Abstract
Subdomains of the cytoplasmic volume in tissue culture cells exclude large tracer particles relative to small. Evidence suggests that exclusion of the large particles is due to molecular sieving by the dense meshwork of microfilaments found in these compartments, but exclusion as a result of the close apposition of the dorsal and ventral plasma membrane of the cell in these regions has not been ruled out conclusively. In principle, these two mechanisms can be distinguished by the dependence of exclusion on tracer particle size. By fluorescence ratio imaging we have measured the partition coefficient (P/PO) into excluding compartments for tracer particles ranging in radius from 1 to 41 nm. The decay of P/PO as a function of particle radius is better fitted by three molecular sieving models than by a slit pore model. The sieving models predict a percolation cutoff radius of the order of 50 nm for partitioning into excluding compartments.
Full text
PDF


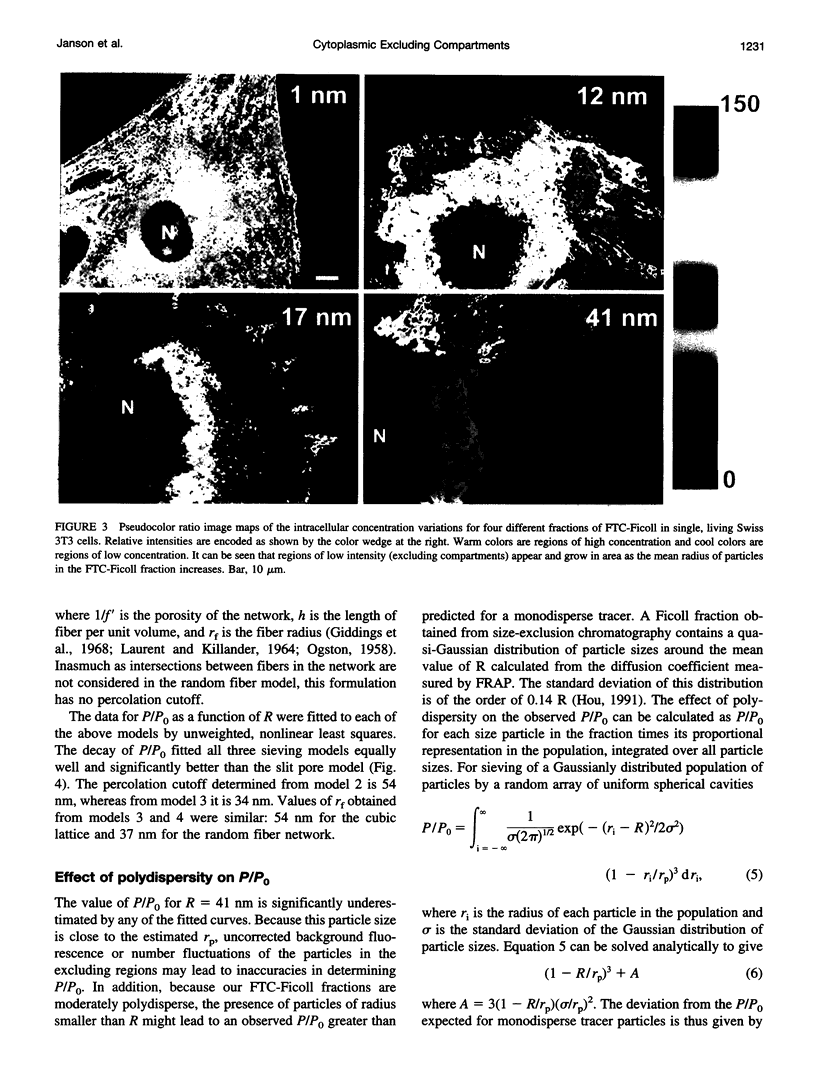
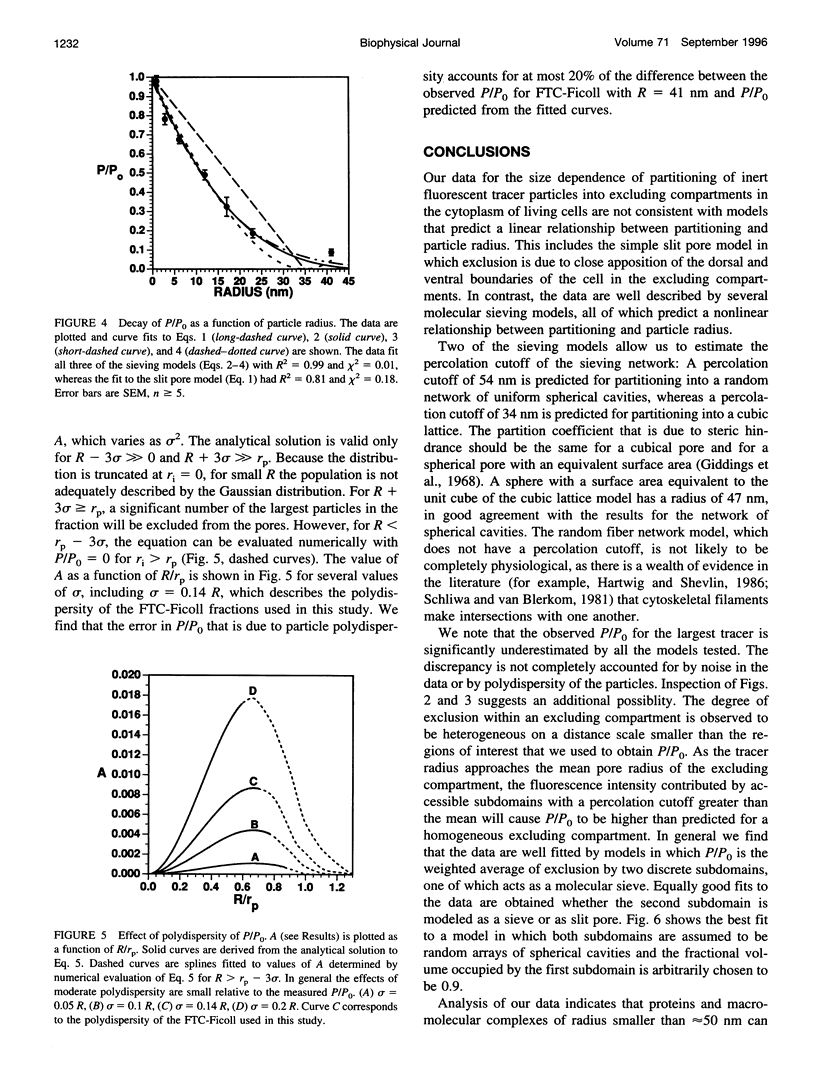

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. L., Quinn J. A. Restricted transport in small pores. A model for steric exclusion and hindered particle motion. Biophys J. 1974 Feb;14(2):130–150. doi: 10.1016/S0006-3495(74)70005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassell G. J., Taneja K. L., Kislauskis E. H., Sundell C. L., Powers C. M., Ross A., Singer R. H. Actin filaments and the spatial positioning of mRNAS. Adv Exp Med Biol. 1994;358:183–189. doi: 10.1007/978-1-4615-2578-3_17. [DOI] [PubMed] [Google Scholar]
- Bridgman P. C., Dailey M. E. The organization of myosin and actin in rapid frozen nerve growth cones. J Cell Biol. 1989 Jan;108(1):95–109. doi: 10.1083/jcb.108.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg J. S. Properties and metabolism of the aqueous cytoplasm and its boundaries. Am J Physiol. 1984 Feb;246(2 Pt 2):R133–R151. doi: 10.1152/ajpregu.1984.246.2.R133. [DOI] [PubMed] [Google Scholar]
- Condeelis J. Elongation factor 1 alpha, translation and the cytoskeleton. Trends Biochem Sci. 1995 May;20(5):169–170. doi: 10.1016/s0968-0004(00)88998-7. [DOI] [PubMed] [Google Scholar]
- Felder S., Elson E. L. Mechanics of fibroblast locomotion: quantitative analysis of forces and motions at the leading lamellas of fibroblasts. J Cell Biol. 1990 Dec;111(6 Pt 1):2513–2526. doi: 10.1083/jcb.111.6.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forscher P., Kaczmarek L. K., Buchanan J. A., Smith S. J. Cyclic AMP induces changes in distribution and transport of organelles within growth cones of Aplysia bag cell neurons. J Neurosci. 1987 Nov;7(11):3600–3611. doi: 10.1523/JNEUROSCI.07-11-03600.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnight J. A., Mischak H., Kolch W., Mushinski J. F. Immunocytochemical localization of eight protein kinase C isozymes overexpressed in NIH 3T3 fibroblasts. Isoform-specific association with microfilaments, Golgi, endoplasmic reticulum, and nuclear and cell membranes. J Biol Chem. 1995 Apr 28;270(17):9991–10001. doi: 10.1074/jbc.270.17.9991. [DOI] [PubMed] [Google Scholar]
- Hartwig J. H., Shevlin P. The architecture of actin filaments and the ultrastructural location of actin-binding protein in the periphery of lung macrophages. J Cell Biol. 1986 Sep;103(3):1007–1020. doi: 10.1083/jcb.103.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hens J. J., Benfenati F., Nielander H. B., Valtorta F., Gispen W. H., De Graan P. N. B-50/GAP-43 binds to actin filaments without affecting actin polymerization and filament organization. J Neurochem. 1993 Oct;61(4):1530–1533. doi: 10.1111/j.1471-4159.1993.tb13649.x. [DOI] [PubMed] [Google Scholar]
- Hou L., Lanni F., Luby-Phelps K. Tracer diffusion in F-actin and Ficoll mixtures. Toward a model for cytoplasm. Biophys J. 1990 Jul;58(1):31–43. doi: 10.1016/S0006-3495(90)82351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil R. A., Menice C. B., Wang C. L., Morgan K. G. Phosphotyrosine-dependent targeting of mitogen-activated protein kinase in differentiated contractile vascular cells. Circ Res. 1995 Jun;76(6):1101–1108. doi: 10.1161/01.res.76.6.1101. [DOI] [PubMed] [Google Scholar]
- Lin C. H., Forscher P. Cytoskeletal remodeling during growth cone-target interactions. J Cell Biol. 1993 Jun;121(6):1369–1383. doi: 10.1083/jcb.121.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby-Phelps K., Castle P. E., Taylor D. L., Lanni F. Hindered diffusion of inert tracer particles in the cytoplasm of mouse 3T3 cells. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4910–4913. doi: 10.1073/pnas.84.14.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby-Phelps K., Hori M., Phelps J. M., Won D. Ca(2+)-regulated dynamic compartmentalization of calmodulin in living smooth muscle cells. J Biol Chem. 1995 Sep 15;270(37):21532–21538. doi: 10.1074/jbc.270.37.21532. [DOI] [PubMed] [Google Scholar]
- Luby-Phelps K. Preparation of fluorescently labeled dextrans and ficolls. Methods Cell Biol. 1989;29:59–73. doi: 10.1016/s0091-679x(08)60187-9. [DOI] [PubMed] [Google Scholar]
- Luby-Phelps K., Taylor D. L. Subcellular compartmentalization by local differentiation of cytoplasmic structure. Cell Motil Cytoskeleton. 1988;10(1-2):28–37. doi: 10.1002/cm.970100107. [DOI] [PubMed] [Google Scholar]
- Minton A. P. Confinement as a determinant of macromolecular structure and reactivity. II. Effects of weakly attractive interactions between confined macrosolutes and confining structures. Biophys J. 1995 Apr;68(4):1311–1322. doi: 10.1016/S0006-3495(95)80304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton A. P. Confinement as a determinant of macromolecular structure and reactivity. Biophys J. 1992 Oct;63(4):1090–1100. doi: 10.1016/S0006-3495(92)81663-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly G., Clarke F. Identification of an actin binding region in aldolase. FEBS Lett. 1993 Apr 19;321(1):69–72. doi: 10.1016/0014-5793(93)80623-3. [DOI] [PubMed] [Google Scholar]
- Provance D. W., Jr, McDowall A., Marko M., Luby-Phelps K. Cytoarchitecture of size-excluding compartments in living cells. J Cell Sci. 1993 Oct;106(Pt 2):565–577. doi: 10.1242/jcs.106.2.565. [DOI] [PubMed] [Google Scholar]
- Schliwa M., van Blerkom J. Structural interaction of cytoskeletal components. J Cell Biol. 1981 Jul;90(1):222–235. doi: 10.1083/jcb.90.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small J. V., Rinnerthaler G., Hinssen H. Organization of actin meshworks in cultured cells: the leading edge. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):599–611. doi: 10.1101/sqb.1982.046.01.056. [DOI] [PubMed] [Google Scholar]