Abstract
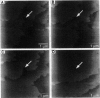
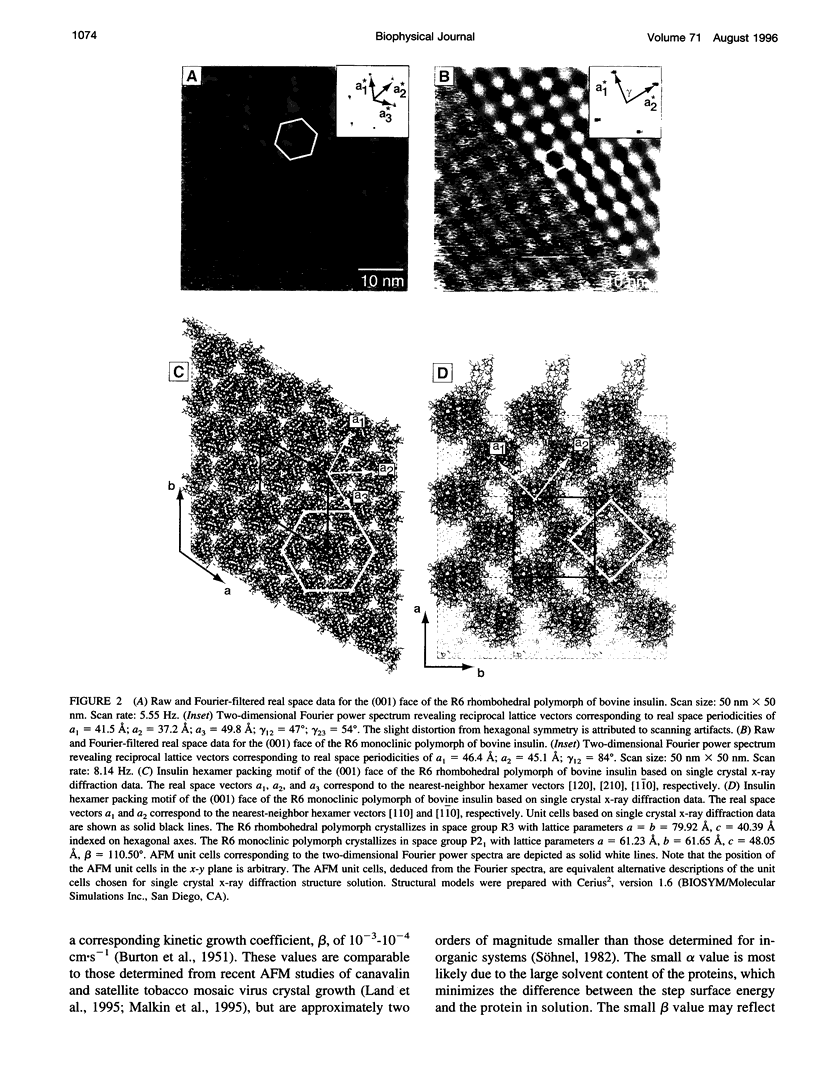
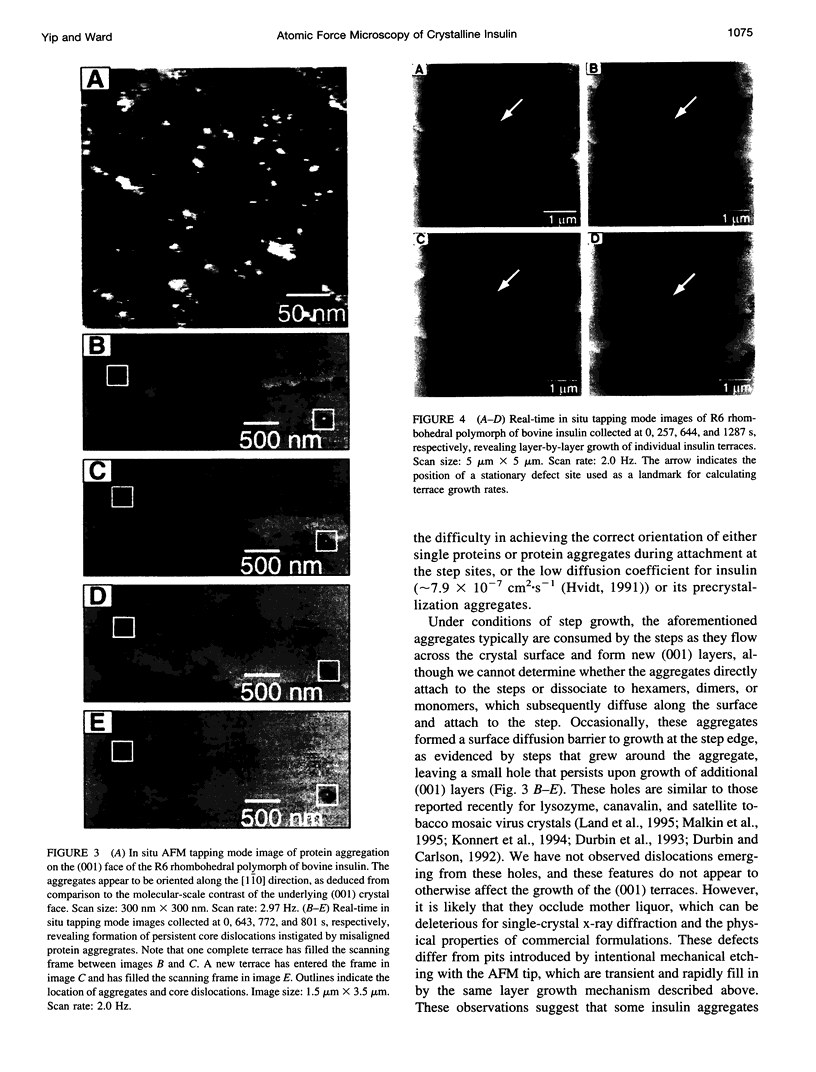
Atomic force microscopy performed on single crystals of three different polymorphs of bovine insulin revealed molecularly smooth (001) layers separated by steps whose heights reflect the dimensions of a single insulin hexamer. Whereas contact mode imaging caused etching that prevented molecular-scale resolution, tapping mode imaging in solution provided molecular-scale contrast that enabled determination of lattice parameters and polymorph identification while simultaneously enabling real-time examination of growth modes and assessment of crystal quality. Crystallization proceeds layer by layer, a process in which the protein molecules assemble homoepitaxially with nearly perfect orientational and translational commensurism. Tapping mode imaging also revealed insulin aggregates attached to the (001) faces, their incorporation into growing terraces, and their role in defect formation. These observations demonstrate that tapping mode imaging is ideal for real-time in situ investigation of the crystallization of soft protein crystals of relatively small proteins such as insulin, which cannot withstand the lateral shear forces exerted by the scanning probe in conventional imaging modes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel J. J. Crystalline Insulin. Proc Natl Acad Sci U S A. 1926 Feb;12(2):132–136. doi: 10.1073/pnas.12.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel J. J., Geiling E. M., Alles G., Raymond A. RESEARCHES ON INSULIN. Science. 1925 Aug 21;62(1599):169–171. doi: 10.1126/science.62.1599.169. [DOI] [PubMed] [Google Scholar]
- Ataka M., Asai M. Analysis of the nucleation and crystal growth kinetics of lysozyme by a theory of self-assembly. Biophys J. 1990 Sep;58(3):807–811. doi: 10.1016/S0006-3495(90)82425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger J., Caspar D. L. Water structure in cubic insulin crystals. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):622–626. doi: 10.1073/pnas.88.2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker E. N., Blundell T. L., Cutfield J. F., Cutfield S. M., Dodson E. J., Dodson G. G., Hodgkin D. M., Hubbard R. E., Isaacs N. W., Reynolds C. D. The structure of 2Zn pig insulin crystals at 1.5 A resolution. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 6;319(1195):369–456. doi: 10.1098/rstb.1988.0058. [DOI] [PubMed] [Google Scholar]
- Berson S. A., Yalow R. S. Insulin in blood and insulin antibodies. Am J Med. 1966 May;40(5):676–690. doi: 10.1016/0002-9343(66)90148-3. [DOI] [PubMed] [Google Scholar]
- Bohidar H. B., Geissler E. Static and dynamic light scattering from dilute insulin solutions. Biopolymers. 1984 Nov;23(11 Pt 2):2407–2417. doi: 10.1002/bip.360231119. [DOI] [PubMed] [Google Scholar]
- Boland T., Ratner B. D. Direct measurement of hydrogen bonding in DNA nucleotide bases by atomic force microscopy. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5297–5301. doi: 10.1073/pnas.92.12.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilkoti A., Boland T., Ratner B. D., Stayton P. S. The relationship between ligand-binding thermodynamics and protein-ligand interaction forces measured by atomic force microscopy. Biophys J. 1995 Nov;69(5):2125–2130. doi: 10.1016/S0006-3495(95)80083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciszak E., Smith G. D. Crystallographic evidence for dual coordination around zinc in the T3R3 human insulin hexamer. Biochemistry. 1994 Feb 15;33(6):1512–1517. doi: 10.1021/bi00172a030. [DOI] [PubMed] [Google Scholar]
- Day J., McPherson A. Macromolecular crystal growth experiments on International Microgravity Laboratory--1. Protein Sci. 1992 Oct;1(10):1254–1268. doi: 10.1002/pro.5560011004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLucas L. J., Smith C. D., Smith H. W., Vijay-Kumar S., Senadhi S. E., Ealick S. E., Carter D. C., Snyder R. S., Weber P. C., Salemme F. R. Protein crystal growth in microgravity. Science. 1989 Nov 3;246(4930):651–654. doi: 10.1126/science.2510297. [DOI] [PubMed] [Google Scholar]
- Derewenda U., Derewenda Z., Dodson E. J., Dodson G. G., Reynolds C. D., Smith G. D., Sparks C., Swenson D. Phenol stabilizes more helix in a new symmetrical zinc insulin hexamer. Nature. 1989 Apr 13;338(6216):594–596. doi: 10.1038/338594a0. [DOI] [PubMed] [Google Scholar]
- Dodson E. J., Dodson G. G., Lewitova A., Sabesan M. Zinc-free cubic pig insulin: crystallization and structure determination. J Mol Biol. 1978 Nov 5;125(3):387–396. doi: 10.1016/0022-2836(78)90409-6. [DOI] [PubMed] [Google Scholar]
- Drake B., Prater C. B., Weisenhorn A. L., Gould S. A., Albrecht T. R., Quate C. F., Cannell D. S., Hansma H. G., Hansma P. K. Imaging crystals, polymers, and processes in water with the atomic force microscope. Science. 1989 Mar 24;243(4898):1586–1589. doi: 10.1126/science.2928794. [DOI] [PubMed] [Google Scholar]
- Durbin S. D., Feher G. Studies of crystal growth mechanisms of proteins by electron microscopy. J Mol Biol. 1990 Apr 20;212(4):763–774. doi: 10.1016/0022-2836(90)90235-E. [DOI] [PubMed] [Google Scholar]
- Florin E. L., Moy V. T., Gaub H. E. Adhesion forces between individual ligand-receptor pairs. Science. 1994 Apr 15;264(5157):415–417. doi: 10.1126/science.8153628. [DOI] [PubMed] [Google Scholar]
- Gursky O., Badger J., Li Y., Caspar D. L. Conformational changes in cubic insulin crystals in the pH range 7-11. Biophys J. 1992 Nov;63(5):1210–1220. doi: 10.1016/S0006-3495(92)81697-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther N., Betzel C., Weber W. The secreted form of the epidermal growth factor receptor. Characterization and crystallization of the receptor-ligand complex. J Biol Chem. 1990 Dec 25;265(36):22082–22085. [PubMed] [Google Scholar]
- Hillier A. C., Ward M. D. Atomic force microscopy of the electrochemical nucleation and growth of molecular crystals. Science. 1994 Mar 4;263(5151):1261–1264. doi: 10.1126/science.263.5151.1261. [DOI] [PubMed] [Google Scholar]
- Hollenberg M. D. Receptor triggering and receptor regulation: structure-activity relationships from the receptor's point of view. J Med Chem. 1990 May;33(5):1275–1281. doi: 10.1021/jm00167a001. [DOI] [PubMed] [Google Scholar]
- Hvidt S. Insulin association in neutral solutions studied by light scattering. Biophys Chem. 1991 Feb;39(2):205–213. doi: 10.1016/0301-4622(91)85023-j. [DOI] [PubMed] [Google Scholar]
- Jeffrey P. D. Polymerization behavior of bovine zinc-insulin at neutral pH. Molecular weight of the subunit and the effect of glucose. Biochemistry. 1974 Oct 8;13(21):4441–4447. doi: 10.1021/bi00718a029. [DOI] [PubMed] [Google Scholar]
- Kaarsholm N. C., Ko H. C., Dunn M. F. Comparison of solution structural flexibility and zinc binding domains for insulin, proinsulin, and miniproinsulin. Biochemistry. 1989 May 16;28(10):4427–4435. doi: 10.1021/bi00436a046. [DOI] [PubMed] [Google Scholar]
- Kadima W., McPherson A., Dunn M. F., Jurnak F. A. Characterization of precrystallization aggregation of canavalin by dynamic light scattering. Biophys J. 1990 Jan;57(1):125–132. doi: 10.1016/S0006-3495(90)82513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam Z., Shore H. B., Feher G. On the crystallization of proteins. J Mol Biol. 1978 Aug 25;123(4):539–555. doi: 10.1016/0022-2836(78)90206-1. [DOI] [PubMed] [Google Scholar]
- Konnert J. H., D'Antonio P., Ward K. B. Observation of growth steps, spiral dislocations and molecular packing on the surface of lysozyme crystals with the atomic force microscope. Acta Crystallogr D Biol Crystallogr. 1994 Jul 1;50(Pt 4):603–613. doi: 10.1107/S0907444994001988. [DOI] [PubMed] [Google Scholar]
- Koszelak S., Day J., Leja C., Cudney R., McPherson A. Protein and virus crystal growth on international microgravity laboratory-2. Biophys J. 1995 Jul;69(1):13–19. doi: 10.1016/S0006-3495(95)79890-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert G., Stura E. A., Wilson I. A. Crystallization and preliminary X-ray diffraction studies of a complex between interleukin-2 and a soluble form of the p55 component of the high affinity interleukin-2 receptor. J Biol Chem. 1989 Jul 25;264(21):12730–12736. [PubMed] [Google Scholar]
- Land TA, Malkin AJ, Kuznetsov YG, McPherson A, De Yoreo JJ Mechanisms of protein crystal growth: An atomic force microscopy study of canavalin crystallization. Phys Rev Lett. 1995 Oct 2;75(14):2774–2777. doi: 10.1103/PhysRevLett.75.2774. [DOI] [PubMed] [Google Scholar]
- Lee G. U., Chrisey L. A., Colton R. J. Direct measurement of the forces between complementary strands of DNA. Science. 1994 Nov 4;266(5186):771–773. doi: 10.1126/science.7973628. [DOI] [PubMed] [Google Scholar]
- Low B. W., Fullerton W. W., Rosen L. S. Insulin-proinsulin, a new crystalline complex. Nature. 1974 Mar 22;248(446):339–340. doi: 10.1038/248339a0. [DOI] [PubMed] [Google Scholar]
- Malkin AJ, Land TA, Kuznetsov YG, McPherson A, DeYoreo JJ. Investigation of virus crystal growth mechanisms by in situ atomic force microscopy. Phys Rev Lett. 1995 Oct 2;75(14):2778–2781. doi: 10.1103/PhysRevLett.75.2778. [DOI] [PubMed] [Google Scholar]
- Matthews B. W. Solvent content of protein crystals. J Mol Biol. 1968 Apr 28;33(2):491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- Milthorpe B. K., Nichol L. W., Jeffrey P. D. The polymerization pattern of zinc(II)-insulin at pH 7.0. Biochim Biophys Acta. 1977 Dec 20;495(2):195–202. doi: 10.1016/0005-2795(77)90376-2. [DOI] [PubMed] [Google Scholar]
- OOSAWA F., KASAI M. A theory of linear and helical aggregations of macromolecules. J Mol Biol. 1962 Jan;4:10–21. doi: 10.1016/s0022-2836(62)80112-0. [DOI] [PubMed] [Google Scholar]
- Ohnesorge F., Heckl W. M., Häberle W., Pum D., Sara M., Schindler H., Schilcher K., Kiener A., Smith D. P., Sleytr U. B. Scanning force microscopy studies of the S-layers from Bacillus coagulans E38-66, Bacillus sphaericus CCM2177 and of an antibody binding process. Ultramicroscopy. 1992 Jul;42-44(Pt B):1236–1242. doi: 10.1016/0304-3991(92)90429-n. [DOI] [PubMed] [Google Scholar]
- Pedersen J. S., Hansen S., Bauer R. The aggregation behavior of zinc-free insulin studied by small-angle neutron scattering. Eur Biophys J. 1994;22(6):379–389. doi: 10.1007/BF00180159. [DOI] [PubMed] [Google Scholar]
- Smith G. D., Ciszak E. The structure of a complex of hexameric insulin and 4'-hydroxyacetanilide. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8851–8855. doi: 10.1073/pnas.91.19.8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. D., Dodson G. G. Structure of a rhombohedral R6 insulin/phenol complex. Proteins. 1992 Nov;14(3):401–408. doi: 10.1002/prot.340140309. [DOI] [PubMed] [Google Scholar]
- Smith G. D., Swenson D. C., Dodson E. J., Dodson G. G., Reynolds C. D. Structural stability in the 4-zinc human insulin hexamer. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7093–7097. doi: 10.1073/pnas.81.22.7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenhorn A. L., Schmitt F. J., Knoll W., Hansma P. K. Streptavidin binding observed with an atomic force microscope. Ultramicroscopy. 1992 Jul;42-44(Pt B):1125–1132. doi: 10.1016/0304-3991(92)90413-e. [DOI] [PubMed] [Google Scholar]
- de Vos A. M., Ultsch M., Kossiakoff A. A. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science. 1992 Jan 17;255(5042):306–312. doi: 10.1126/science.1549776. [DOI] [PubMed] [Google Scholar]