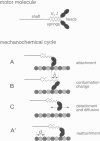
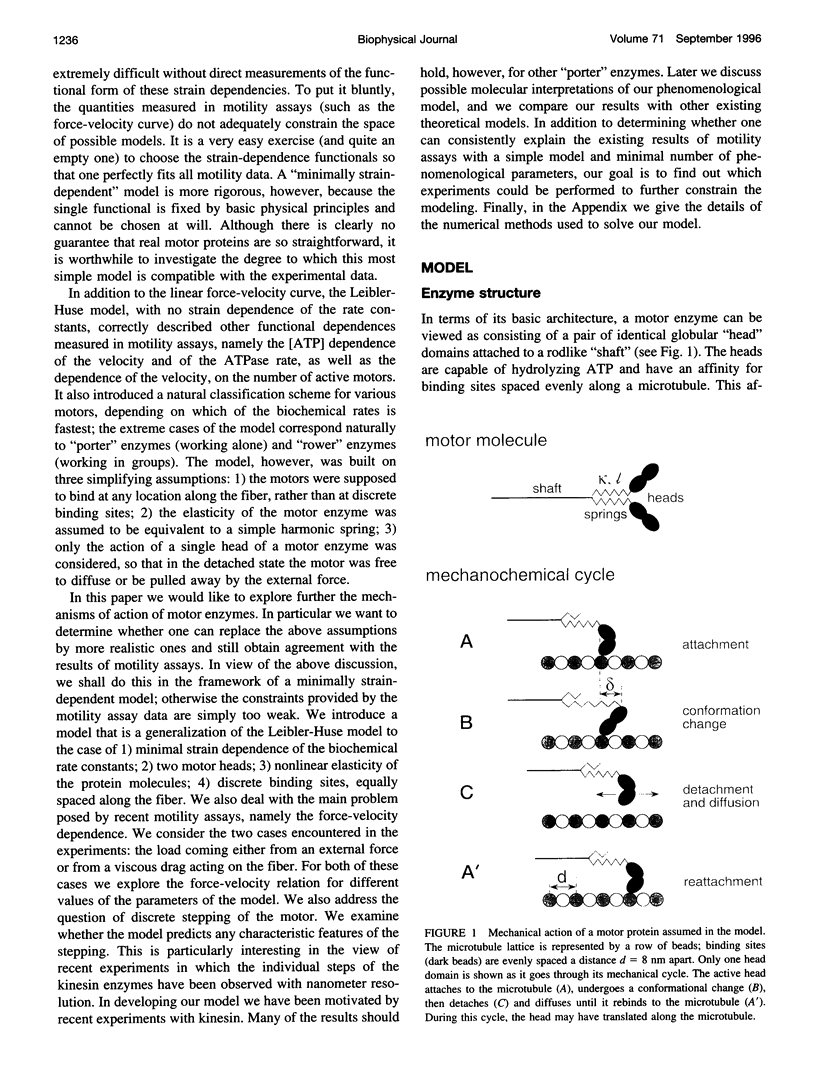
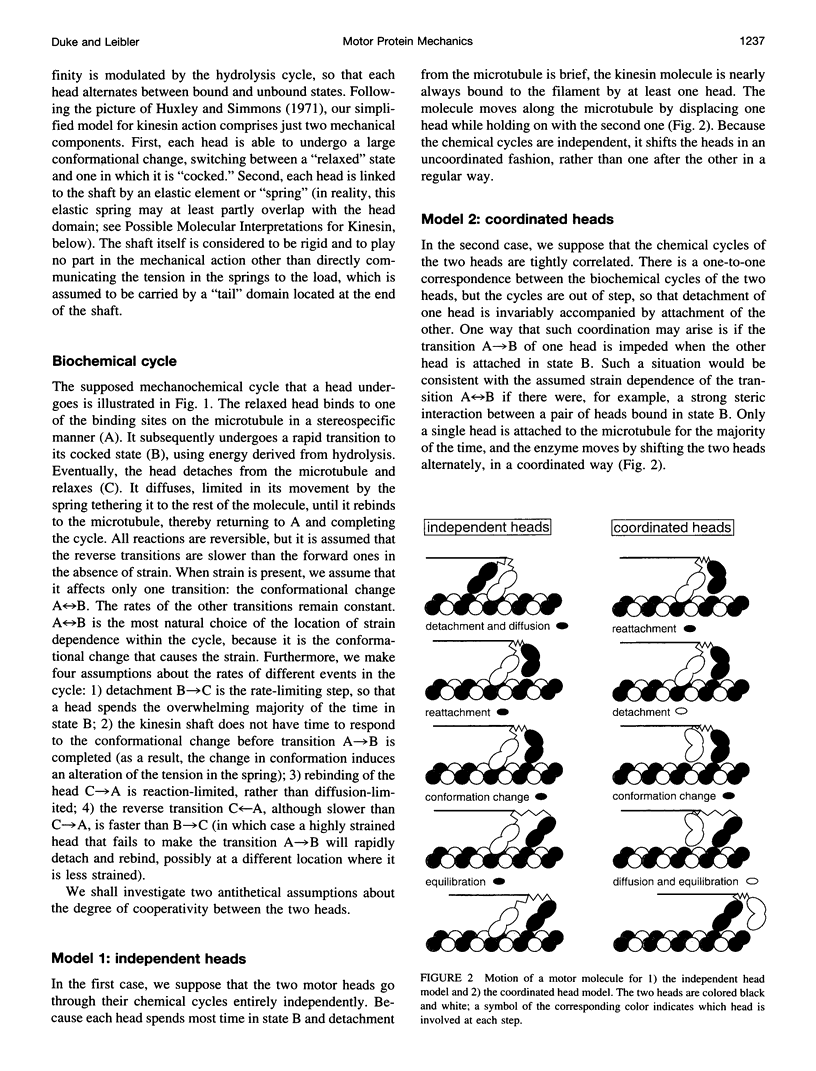
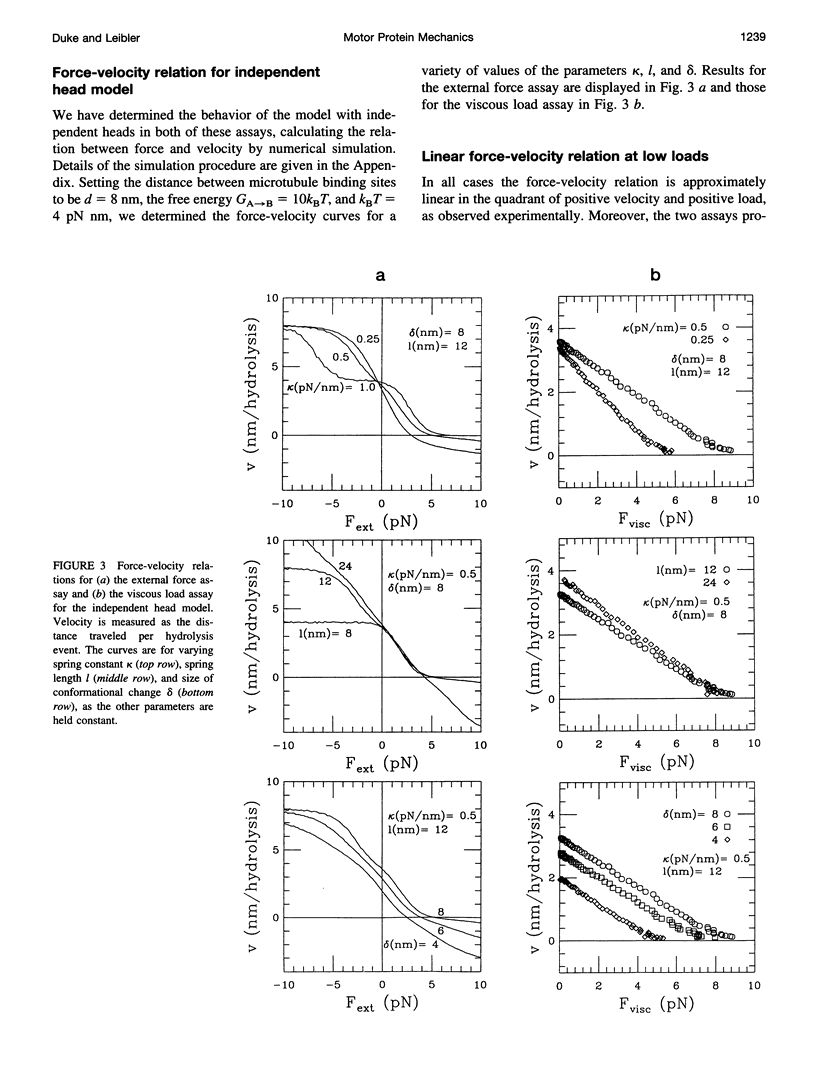
Abstract
A stochastic model for the action of motor proteins such as kinesin is presented. The mechanical components of the enzyme are 1) two identical head domains that bind to discrete sites on a microtubule and that are capable of undergoing a conformational change; and 2) an elastic element that connects each head to the rest of the molecule. We investigate the situation in which the strain dependence of the chemical reaction rates is minimal and the heads have independent biochemical cycles. The enzyme advances stochastically along a filament when one head detaches and diffuses to a new binding site, while the other head remains bound to the microtubule. We also investigate the case in which the chemical cycles of the heads are correlated so that the molecule shifts each head alternately. The predictions of the model are found to be in agreement with experimentally measured force-velocity relationships for kinesin-both when the force is applied externally and when the enzyme is loaded by a viscous drag. For reasonable values of the parameters, this agreement is quantitative. The molecular stepping characteristics observed in recent motility assays are also reproduced. A number of experiments are suggested that would provide a more stringent test of the model and help determine whether this simple picture is an appropriate description of motor proteins or whether models that include strain-dependent reaction rates or more complicated types of cooperation of the two heads need to be considered.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astumian RD, Bier M. Fluctuation driven ratchets: Molecular motors. Phys Rev Lett. 1994 Mar 14;72(11):1766–1769. doi: 10.1103/PhysRevLett.72.1766. [DOI] [PubMed] [Google Scholar]
- Berliner E., Young E. C., Anderson K., Mahtani H. K., Gelles J. Failure of a single-headed kinesin to track parallel to microtubule protofilaments. Nature. 1995 Feb 23;373(6516):718–721. doi: 10.1038/373718a0. [DOI] [PubMed] [Google Scholar]
- Funatsu T., Harada Y., Tokunaga M., Saito K., Yanagida T. Imaging of single fluorescent molecules and individual ATP turnovers by single myosin molecules in aqueous solution. Nature. 1995 Apr 6;374(6522):555–559. doi: 10.1038/374555a0. [DOI] [PubMed] [Google Scholar]
- Gilbert S. P., Webb M. R., Brune M., Johnson K. A. Pathway of processive ATP hydrolysis by kinesin. Nature. 1995 Feb 23;373(6516):671–676. doi: 10.1038/373671a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney D. D. Evidence for alternating head catalysis by kinesin during microtubule-stimulated ATP hydrolysis. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):6865–6869. doi: 10.1073/pnas.91.15.6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney D. D. The rate-limiting step in microtubule-stimulated ATP hydrolysis by dimeric kinesin head domains occurs while bound to the microtubule. J Biol Chem. 1994 Jun 10;269(23):16508–16511. [PubMed] [Google Scholar]
- Harrison B. C., Marchese-Ragona S. P., Gilbert S. P., Cheng N., Steven A. C., Johnson K. A. Decoration of the microtubule surface by one kinesin head per tubulin heterodimer. Nature. 1993 Mar 4;362(6415):73–75. doi: 10.1038/362073a0. [DOI] [PubMed] [Google Scholar]
- Hunt A. J., Gittes F., Howard J. The force exerted by a single kinesin molecule against a viscous load. Biophys J. 1994 Aug;67(2):766–781. doi: 10.1016/S0006-3495(94)80537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Leibler S., Huse D. A. Porters versus rowers: a unified stochastic model of motor proteins. J Cell Biol. 1993 Jun;121(6):1357–1368. doi: 10.1083/jcb.121.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnasco MO. Forced thermal ratchets. Phys Rev Lett. 1993 Sep 6;71(10):1477–1481. doi: 10.1103/PhysRevLett.71.1477. [DOI] [PubMed] [Google Scholar]
- Meyhöfer E., Howard J. The force generated by a single kinesin molecule against an elastic load. Proc Natl Acad Sci U S A. 1995 Jan 17;92(2):574–578. doi: 10.1073/pnas.92.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskin C. S., Oster G. Coordinated hydrolysis explains the mechanical behavior of kinesin. Biophys J. 1995 Apr;68(4 Suppl):202S–211S. [PMC free article] [PubMed] [Google Scholar]
- Prost J, Chauwin JF, Peliti L, Ajdari A. Asymmetric pumping of particles. Phys Rev Lett. 1994 Apr 18;72(16):2652–2655. doi: 10.1103/PhysRevLett.72.2652. [DOI] [PubMed] [Google Scholar]
- Ray S., Meyhöfer E., Milligan R. A., Howard J. Kinesin follows the microtubule's protofilament axis. J Cell Biol. 1993 Jun;121(5):1083–1093. doi: 10.1083/jcb.121.5.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayment I., Holden H. M., Whittaker M., Yohn C. B., Lorenz M., Holmes K. C., Milligan R. A. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993 Jul 2;261(5117):58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- Svoboda K., Block S. M. Force and velocity measured for single kinesin molecules. Cell. 1994 Jun 3;77(5):773–784. doi: 10.1016/0092-8674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Svoboda K., Mitra P. P., Block S. M. Fluctuation analysis of motor protein movement and single enzyme kinetics. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):11782–11786. doi: 10.1073/pnas.91.25.11782. [DOI] [PMC free article] [PubMed] [Google Scholar]