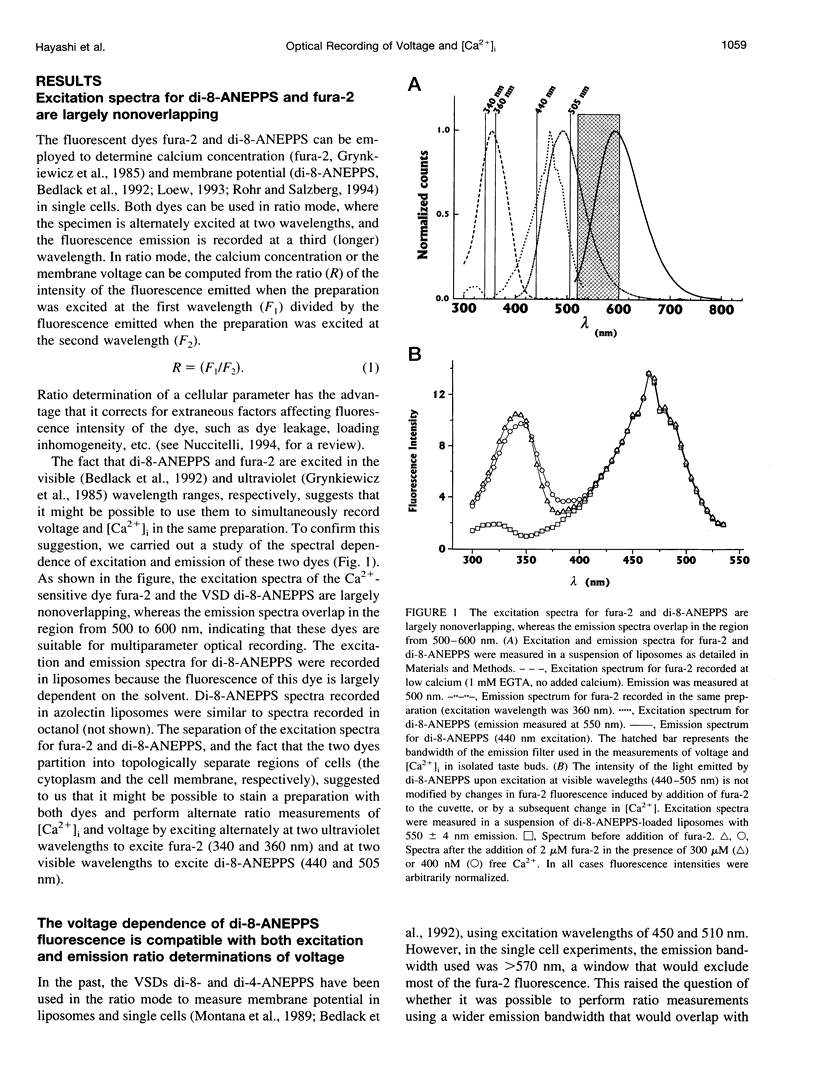
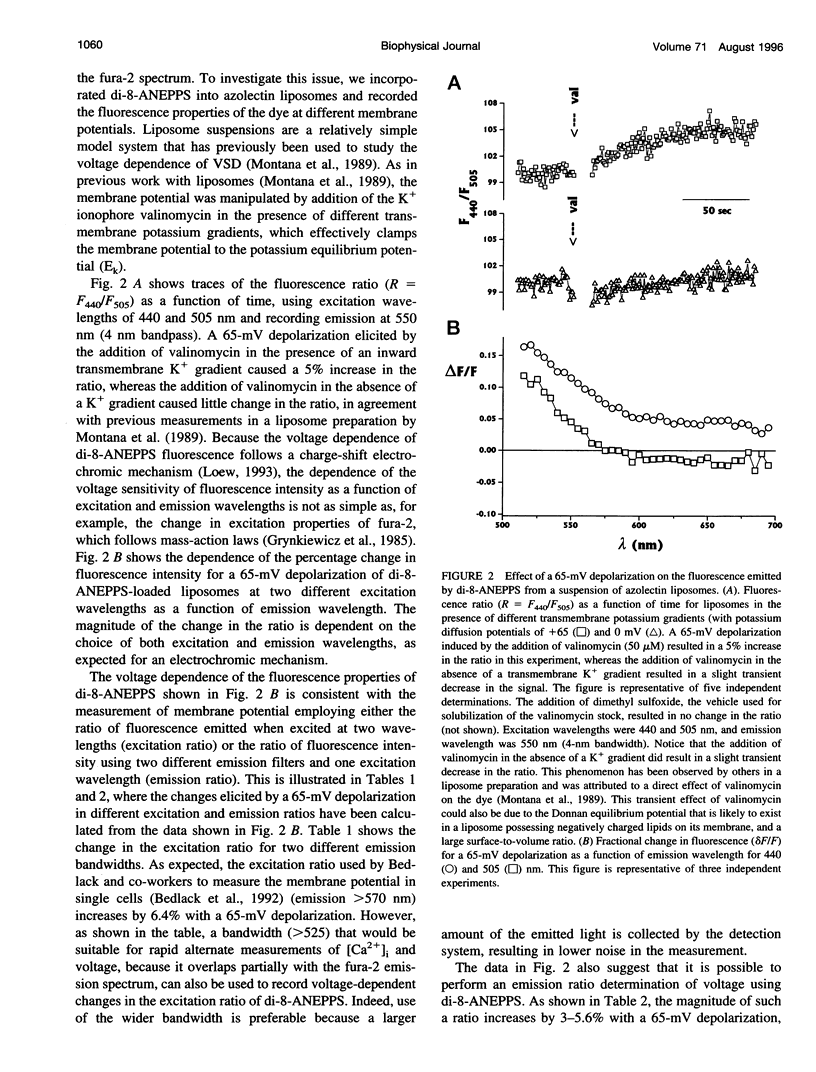
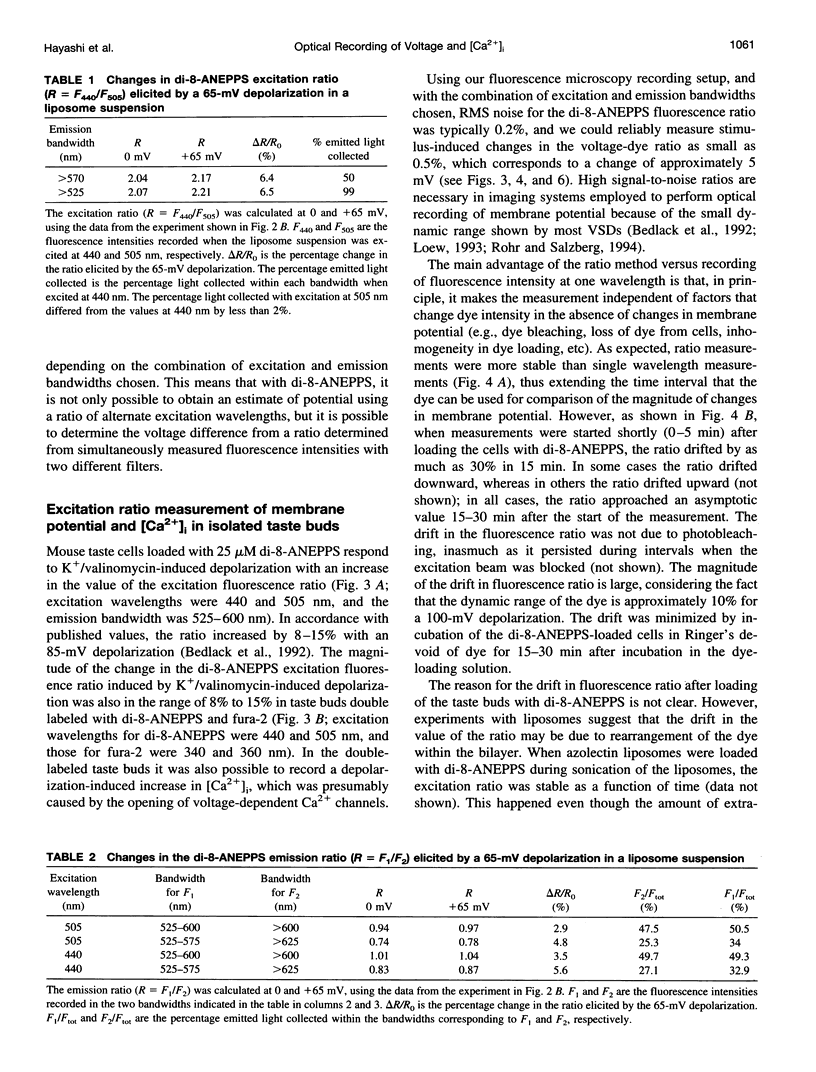
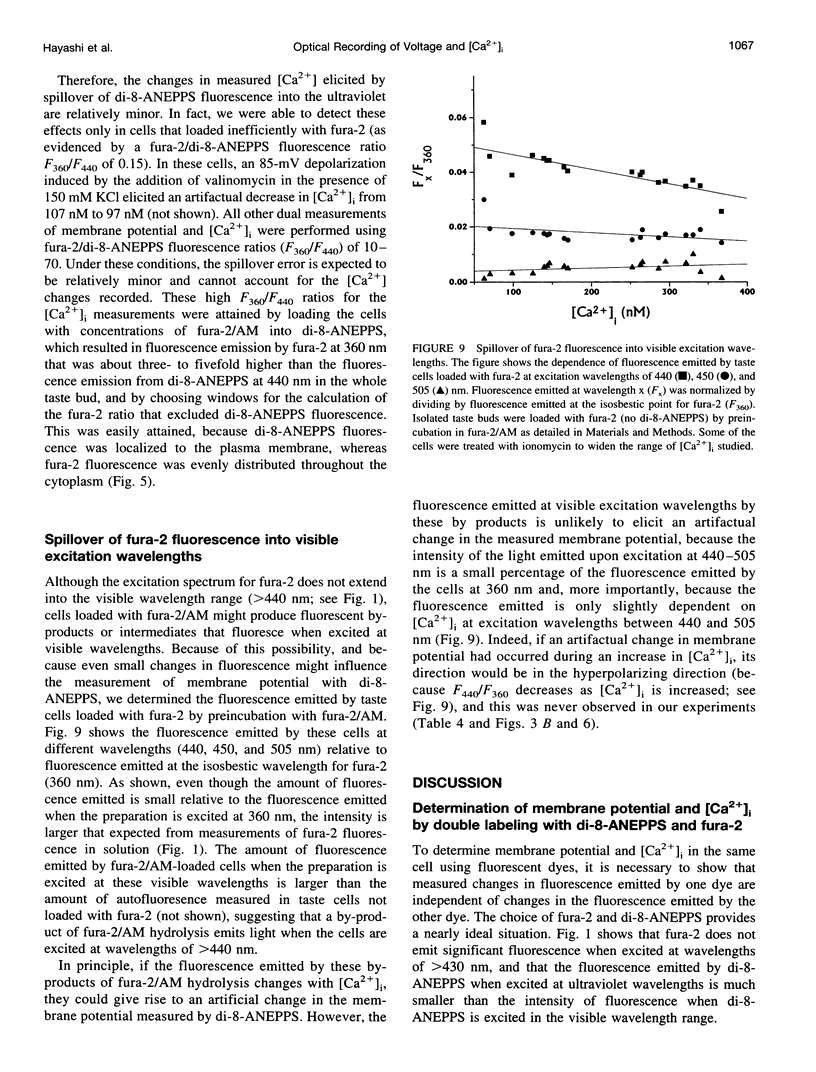



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedlack R. S., Jr, Wei M., Loew L. M. Localized membrane depolarizations and localized calcium influx during electric field-guided neurite growth. Neuron. 1992 Sep;9(3):393–403. doi: 10.1016/0896-6273(92)90178-g. [DOI] [PubMed] [Google Scholar]
- Bernhardt S. J., Naim M., Zehavi U., Lindemann B. Changes in IP3 and cytosolic Ca2+ in response to sugars and non-sugar sweeteners in transduction of sweet taste in the rat. J Physiol. 1996 Jan 15;490(Pt 2):325–336. doi: 10.1113/jphysiol.1996.sp021147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacon E., Reece J. M., Nieminen A. L., Zahrebelski G., Herman B., Lemasters J. J. Distribution of electrical potential, pH, free Ca2+, and volume inside cultured adult rabbit cardiac myocytes during chemical hypoxia: a multiparameter digitized confocal microscopic study. Biophys J. 1994 Apr;66(4):942–952. doi: 10.1016/S0006-3495(94)80904-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delay R. J., Taylor R., Roper S. D. Merkel-like basal cells in Necturus taste buds contain serotonin. J Comp Neurol. 1993 Sep 22;335(4):606–613. doi: 10.1002/cne.903350411. [DOI] [PubMed] [Google Scholar]
- Ewald D. A., Roper S. D. Intercellular signaling in Necturus taste buds: chemical excitation of receptor cells elicits responses in basal cells. J Neurophysiol. 1992 May;67(5):1316–1324. doi: 10.1152/jn.1992.67.5.1316. [DOI] [PubMed] [Google Scholar]
- Gilbertson T. A., Roper S. D., Kinnamon S. C. Proton currents through amiloride-sensitive Na+ channels in isolated hamster taste cells: enhancement by vasopressin and cAMP. Neuron. 1993 May;10(5):931–942. doi: 10.1016/0896-6273(93)90208-9. [DOI] [PubMed] [Google Scholar]
- Gilbertson T. A. The physiology of vertebrate taste reception. Curr Opin Neurobiol. 1993 Aug;3(4):532–539. doi: 10.1016/0959-4388(93)90052-z. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Jackson A. P., Timmerman M. P., Bagshaw C. R., Ashley C. C. The kinetics of calcium binding to fura-2 and indo-1. FEBS Lett. 1987 May 25;216(1):35–39. doi: 10.1016/0014-5793(87)80752-4. [DOI] [PubMed] [Google Scholar]
- Kinnamon S. C., Cummings T. A. Chemosensory transduction mechanisms in taste. Annu Rev Physiol. 1992;54:715–731. doi: 10.1146/annurev.ph.54.030192.003435. [DOI] [PubMed] [Google Scholar]
- Kremer S. G., Zeng W., Skorecki K. L. Simultaneous fluorescence measurement of calcium and membrane potential responses to endothelin. Am J Physiol. 1992 Dec;263(6 Pt 1):C1302–C1309. doi: 10.1152/ajpcell.1992.263.6.C1302. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Silver R. B. Microdomains of high calcium concentration in a presynaptic terminal. Science. 1992 May 1;256(5057):677–679. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- Loew L. M., Tuft R. A., Carrington W., Fay F. S. Imaging in five dimensions: time-dependent membrane potentials in individual mitochondria. Biophys J. 1993 Dec;65(6):2396–2407. doi: 10.1016/S0006-3495(93)81318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Zaguilán R., Martínez G. M., Lattanzio F., Gillies R. J. Simultaneous measurement of intracellular pH and Ca2+ using the fluorescence of SNARF-1 and fura-2. Am J Physiol. 1991 Feb;260(2 Pt 1):C297–C307. doi: 10.1152/ajpcell.1991.260.2.C297. [DOI] [PubMed] [Google Scholar]
- Monck J. R., Oberhauser A. F., Keating T. J., Fernandez J. M. Thin-section ratiometric Ca2+ images obtained by optical sectioning of fura-2 loaded mast cells. J Cell Biol. 1992 Feb;116(3):745–759. doi: 10.1083/jcb.116.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montana V., Farkas D. L., Loew L. M. Dual-wavelength ratiometric fluorescence measurements of membrane potential. Biochemistry. 1989 May 30;28(11):4536–4539. doi: 10.1021/bi00437a003. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y., Funakoshi M. Peripheral neural basis for behavioural discrimination between glutamate and the four basic taste substances in mice. Comp Biochem Physiol A Comp Physiol. 1989;92(3):371–376. doi: 10.1016/0300-9629(89)90578-1. [DOI] [PubMed] [Google Scholar]
- Ochsner M., Fleck T., Waldmeier P. Simultaneous measurement of Ca2+ transients and of membrane depolarizations in synaptosomes. Biochem Biophys Res Commun. 1991 Dec 16;181(2):797–803. doi: 10.1016/0006-291x(91)91260-j. [DOI] [PubMed] [Google Scholar]
- Reutter K. Die geschmacksknospen des zwergwelses amiurus nebulosus (Lesueur). Morphologische und histochemische untersuchungen. Z Zellforsch Mikrosk Anat. 1971;120(2):280–308. [PubMed] [Google Scholar]
- Rohr S., Salzberg B. M. Multiple site optical recording of transmembrane voltage (MSORTV) in patterned growth heart cell cultures: assessing electrical behavior, with microsecond resolution, on a cellular and subcellular scale. Biophys J. 1994 Sep;67(3):1301–1315. doi: 10.1016/S0006-3495(94)80602-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeter J. H., Kumazawa T., Brand J. G., Kalinoski D. L., Honda E., Smutzer G. Amino acid receptor channels in taste cells. Soc Gen Physiol Ser. 1992;47:291–306. [PubMed] [Google Scholar]
- Yang J., Roper S. D. Dye-coupling in taste buds in the mudpuppy, Necturus maculosus. J Neurosci. 1987 Nov;7(11):3561–3565. doi: 10.1523/JNEUROSCI.07-11-03561.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zviman M. M., Restrepo D., Teeter J. H. Single taste stimuli elicit either increases or decreases in intracellular calcium in isolated catfish taste cells. J Membr Biol. 1996 Jan;149(2):81–88. doi: 10.1007/s002329900009. [DOI] [PubMed] [Google Scholar]