Abstract
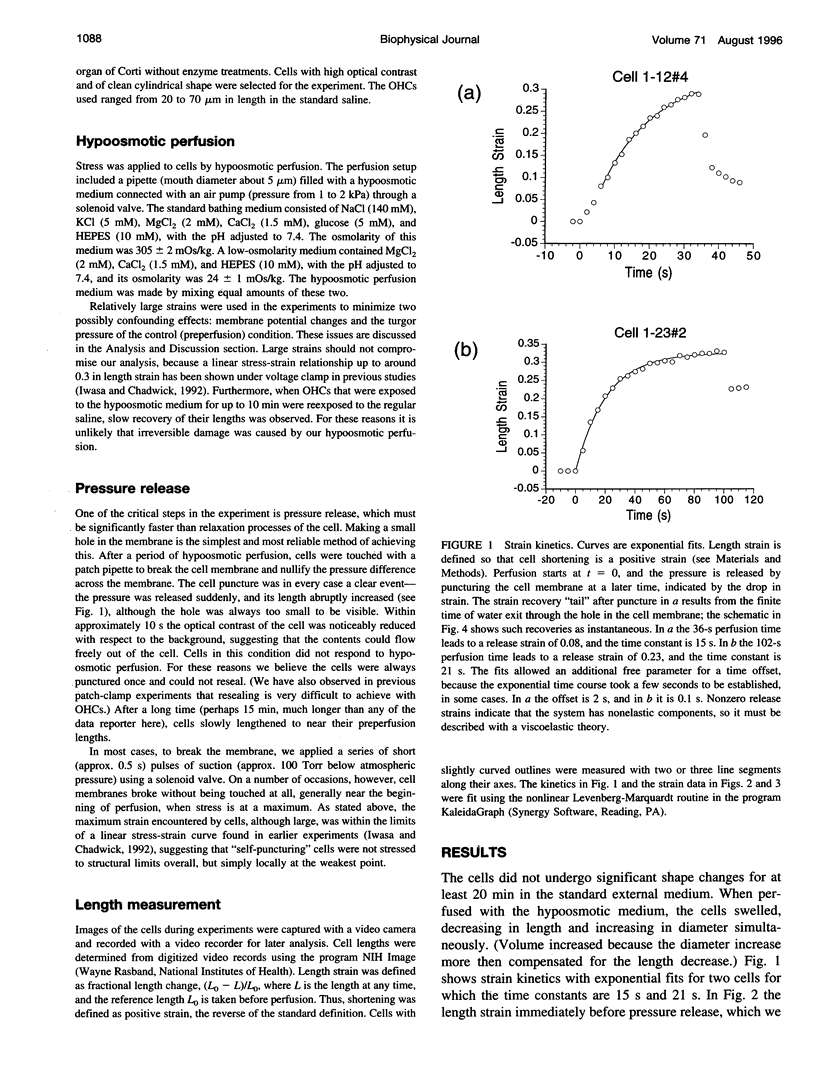
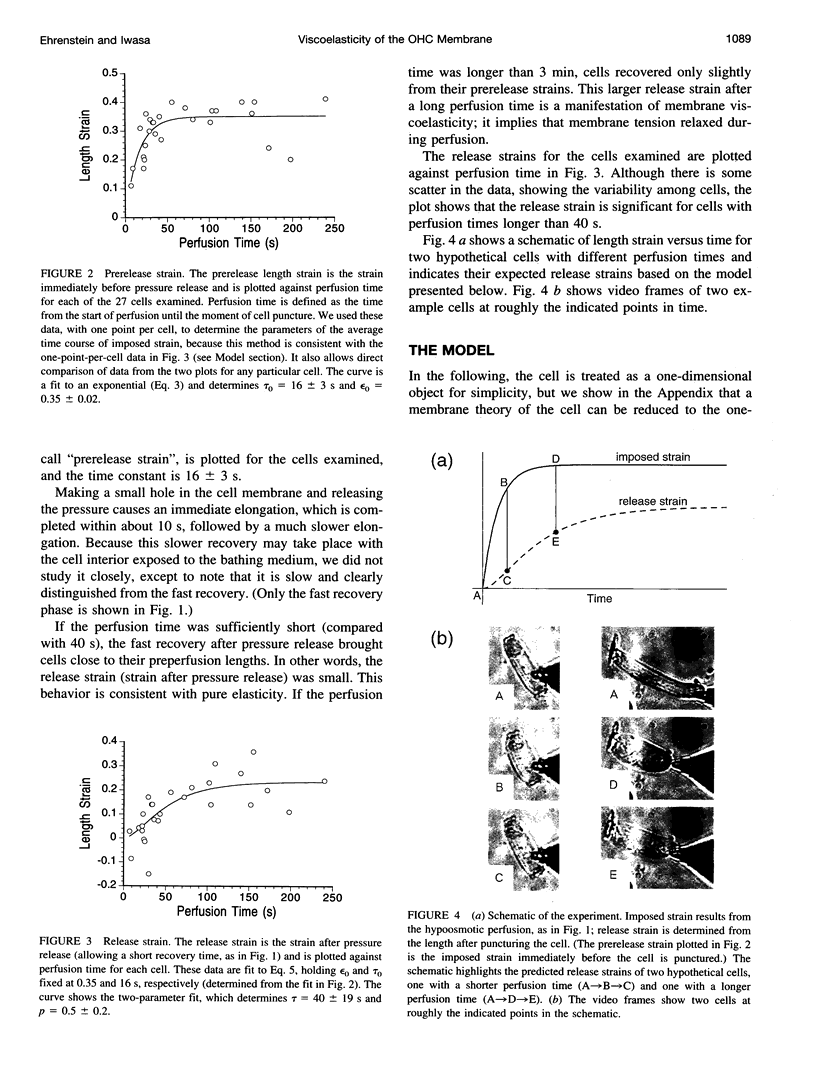
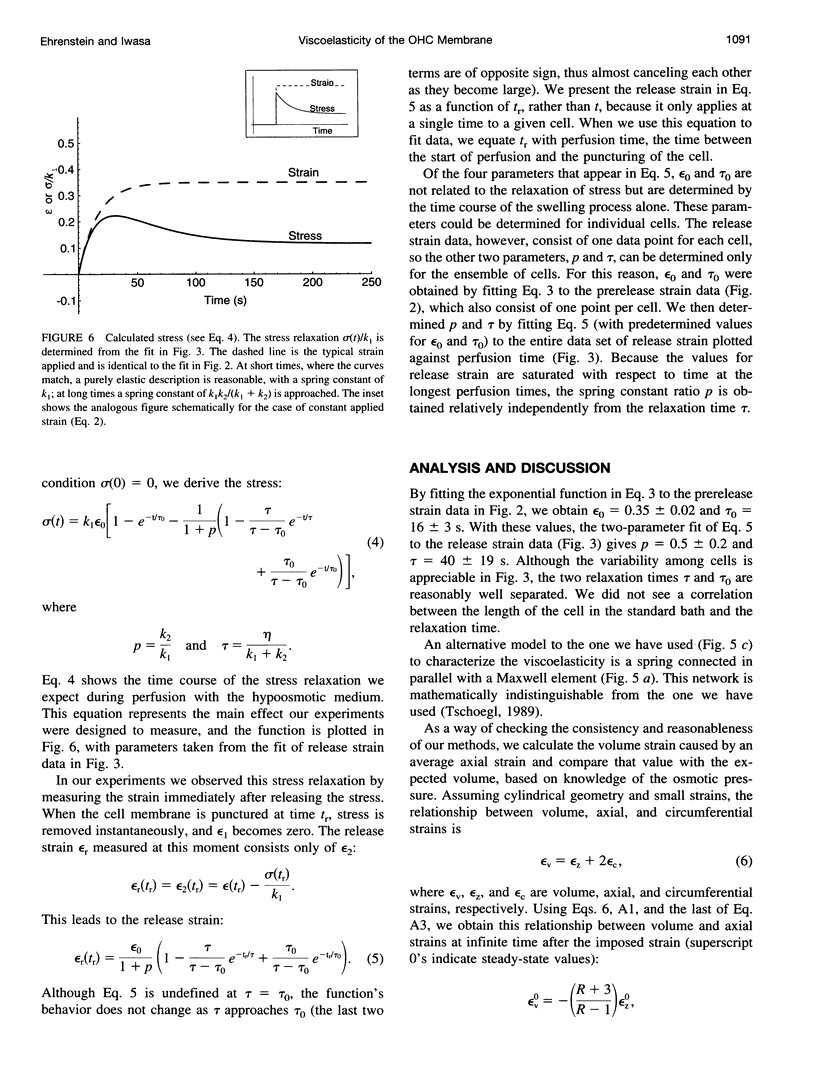
The outer hair cell (OHC) in the mammalian ear has a unique membrane potential-dependent motility, which is considered to be important for frequency discrimination (tuning). The OHC motile mechanism is located at the cell membrane and is strongly influenced by its passive mechanical properties. To study the viscoelastic properties of OHCs, we exposed cells to a hypoosmotic solution for varying durations and then punctured them, to immediately release the osmotic stress. Using video records of the cells, we determined both the imposed strain and the strain after puncturing, when stress was reset to zero. The strain data were described by a simple rheological model consisting of two springs and a dashpot, and the fit to this model gave a time constant of 40 +/- 19 s for the relaxation (reduction) of tension during prolonged strain. For time scales much shorter or longer than this, we would expect essentially elastic behavior. This relaxation process affects the membrane tension of the cell, and because it has been shown that membrane tension has a modulatory role in the OHC's motility, this relaxation process could be part of an adaptation mechanism, with which the motility system of the OHC can adjust to changing conditions and maintain optimum membrane tension.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmore J. F. A fast motile response in guinea-pig outer hair cells: the cellular basis of the cochlear amplifier. J Physiol. 1987 Jul;388:323–347. doi: 10.1113/jphysiol.1987.sp016617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore J. F. The G.L. Brown Prize Lecture. The cellular machinery of the cochlea. Exp Physiol. 1994 Mar;79(2):113–134. doi: 10.1113/expphysiol.1994.sp003746. [DOI] [PubMed] [Google Scholar]
- Assad J. A., Corey D. P. An active motor model for adaptation by vertebrate hair cells. J Neurosci. 1992 Sep;12(9):3291–3309. doi: 10.1523/JNEUROSCI.12-09-03291.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell W. E., Bader C. R., Bertrand D., de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985 Jan 11;227(4683):194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- Brundin L., Flock A., Khanna S. M., Ulfendahl M. Frequency-specific position shift in the guinea pig organ of Corti. Neurosci Lett. 1991 Jul 8;128(1):77–80. doi: 10.1016/0304-3940(91)90763-j. [DOI] [PubMed] [Google Scholar]
- Dallos P., Evans B. N., Hallworth R. Nature of the motor element in electrokinetic shape changes of cochlear outer hair cells. Nature. 1991 Mar 14;350(6314):155–157. doi: 10.1038/350155a0. [DOI] [PubMed] [Google Scholar]
- Dallos P., Hallworth R., Evans B. N. Theory of electrically driven shape changes of cochlear outer hair cells. J Neurophysiol. 1993 Jul;70(1):299–323. doi: 10.1152/jn.1993.70.1.299. [DOI] [PubMed] [Google Scholar]
- Dulon D., Schacht J. Motility of cochlear outer hair cells. Am J Otol. 1992 Mar;13(2):108–112. [PubMed] [Google Scholar]
- Harada N., Ernst A., Zenner H. P. Hyposmotic activation hyperpolarizes outer hair cells of guinea pig cochlea. Brain Res. 1993 Jun 18;614(1-2):205–211. doi: 10.1016/0006-8993(93)91036-r. [DOI] [PubMed] [Google Scholar]
- Hochmuth R. M. Measuring the mechanical properties of individual human blood cells. J Biomech Eng. 1993 Nov;115(4B):515–519. doi: 10.1115/1.2895533. [DOI] [PubMed] [Google Scholar]
- Holley M. C., Ashmore J. F. On the mechanism of a high-frequency force generator in outer hair cells isolated from the guinea pig cochlea. Proc R Soc Lond B Biol Sci. 1988 Jan 22;232(1269):413–429. doi: 10.1098/rspb.1988.0004. [DOI] [PubMed] [Google Scholar]
- Huang G., Santos-Sacchi J. Mapping the distribution of the outer hair cell motility voltage sensor by electrical amputation. Biophys J. 1993 Nov;65(5):2228–2236. doi: 10.1016/S0006-3495(93)81248-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth A. J. How the ear's works work. Nature. 1989 Oct 5;341(6241):397–404. doi: 10.1038/341397a0. [DOI] [PubMed] [Google Scholar]
- Iwasa K. H. A membrane motor model for the fast motility of the outer hair cell. J Acoust Soc Am. 1994 Oct;96(4):2216–2224. doi: 10.1121/1.410094. [DOI] [PubMed] [Google Scholar]
- Iwasa K. H., Chadwick R. S. Elasticity and active force generation of cochlear outer hair cells. J Acoust Soc Am. 1992 Dec;92(6):3169–3173. doi: 10.1121/1.404194. [DOI] [PubMed] [Google Scholar]
- Iwasa K. H., Chadwick R. S. Factors influencing the length change of an auditory outer hair cell in a tight-fitting capillary. J Acoust Soc Am. 1993 Aug;94(2 Pt 1):1156–1159. doi: 10.1121/1.406965. [DOI] [PubMed] [Google Scholar]
- Iwasa K. H. Effect of stress on the membrane capacitance of the auditory outer hair cell. Biophys J. 1993 Jul;65(1):492–498. doi: 10.1016/S0006-3495(93)81053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa K. H., Kachar B. Fast in vitro movement of outer hair cells in an external electric field: effect of digitonin, a membrane permeabilizing agent. Hear Res. 1989 Jul;40(3):247–254. doi: 10.1016/0378-5955(89)90165-2. [DOI] [PubMed] [Google Scholar]
- Iwasa K. H., Li M. X., Jia M., Kachar B. Stretch sensitivity of the lateral wall of the auditory outer hair cell from the guinea pig. Neurosci Lett. 1991 Dec 9;133(2):171–174. doi: 10.1016/0304-3940(91)90562-8. [DOI] [PubMed] [Google Scholar]
- Iwasa K. H., Li M., Jia M. Can membrane proteins drive a cell? Biophys J. 1995 Apr;68(4 Suppl):214S–214S. [PMC free article] [PubMed] [Google Scholar]
- Kachar B., Brownell W. E., Altschuler R., Fex J. Electrokinetic shape changes of cochlear outer hair cells. Nature. 1986 Jul 24;322(6077):365–368. doi: 10.1038/322365a0. [DOI] [PubMed] [Google Scholar]
- Kakehata S., Santos-Sacchi J. Membrane tension directly shifts voltage dependence of outer hair cell motility and associated gating charge. Biophys J. 1995 May;68(5):2190–2197. doi: 10.1016/S0006-3495(95)80401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinec F., Holley M. C., Iwasa K. H., Lim D. J., Kachar B. A membrane-based force generation mechanism in auditory sensory cells. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8671–8675. doi: 10.1073/pnas.89.18.8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman M. C., Dodds L. W. Single-neuron labeling and chronic cochlear pathology. III. Stereocilia damage and alterations of threshold tuning curves. Hear Res. 1984 Oct;16(1):55–74. doi: 10.1016/0378-5955(84)90025-x. [DOI] [PubMed] [Google Scholar]
- Poznański J., Pawłowski P., Fikus M. Bioelectrorheological model of the cell. 3. Viscoelastic shear deformation of the membrane. Biophys J. 1992 Mar;61(3):612–620. doi: 10.1016/S0006-3495(92)81866-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J., Dilger J. P. Whole cell currents and mechanical responses of isolated outer hair cells. Hear Res. 1988 Sep 15;35(2-3):143–150. doi: 10.1016/0378-5955(88)90113-x. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J. Harmonics of outer hair cell motility. Biophys J. 1993 Nov;65(5):2217–2227. doi: 10.1016/S0006-3495(93)81247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung K. L., Chien S. Influence of temperature on rheology of human erythrocytes. Chin J Physiol. 1992;35(2):81–94. [PubMed] [Google Scholar]
- Tolomeo J. A., Steele C. R. Orthotropic piezoelectric properties of the cochlear outer hair cell wall. J Acoust Soc Am. 1995 May;97(5 Pt 1):3006–3011. doi: 10.1121/1.411865. [DOI] [PubMed] [Google Scholar]



