Abstract
Background
MDM2, a negative regulator of p53, has emerged as a potential therapeutic target as its inhibition can restore p53 tumor suppressor activity in MDM2-amplified tumors, including subsets of biliary tract cancer (BTC). However, the genomic and clinical characteristics of MDM2-amplified BTC remain poorly understood.
Materials and methods
Patients with advanced BTC who underwent tissue-based targeted next-generation sequencing and received first-line gemcitabine plus cisplatin (GemCis)-based chemotherapy at Asan Medical Center, Seoul, Korea, between January 2016 and December 2023 were included. Clinicogenomic characteristics and survival outcomes were compared according to the presence of MDM2 amplification with wild-type TP53 (MDM2-amp/TP53-WT). Propensity score (PS) matching was carried out to balance baseline characteristics between patients with and without MDM2-amp/TP53-WT.
Results
Among 813 patients, there were 41 patients (5.0%) with MDM2-amp/TP53-WT, demonstrating no significant association with the primary tumor site: intrahepatic cholangiocarcinoma (4.7%), extrahepatic cholangiocarcinoma (3.7%), and gall-bladder cancer (8.0%) (P = 0.111). Overall, clinical characteristics did not differ according to MDM2-amp/TP53-WT status. Actionable alterations, including ERBB2 amplification (2.4% versus 10.6%) and IDH1 mutation (2.4% versus 6.9%), were less common in MDM2-amp/TP53-WT tumors. In both unmatched and PS-matched populations, MDM2-amp/TP53-WT was associated with significantly longer progression-free survival with first-line GemCis-based therapy [in PS-matched analysis: median 9.6 versus 6.9 months, hazard ratio (HR) 0.63, P = 0.035] and longer, but not statistically significant, overall survival (in PS-matched analysis: median 20.3 versus 16.4 months, HR 0.81, P = 0.267).
Conclusions
In advanced BTC, tumors harboring MDM2-amp/TP53-WT exhibited distinct mutational patterns with limited other actionable alterations and were associated with better survival outcomes following first-line GemCis-containing chemotherapy. Further investigation of MDM2-targeted therapies for this subset is warranted.
Key words: biliary tract cancer, cholangiocarcinoma, MDM2, TP53, gemcitabine, cisplatin
Highlights
-
•
Patients with MDM2-amp/TP53-WT account for 5.0% of advanced BTC.
-
•
MDM2-amp/TP53-WT status is associated with favorable PFS following GemCis-based chemotherapy.
-
•
MDM2-amp/TP53-WT tumors exhibit distinct mutational profiles with limited other actionable alterations.
Introduction
Biliary tract cancer (BTC) refers to a group of aggressive malignancies arising from epithelial cells lining the biliary tree, including intrahepatic cholangiocarcinoma (CCA), extrahepatic CCA, and gall-bladder cancer, each harboring distinct genomic alterations contributing to its molecular heterogeneity.1 Surgery is the cornerstone of cure; however, most patients present at an advanced stage.1,2 Systemic chemotherapy is the mainstay of treatment for patients with advanced BTC. A combination of immune checkpoint inhibitors (ICIs) with gemcitabine plus cisplatin (GemCis) is the current standard of care; however, the median overall survival is limited to around 1 year.3,4
In recent years, a growing body of evidence has highlighted the feasibility and efficacy of targeted therapies in advanced BTC. Advances in next-generation sequencing (NGS) have enabled the identification of actionable genetic alterations, with nearly 40% of patients known to harbor targetable alterations.5,6 Many targeted agents are being actively investigated, and several, including those for IDH1 mutation, FGFR2 fusion, and ERBB2 amplification, have demonstrated clinical efficacy and received approval for the later-line treatment of advanced BTC.7, 8, 9
Among targetable alterations, amplification of the Mouse double minute 2 homolog (MDM2) gene, which codes for the oncoprotein MDM2, is present in 5%-8% of cases of BTC.5,10 MDM2 primarily exerts its effects by inhibiting the p53 tumor suppressor.11 p53 induces cell death by apoptosis under stress conditions such as DNA damage.12 Consequently, overexpressed MDM2 inhibits p53 under stress conditions, leading to uncontrolled cell proliferation.13 Accordingly, inhibition of the MDM2–p53 interaction and reactivation of p53-dependent pathways have emerged as a potential therapeutic strategy in the presence of wild-type (WT) p53,14 and novel agents targeting MDM2 are currently under development.15
At present, there is a lack of literature on the clinicogenomic characteristics of MDM2-amplified BTC. Given the therapeutic potential of MDM2-targeted therapies for tumors harboring MDM2-amplification/TP53-WT (MDM2-amp/TP53-WT) status, it is necessary to understand the disease characteristics and biology of this subset. In this study, we investigated the clinicogenomic implications of MDM2-amp/TP53-WT status in a homogeneous group of patients with advanced BTC treated with first-line GemCis-containing chemotherapy.
Materials and methods
Study design and patients
Patients were retrospectively identified and included in the analysis if they had histologically confirmed adenocarcinoma or adenosquamous carcinoma originating from the intrahepatic or extrahepatic bile duct, or the gall-bladder. Eligible patients had locally advanced unresectable or metastatic disease, or recurrent disease following curative-intent surgery and were treated with first-line GemCis-containing chemotherapy at Asan Medical Center, Seoul, Republic of Korea, between 1 January 2016 and 31 December 2023. Available tissue-based NGS results were required for inclusion. Patients with tumor histology other than adenocarcinoma or adenosquamous carcinoma, as well as those with concurrent active malignancy, were excluded.
The study protocol was approved by the Institutional Review Board of Asan Medical Center and was conducted in accordance with the ethical standards of the Declaration of Helsinki. All authors had access to the study data and reviewed and approved the final manuscript.
Tissue-based targeted NGS data analysis
Targeted NGS was carried out using the NextSeq platform (Illumina, San Diego, CA) with OncoPanel AMC version 4, an in-house panel developed at Asan Medical Center, Seoul, Republic of Korea. This panel captured 323 cancer-related genes, including 225 genes for entire exons, 6 genes for partial introns, and 99 hotspots involved in rearrangements, and tumor samples were evaluated for genomic alterations, including base substitutions, insertions, deletions, copy number variations, and gene fusions and rearrangements. The tumor mutational burden was calculated as the number of nonsynonymous alterations per megabase of the analyzed genome. The Maftools package was used to summarize, visualize, and analyze the NGS-derived maf files from the entire study population.16 Oncogenic alterations were defined as oncogenic or likely oncogenic alterations according to the OncoKB database.17 Actionable alterations were defined as oncogenic alterations with European Society for Medical Oncology Scale for Clinical Actionability of molecular Targets (ESCAT) tiers I-III,18 with the addition of MTAP deletion given its potential therapeutic implications.19 Alterations in oncogenic signaling pathways were analyzed according to the 10 canonical pathways defined by Sanchez-Vega et al.20 A set of 127 genes associated with six key DNA damage repair (DDR) pathways were identified based on the data from Qin et al.21 This list consisted of 42 genes related to homologous recombination (HR), 12 genes involved in mismatch repair (MMR), 33 genes associated with nucleotide excision repair (NER), 20 genes linked to Fanconi anemia (FA), 9 genes involved in nonhomologous end joining (NHEJ), and 22 genes involved in base excision repair (BER). Core HR gene alterations comprised oncogenic alterations in three key HR genes (BRCA1, BRCA2, and PALB2), whereas non-core HR gene alterations included oncogenic alterations in the remaining 39 HR genes.22
Assessments
MDM2 amplification and TP53 mutation status were determined based on targeted NGS results, with MDM2 amplification defined as five or more copies per tumor cell. Baseline characteristics, clinical outcomes, and the genomic landscape were analyzed and compared according to the presence or absence of MDM2-amp/TP53-WT. Tumor response was assessed according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1). Progression-free survival (PFS) was defined as the time from the start of first-line GemCis-based therapy to the date of disease progression or death, whichever occurred first. Overall survival (OS) was defined as the time from the start of the GemCis-based therapy to death from any cause. The objective response rate (ORR) was defined as the proportion of patients who achieved complete and partial responses, and the disease control rate (DCR) was defined as the proportion of patients who achieved complete response, partial response, and stable disease.
Statistical analysis
In the overall cohort, chi-square test or Fisher’s exact test was used to compare categorical variables between groups, and Student’s t-test or Wilcoxon rank sum test was used to compare continuous variables between groups. Survival curves were estimated using the Kaplan–Meier method and compared by the log-rank test. The association between survival endpoints and potential prognostic factors was assessed using Cox regression models. Univariate analysis was carried out, and variables with a P value of <0.10 or that were clinically relevant were included in a multivariate model.
Propensity score (PS) matching was used to compare the clinical outcomes of first-line GemCis-containing therapy while reducing differences in the baseline characteristics between groups. For PS matching, missing values in the baseline characteristics were filled by single imputation using sequential regression (also known as chained equations).23 The PS, which could estimate the probability of MDM2-amp/TP53-WT based on baseline covariates, was derived using a multivariable logistic regression model. Matching was carried out using a 1 : 4 nearest neighbor matching algorithm without replacement, with a caliper width equal to 0.2 of the standard deviation of the logit of the PS. Absolute standardized differences (ASDs) were assessed pre- and post-matching, with ASD <0.1 indicating a relatively small imbalance. Clinical outcomes in the matched cohort were further adjusted using logistic regression (ORR and DCR) and Cox proportional hazards regression (PFS and OS), both with robust standard errors to account for clustering in matched pairs. In the matched cohort, P values for survival outcomes were reported from Cox regression analyses.
All reported P values were two-sided, and P < 0.05 was considered statistically significant. All statistical analyses were carried out using R software, version 4.2.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
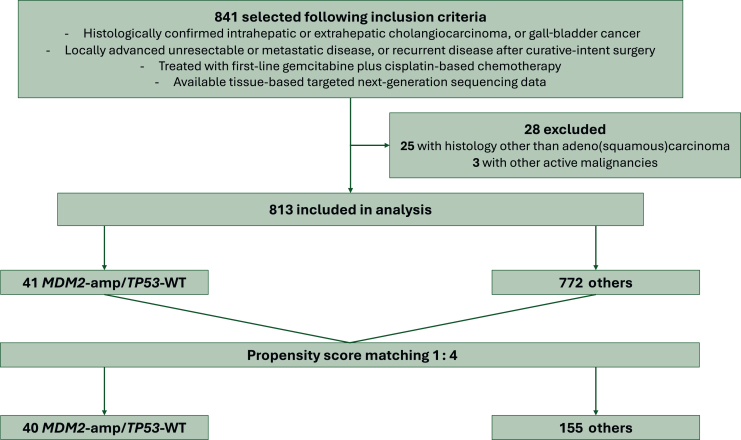
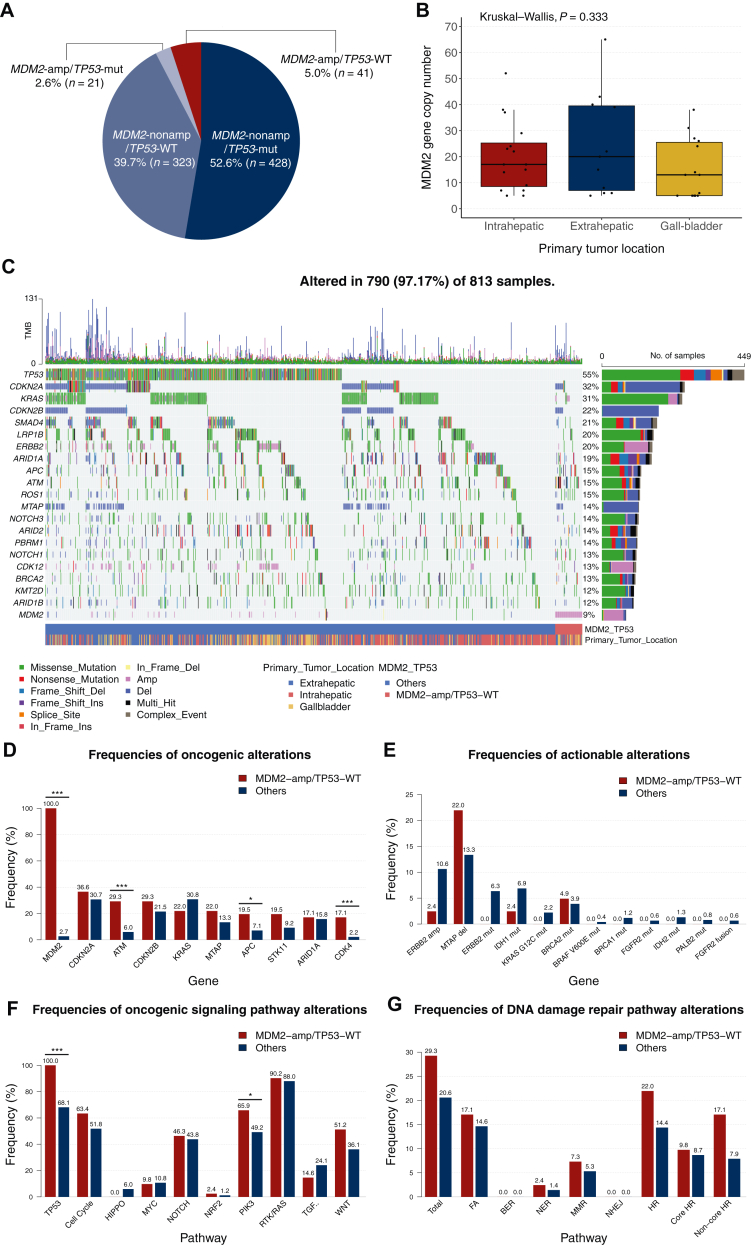
From January 2016 to December 2023, a total of 841 patients with advanced BTC who received palliative first-line GemCis-based chemotherapy were identified, of whom 813 patients were included in the analysis (Figure 1). Among the 813 patients, there were 41 patients (5.0%) with MDM2-amp/TP53-WT (Figure 2A).
Figure 1.
Study diagram. amp, amplification; WT, wild-type.
Figure 2.
Genomic landscape highlighting MDM2 amplification in the overall population. (A) MDM2 amplification and TP53 mutation status in the overall population. (B) MDM2 gene copy number according to the primary tumor location among patients with MDM2-amp/TP53-WT. (C) Genomic landscape of alterations detected with tissue-based targeted next-generation sequencing (NGS) in the overall population. (D-G) Gene- and pathway-level alterations according to the presence of MDM2-amp/TP53-WT. (D) Top 10 oncogenic alterations in patients with MDM2-amp/TP53-WT. (E) Actionable alterations. (F) Oncogenic signaling pathways. (G) DNA damage repair (DDR) pathways. Statistically significant findings (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001) were obtained with Pearson’s chi-square test or Fisher’s exact test as appropriate. amp, amplification; BER, base excision repair; del, deletion; FA, Fanconi anemia; HR, homologous recombination; MMR, mismatch repair; mut, mutation; NER, nucleotide excision repair; NHEJ, nonhomologous end joining; nonamp, non-amplification; TMB, tumor mutational burden; WT, wild-type.
The baseline patient and tumor characteristics are summarized in Table 1. Overall, baseline characteristics including age at diagnosis, sex, primary tumor site, carbohydrate antigen 19-9 level, and microsatellite instability (MSI) status did not differ according to the presence of MDM2-amp/TP53-WT tumors. However, viral hepatitis B infection (2.4% versus 18.4%, P = 0.009) and lung metastasis (2.4% versus 13.2%, P = 0.043) were less common among patients with MDM2-amp/TP53-WT. The prevalence of MDM2-amp/TP53-WT tumors was 4.7%, 3.7%, and 8.0% in intrahepatic CCA, extrahepatic CCA, and gall-bladder cancer, respectively (P = 0.111), and the median amplified MDM2 gene copy number did not differ according to the primary site (P = 0.333) (Figure 2B). Patient characteristics were well balanced after PS matching with a 1 : 4 ratio, resulting in ASD <0.1 for most variables (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2025.105540).
Table 1.
Baseline patient and tumor characteristics in the overall population
| Characteristic | MDM2-amp/TP53-WT (n = 41) | Others (n = 772) | P |
|---|---|---|---|
| Age, years | 67.0 (40.0-83.0) | 64.0 (28.0-84.0) | 0.164 |
| Sex | 0.886 | ||
| Female | 16 (39.0) | 310 (40.2) | |
| Male | 25 (61.0) | 462 (59.8) | |
| ECOG performance status | 0.567 | ||
| 0 | 7 (17.1) | 190 (24.6) | |
| 1 | 17 (41.5) | 310 (40.2) | |
| 2 | 0 (0.0) | 9 (1.2) | |
| Not available | 17 (41.5) | 263 (34.1) | |
| Virology status | |||
| Any viral hepatitis B | 1 (2.4) | 142 (18.4) | 0.009 |
| Any viral hepatitis C | 1 (2.4) | 4 (0.5) | 0.228 |
| Level of carbohydrate antigen 19-9, proportion | 0.453 | ||
| Normal | 16 (39.0) | 263 (34.1) | |
| Elevated | 20 (48.8) | 446 (57.8) | |
| Not available | 5 (12.2) | 63 (8.2) | |
| Level of carbohydrate antigen 19-9, valuea | 1265.0 [141.5-2392.5] | 344.0 [100.3-1702.0] | 0.097 |
| Primary tumor location | 0.111 | ||
| Intrahepatic | 16 (39.0) | 325 (42.1) | |
| Extrahepatic | 11 (26.8) | 286 (37.0) | |
| Gall-bladder | 14 (34.1) | 161 (20.9) | |
| Tumor histological type | 0.518 | ||
| Adenocarcinoma | 40 (97.6) | 759 (98.3) | |
| Adenosquamous carcinoma | 1 (2.4) | 13 (1.7) | |
| Degree of differentiation | 0.513 | ||
| Well | 5 (12.2) | 60 (7.8) | |
| Moderately | 27 (65.9) | 467 (60.5) | |
| Poorly or undifferentiated | 7 (17.1) | 194 (25.1) | |
| Not available | 2 (4.9) | 51 (6.6) | |
| Disease status | 0.973 | ||
| Locally advanced | 6 (14.6) | 105 (13.6) | |
| Initially metastatic | 19 (46.3) | 354 (45.9) | |
| Recurrent after curative-intent surgery | 16 (39.0) | 313 (40.5) | |
| Sites of metastasis at diagnosis or first site of recurrence | |||
| Distant lymph node | 17 (41.5) | 309 (40.0) | 0.855 |
| Liver | 16 (39.0) | 322 (41.7) | 0.734 |
| Lung | 1 (2.4) | 102 (13.2) | 0.043 |
| Peritoneum | 12 (29.3) | 184 (23.8) | 0.428 |
| Bone | 1 (2.4) | 42 (5.4) | 0.718 |
| MSI status | 0.220 | ||
| MSS | 39 (95.1) | 751 (97.3) | |
| MSI-H | 1 (2.4) | 15 (1.9) | |
| Not available | 1 (2.4) | 6 (0.8) | |
| Tumor mutational burden | 12.5 [10.2-17.2] | 14.1 [10.9-18.8] | 0.112 |
| First-line chemotherapy regimen | 0.358 | ||
| GemCis with ICIs | 1 (2.4) | 61 (7.9) | |
| GemCis with or without non-ICIs | 40 (97.6) | 711 (92.1) | |
| Second-line therapy regimen | 0.084 | ||
| No | 8 (19.5) | 225 (29.1) | |
| 5-FU-based chemotherapy | 11 (26.8) | 223 (28.9) | |
| ICI monotherapy | 12 (29.3) | 134 (17.4) | |
| Others | 10 (24.4) | 190 (24.6) | |
| Number of palliative chemotherapy lines | 2.0 [2.0-3.0] | 2.0 [1.0-3.0] | 0.239 |
Data are presented as medians (ranges), medians [interquartile ranges], or n (%). P value based on Pearson’s chi-square test, Fisher’s exact test, or Wilcoxon rank sum test as appropriate.
5-FU, 5-fluorouracil; amp, amplification; ECOG, Eastern Cooperative Oncology Group; GemCis, gemcitabine plus cisplatin; ICI, immune checkpoint inhibitor; MSI, microsatellite instability; MSI-H, microsatellite instability-high; MSS, microsatellite stable; WT, wild-type.
Value of patients with elevated carbohydrate antigen 19-9.
Genomic landscape based on tissue-based targeted NGS
The genomic landscape of the overall study population is shown in Figure 2C. The most frequently altered gene was TP53 (n = 449, 55.2%), followed by CDKN2A (n = 261, 32.1%) and KRAS (n = 253, 31.1%). In the overall cohort, the most frequent actionable alteration was MTAP deletion (n = 112, 13.8%), followed by ERBB2 amplification (n = 83, 10.2%) and IDH1 mutation (n = 54, 6.6%) (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2025.105540).
Alterations in the ATM (29.3% versus 6.0%), APC (19.5% versus 7.1%), and CDK4 genes (17.1% versus 2.2%) were more common in the MDM2-amp/TP53-WT group than in other groups (Figure 2D). Actionable alterations with approved therapies, including ERBB2 amplification (2.4% versus 10.6%) and IDH1 mutation (2.4% versus 6.9%), were less common in the MDM2-amp/TP53-WT group; however, MTAP deletion and BRCA2 mutation were more common in the MDM2-amp/TP53-WT group (Figure 2E).
Among oncogenic signaling pathways excluding the TP53 pathway, alterations in the PI3K pathway were significantly more common among patients with MDM2-amp/TP53-WT (65.9% versus 49.2%), and no significant differences were observed in other pathways (Figure 2F). The frequency of gene alterations in DDR pathways was not different among the groups (Figure 2G).
Clinical outcomes of GemCis-containing therapy according to the presence of MDM2-amp/TP53-WT in the overall population
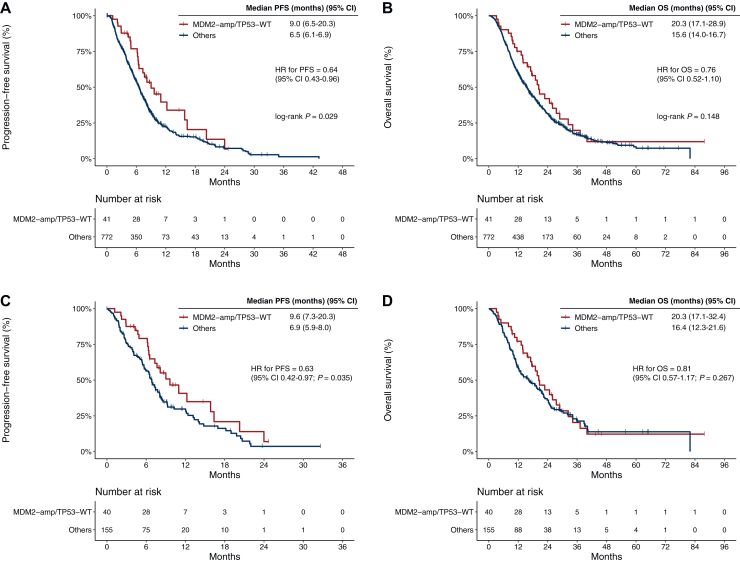
The median follow-up duration was 33.3 months [95% confidence interval (CI) 28.8-43.5 months] in the overall study population. Before matching, when compared with patients without MDM2-amp/TP53-WT, patients with MDM2-amp/TP53-WT showed significantly longer PFS [median 9.0 versus 6.5 months, log-rank P = 0.029; hazard ratio (HR) 0.64, 95% CI 0.43-0.96] and exhibited a trend toward longer OS (median 20.3 versus 15.6 months, log-rank P = 0.148; HR 0.76, 95% CI 0.52-1.10) (Figure 3A and B).
Figure 3.
Kaplan-Meier estimates of progression-free survival (PFS) and overall survival (OS) following first-line GemCis-containing chemotherapy. (A, B) PFS and OS in the overall population. (C, D) PFS and OS in the propensity score (PS)-matched population. MDM2-amp/TP53-WT versus others. amp, amplification; CI, confidence interval; HR, hazard ratio; WT, wild-type.
In multivariate Cox regression analysis for PFS and OS, MDM2-amp/TP53-WT remained significantly associated with favorable PFS (HR 0.63, 95% CI 0.42-0.94, P = 0.025) after adjusting for clinical variables. For OS, MDM2-amp/TP53-WT status showed a trend toward favorable survival; however, the result was not statistically significant (HR 0.70, 95% CI 0.48-1.02, P = 0.065) (Table 2). Among 749 patients with measurable disease, ORR and DCR did not significantly differ between the two groups (ORR 22.0% versus 19.6%, P = 0.707; DCR 85.4% versus 73.4%, P = 0.090) (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2025.105540).
Table 2.
Prognostic factor analysis for progression-free survival (PFS) and overall survival (OS) in the overall population
| Variable | Category | Univariate Cox regression for PFS |
Multivariate Cox regression for PFS |
Univariate Cox regression for OS |
Multivariate Cox regression for OS |
||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | ||
| Age | 1.00 (0.99-1.01) | 0.756 | 1.01 (1.00-1.02) | 0.007 | 1.01 (1.00-1.02) | 0.003 | |||
| Sex | Female | Reference | Reference | Reference | |||||
| Male | 0.86 (0.73-1.02) | 0.086 | 0.95 (0.80-1.13) | 0.561 | 0.99 (0.84-1.17) | 0.939 | |||
| ECOG performance status | 0 | Reference | Reference | Reference | |||||
| 1 | 1.12 (0.94-1.33) | 0.198 | 1.19 (1.01-1.40) | 0.038 | 1.06 (0.90-1.25) | 0.503 | |||
| Any viral hepatitis B | No | Reference | Reference | ||||||
| Yes | 0.88 (0.71-1.10) | 0.269 | 0.90 (0.73-1.11) | 0.336 | |||||
| Any viral hepatitis C | No | Reference | Reference | ||||||
| Yes | 1.01 (0.38-2.71) | 0.980 | 1.23 (0.46-3.30) | 0.677 | |||||
| Level of carbohydrate antigen 19-9 | Normal | Reference | Reference | Reference | Reference | ||||
| Elevated | 1.46 (1.22-1.75) | <0.001 | 1.50 (1.25-1.80) | <0.001 | 1.71 (1.43-2.03) | <0.001 | 1.66 (1.39-1.98) | <0.001 | |
| Primary tumor location | Intrahepatic | Reference | Reference | Reference | |||||
| Extrahepatic | 1.01 (0.84-1.23) | 0.889 | 1.08 (0.88-1.33) | 0.469 | 1.13 (0.94-1.35) | 0.197 | |||
| Gall-bladder | 1.35 (1.09-1.67) | 0.006 | 1.41 (1.14-1.76) | 0.002 | 1.02 (0.83-1.26) | 0.839 | |||
| Tumor histological type | Adenocarcinoma | Reference | Reference | Reference | Reference | ||||
| Adenosquamous carcinoma | 2.34 (1.32-4.15) | 0.004 | 2.08 (1.15-3.77) | 0.016 | 2.03 (1.12-3.68) | 0.020 | 2.54 (1.37-4.70) | 0.003 | |
| Degree of differentiation | Well | Reference | Reference | Reference | Reference | ||||
| Moderately | 1.52 (1.10-2.09) | 0.011 | 1.48 (1.06-2.05) | 0.020 | 1.17 (0.87-1.58) | 0.289 | 1.06 (0.78-1.43) | 0.716 | |
| Poorly or undifferentiated | 1.94 (1.39-2.71) | <0.001 | 1.69 (1.19-2.40) | 0.003 | 1.34 (0.98-1.83) | 0.068 | 1.10 (0.80-1.52) | 0.554 | |
| Disease status | Locally advanced | Reference | Reference | Reference | Reference | ||||
| Initially metastatic | 1.96 (1.46-2.64) | <0.001 | 1.50 (1.09-2.07) | 0.012 | 1.76 (1.36-2.28) | <0.001 | 1.60 (1.21-2.12) | 0.001 | |
| Recurrent after curative-intent surgery | 1.58 (1.17-2.14) | 0.003 | 1.48 (1.08-2.04) | 0.014 | 1.25 (0.96-1.63) | 0.099 | 1.21 (0.92-1.60) | 0.178 | |
| Liver metastasis | No | Reference | Reference | Reference | Reference | ||||
| Yes | 1.69 (1.43-2.00) | <0.001 | 1.49 (1.24-1.78) | <0.001 | 1.54 (1.31-1.81) | <0.001 | 1.35 (1.14-1.61) | <0.001 | |
| MSI status | MSS | Reference | Reference | Reference | |||||
| MSI-H | 1.62 (0.89-2.94) | 0.116 | 0.45 (0.22-0.90) | 0.024 | 0.44 (0.22-0.90) | 0.024 | |||
| First-line chemotherapy regimen | GemCis with ICIs | Reference | Reference | ||||||
| GemCis with or without non-ICIs | 1.11 (0.80-1.55) | 0.538 | 1.30 (0.93-1.82) | 0.120 | |||||
| MDM2-amp/TP53-WT status | No | Reference | Reference | Reference | Reference | ||||
| Yes | 0.64 (0.43-0.96) | 0.031 | 0.63 (0.42-0.94) | 0.025 | 0.76 (0.52-1.10) | 0.149 | 0.70 (0.48-1.02) | 0.065 | |
amp, amplification; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; GemCis, gemcitabine plus cisplatin; HR, hazard ratio; ICI, immune checkpoint inhibitor; MSI, microsatellite instability; MSI-H, microsatellite instability-high; MSS, microsatellite stable; WT, wild-type.
Clinical outcomes of first-line GemCis-containing therapy according to the presence of MDM2-amp/TP53-WT in the PS-matched population
PS-matched analyses showed consistent results with unmatched analyses. After PS matching, patients with MDM2-amp/TP53-WT showed significantly longer PFS compared with those in the matched group (median 9.6 versus 6.9 months; HR 0.63, 95% CI 0.42-0.97, P = 0.035) and a non-significant tendency toward longer OS (median 20.3 versus 16.4 months; HR 0.81, 95% CI 0.57-1.17, P = 0.267) (Figure 3C and D). Among 179 patients with measurable disease in the PS-matched cohort, ORR and DCR did not significantly differ between the two groups (ORR 22.5% versus 23.2%, P = 0.943; DCR 85.0% versus 74.8%, P = 0.056) (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2025.105540).
Clinical outcomes of second-line therapy according to the presence of MDM2-amp/TP53-WT in the overall population
In the overall population, 588 patients (72.3%) received second-line therapy, including 33 patients (5.6%) with MDM2-amp/TP53-WT. Among patients who received second-line 5-fluorouracil-based therapy (n = 234, 28.8%), PFS did not differ according to the presence of MDM2-amp/TP53-WT (median 4.1 versus 2.1 months, log-rank P = 0.227) (Supplementary Figure S1A, available at https://doi.org/10.1016/j.esmoop.2025.105540). Among patients who received second-line ICI monotherapy (n = 146, 18.0%), there was no statistically significant difference in PFS between the two groups (median 2.9 versus 2.0 months, log-rank P = 0.568) (Supplementary Figure S1B, available at https://doi.org/10.1016/j.esmoop.2025.105540).
Discussion
Although novel agents targeting MDM2 amplification are currently under development,15 the genomic and clinical characteristics of BTC with MDM2-amp/TP53-WT have been poorly defined. In this study, we aimed to elucidate the genomic and clinical characteristics of patients with advanced BTC harboring MDM2-amp/TP53-WT. In our large-sized cohort of patients with advanced BTC homogeneously treated with first-line GemCis, we observed that patients with MDM2-amp/TP53-WT, comprising ∼5% of the cohort, demonstrated distinct molecular features and favorable clinical outcomes. The favorable survival outcomes associated with MDM2-amp/TP53-WT status were consistently observed in PS-matched analyses and multivariate survival analyses after adjusting for clinical characteristics.
In our cohort, MDM2 amplification was observed in 7.6% of patients with advanced BTC, with MDM2-amp/TP53-WT accounting for 5.0% of the patients. The prevalence was consistent with the results of previous studies on advanced BTC (4%-8%).10,24 Neither the prevalence of MDM2-amp/TP53-WT status nor the copy number of MDM2 amplification differed significantly according to the primary tumor site.25 Furthermore, baseline patient characteristics were similar regardless of MDM2-amp/TP53-WT status, consistent with previous reports.26
In the current study, MDM2-amp/TP53-WT status was associated with longer PFS following first-line GemCis-based chemotherapy, with a median PFS of 9.0 months compared with 6.5 months for patients without MDM2-amp/TP53-WT. Favorable PFS was consistently observed in PS-matched analyses, which further balanced clinical characteristics between the two groups. Moreover, in multivariate Cox regression analyses adjusted for clinically relevant prognostic variables, MDM2-amp/TP53-WT status remained significantly associated with improved PFS. Although previous studies have reported that MDM2 amplification may be associated with chemotherapy resistance and poor prognosis, the literature regarding survival implications in BTC remains limited and inconsistent.24,26, 27, 28 Our findings, which were based on a homogeneous patient cohort with detailed clinical annotation and robust statistical analyses, suggest that MDM2-amp/TP53-WT may represent a favorable prognostic subset in advanced BTC.
In terms of genomic alterations, patients with MDM2-amp/TP53-WT tumors exhibited more frequent ATM alterations, a finding consistent with observations in a large cohort of patients with gastrointestinal cancer.29 ATM is known to indirectly phosphorylate MDM2 in response to DNA damage and promote p53 accumulation.30 We also observed that CDK4, located near MDM2 on chromosome 12q13-15, was more frequently altered in MDM2-amp/TP53-WT tumors.31 These two genes function synergistically to promote tumorigenesis by inactivating the Rb and p53 pathways, respectively,32 and they often are co-amplified in several malignancies, including BTC, liposarcoma, and neuroblastoma.33, 34, 35 These findings suggest the potential of dual MDM2 and CDK4 inhibition as a therapeutic strategy in BTC, a concept currently being investigated in early-phase clinical trials for liposarcoma (NCT04116541). In addition, APC alterations were more common in the MDM2-amp/TP53-WT group. Preclinical studies have demonstrated that the RP-MDM2-p53 pathway plays a tumor-suppressive role in colorectal carcinogenesis following APC loss.36 However, the clinical significance of co-occurring alterations in these genes remains unknown and warrants further studies.
To better characterize the molecular features of MDM2-amp/TP53-WT tumors, we further investigated alterations at the pathway level as well as druggable targets in BTC. Among oncogenic signaling pathways, the PI3K pathway was more frequently altered in MDM2-amp/TP53-WT tumors. AKT-mediated phosphorylation of MDM2 has been reported to lead to p53 suppression and tumorigenesis,37 and a preclinical study has suggested that combining PI3K/AKT/mTOR inhibitors with MDM2 inhibitors may have synergistic effects.38 However, alterations in DDR pathways were not significantly different between groups. The prevalence of alterations in genes associated with HR deficiency was similar, suggesting that differences in sensitivity to cisplatin are unlikely to explain the improved PFS with GemCis in MDM2-amp/TP53-WT tumors.39 Additionally, the prevalence of actionable alterations with currently approved therapies, including ERBB2 amplification or IDH1 mutation (2.4% each), FGFR2 gene fusion, and KRAS G12C or BRAF V600E mutations (0%), was especially low in patients with MDM2-amp/TP53-WT. This finding further supports the hypothesis of oncogenic dependency on the MDM2–p53 axis in this subset and underscores the potential need for MDM2-targeted therapeutic strategies.
A limitation of this study is its retrospective design in a single-center setting. However, the analysis was conducted in a large, homogeneous cohort of patients with advanced BTC, which could enhance the robustness of the findings. Furthermore, through PS matching, we aimed to reduce potential confounding factors in comparing the clinical outcomes between patients with and without MDM2-amp/TP53-WT. In addition, the small number of MDM2-amp/TP53-WT patients who received second-line therapy limits the statistical power of the analysis and warrants caution in interpreting the observed lack of association between MDM2-amp/TP53-WT status and PFS in the second-line setting. Moreover, we used a copy number of five as the cut-off for MDM2-amp, based on the general reporting criteria of the targeted sequencing panel used; however, the available data remain insufficient to draw definitive conclusions regarding the optimal cut-off value for MDM2-amp. Lastly, our findings may be limited in their applicability to the current standard of care (i.e. combination of ICIs with GemCis) as only a small number of patients in this study received GemCis with ICIs. Nevertheless, our study uniquely provides detailed clinical annotation in association with the genomic landscape of MDM2-amp/TP53-WT BTC, a subset for which published data are scarce. Therefore, our data may serve as a useful reference for future investigation of the therapeutic efficacy of MDM2 inhibitors in this patient population.
In conclusion, our study demonstrates that in advanced BTCs, tumors harboring MDM2-amp/TP53-WT are associated with better survival outcomes following first-line GemCis-containing chemotherapy. Our findings suggest that MDM2-amp/TP53-WT may be a distinct subgroup of BTC with limited other actionable alterations, warranting further investigation of MDM2 inhibitors for this subgroup.
Funding
This work was supported by Boehringer Ingelheim (no grant number).
Disclosure
CY received honoraria from Servier, Bayer, AstraZeneca, Merck Sharp & Dohme, Eisai, Roche, Celgene, Bristol Myers Squibb, Ipsen, Novartis, Boehringer Ingelheim, Boryung, Mundipharma, Autem Therapeutics, Qurient, HLB, and Elevar Therapeutics; and received research grants from Servier, Bayer, AstraZeneca, Ono Pharmaceuticals, Ipsen, Boryung, CKD Pharm, Lunit Inc., and Boehringer Ingelheim. All other authors have declared no conflicts of interest.
Data sharing
The data collected for this study cannot be made available to the public based on the Personal Information Protection Act in Korea. All requests for raw and analyzed data and materials should be directed to contact the corresponding authors (CY) and will be responded within 4 weeks. The requests will be promptly reviewed by the IRB of Asan Medical Center to determine whether it is subject to any intellectual property or confidentiality obligations.
Supplementary data
Kaplan-Meier estimates of progression-free survival (PFS). (A) PFS for patients treated with second-line 5-FU-based chemotherapy. (B) PFS for patients treated with second-line immune-checkpoint-inhibitor monotherapy. MDM2-amp/TP53-WT versus others. amp, amplification; CI, confidence interval; HR, hazard ratio; WT, wild-type.
References
- 1.Valle J.W., Kelley R.K., Nervi B., Oh D.-Y., Zhu A.X. Biliary tract cancer. Lancet. 2021;397(10272):428–444. doi: 10.1016/S0140-6736(21)00153-7. [DOI] [PubMed] [Google Scholar]
- 2.Banales J.M., Marin J.J.G., Lamarca A., et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17(9):557–588. doi: 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh D.-Y., Ruth He A., Qin S., et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022;1(8) doi: 10.1056/EVIDoa2200015. [DOI] [PubMed] [Google Scholar]
- 4.Kelley R.K., Ueno M., Yoo C., et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401(10391):1853–1865. doi: 10.1016/S0140-6736(23)00727-4. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura H., Arai Y., Totoki Y., et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47(9):1003–1010. doi: 10.1038/ng.3375. [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarti S., Kamgar M., Mahipal A. Targeted therapies in advanced biliary tract cancer: an evolving paradigm. Cancers (Basel) 2020;12(8):2039. doi: 10.3390/cancers12082039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abou-Alfa G.K., Macarulla T., Javle M.M., et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(6):796–807. doi: 10.1016/S1470-2045(20)30157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abou-Alfa G.K., Sahai V., Hollebecque A., et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21(5):671–684. doi: 10.1016/S1470-2045(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harding J.J., Fan J., Oh D.-Y., et al. Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer (HERIZON-BTC-01): a multicentre, single-arm, phase 2b study. Lancet Oncol. 2023;24(7):772–782. doi: 10.1016/S1470-2045(23)00242-5. [DOI] [PubMed] [Google Scholar]
- 10.Bouattour M., Valle J., Vogel A., et al. Characterization of long-term survivors in the TOPAZ-1 study of durvalumab or placebo plus gemcitabine and cisplatin in advanced biliary tract cancer. J Clin Oncol. 2023;41:531. [Google Scholar]
- 11.Oliner J.D., Pietenpol J.A., Thiagalingam S., Gyuris J., Kinzler K.W., Vogelstein B. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993;362(6423):857–860. doi: 10.1038/362857a0. [DOI] [PubMed] [Google Scholar]
- 12.Aubrey B.J., Kelly G.L., Janic A., Herold M.J., Strasser A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018;25(1):104–113. doi: 10.1038/cdd.2017.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W., Albadari N., Du Y., et al. MDM2 inhibitors for cancer therapy: the past, present, and future. Pharmacol Rev. 2024;76(3):414–453. doi: 10.1124/pharmrev.123.001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wade M., Li Y.C., Wahl G.M. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat Rev Cancer. 2013;13(2):83–96. doi: 10.1038/nrc3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoo C., Lamarca A., Choi H.J., et al. Brightline-2: a phase IIa/IIb trial of brigimadlin (BI 907828) in advanced biliary tract cancer, pancreatic ductal adenocarcinoma or other solid tumors. Future Oncol. 2024;20(16):1069–1077. doi: 10.2217/fon-2023-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayakonda A., Lin D.C., Assenov Y., Plass C., Koeffler H.P. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 2018;28(11):1747–1756. doi: 10.1101/gr.239244.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakravarty D., Gao J., Phillips S.M., et al. OncoKB: a precision oncology knowledge base. JCO Precis Oncol. 2017 doi: 10.1200/PO.17.00011. PO.17.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogel A., Ducreux M. ESMO Clinical Practice Guideline interim update on the management of biliary tract cancer. ESMO Open. 2025;10(1) doi: 10.1016/j.esmoop.2024.104003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engstrom L.D., Aranda R., Waters L., et al. MRTX1719 is an MTA-cooperative PRMT5 inhibitor that exhibits synthetic lethality in preclinical models and patients with MTAP-deleted cancer. Cancer Discov. 2023;13(11):2412–2431. doi: 10.1158/2159-8290.CD-23-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez-Vega F., Mina M., Armenia J., et al. Oncogenic signaling pathways in the cancer genome atlas. Cell. 2018;173(2):321–337.e10. doi: 10.1016/j.cell.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin N., Wang Z., Liu Q., et al. Pathogenic germline mutations in DNA repair genes in combination with cancer treatment exposures and risk of subsequent neoplasms among long-term survivors of childhood cancer. J Clin Oncol. 2020;38(24):2728–2740. doi: 10.1200/JCO.19.02760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X., Heyer W.-D. Homologous recombination in DNA repair and DNA damage tolerance. Cell Res. 2008;18(1):99–113. doi: 10.1038/cr.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raghunathan T. IVEware: Imputation and Variance Estimation Software. https://src.isr.umich.edu/iveware/ Available at.
- 24.Iyer R., Evans K., Klein A.B., et al. Real-world assessment of MDM2 amplification and survival among patients with advanced or metastatic biliary tract cancer in the United States. J Clin Oncol. 2025;43(suppl 4):544. [Google Scholar]
- 25.Kratz J.D., Klein A.B., Gray C.B., et al. The epidemiology of biliary tract cancer and associated prevalence of MDM2 amplification: a targeted literature review. Target Oncol. 2024;19(6):833–844. doi: 10.1007/s11523-024-01086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim S.J., Akita M., Sung Y.N., et al. MDM2 amplification in intrahepatic cholangiocarcinomas: its relationship with large-duct type morphology and uncommon KRAS mutations. Am J Surg Pathol. 2018;42(4):512–521. doi: 10.1097/PAS.0000000000001006. [DOI] [PubMed] [Google Scholar]
- 27.Hou H., Sun D., Zhang X. The role of MDM2 amplification and overexpression in therapeutic resistance of malignant tumors. Cancer Cell Int. 2019;19(1):216. doi: 10.1186/s12935-019-0937-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dworakowska D., Jassem E., Jassem J., et al. MDM2 gene amplification: a new independent factor of adverse prognosis in non-small cell lung cancer (NSCLC) Lung Cancer. 2004;43(3):285–295. doi: 10.1016/j.lungcan.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Battaglin F., Xiu J., Baca Y., et al. 1952P Comprehensive profiling of MDM2 amplified gastrointestinal (GI) cancers. Ann Oncol. 2020;31 [Google Scholar]
- 30.Maya R., Balass M., Kim S.T., et al. ATM-dependent phosphorylation of Mdm2 on serine 395: role in p53 activation by DNA damage. Genes Dev. 2001;15(9):1067–1077. doi: 10.1101/gad.886901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pilotti S., Della Torre G., Lavarino C., et al. Molecular abnormalities in liposarcoma: role of MDM2 and CDK4-containing amplicons at 12q13-22. J Pathol. 1998;185(2):188–190. doi: 10.1002/(SICI)1096-9896(199806)185:2<188::AID-PATH53>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 32.Sherr C.J., McCormick F. The RB and p53 pathways in cancer. Cancer Cell. 2002;2(2):103–112. doi: 10.1016/s1535-6108(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 33.Italiano A., Bianchini L., Gjernes E., et al. Clinical and biological significance of CDK4 amplification in well-differentiated and dedifferentiated liposarcomas. Clin Cancer Res. 2009;15(18):5696–5703. doi: 10.1158/1078-0432.CCR-08-3185. [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Monleon A., Kryh Öberg H., Gaarder J., et al. Amplification of CDK4 and MDM2: a detailed study of a high-risk neuroblastoma subgroup. Sci Rep. 2022;12(1) doi: 10.1038/s41598-022-16455-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kendre G., Murugesan K., Brummer T., Segatto O., Saborowski A., Vogel A. Charting co-mutation patterns associated with actionable drivers in intrahepatic cholangiocarcinoma. J Hepatol. 2023;78(3):614–626. doi: 10.1016/j.jhep.2022.11.030. [DOI] [PubMed] [Google Scholar]
- 36.Liu S., Tackmann N.R., Yang J., Zhang Y. Disruption of the RP-MDM2-p53 pathway accelerates APC loss-induced colorectal tumorigenesis. Oncogene. 2017;36(10):1374–1383. doi: 10.1038/onc.2016.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayo L.D., Donner D.B. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci U S A. 2001;98(20):11598–11603. doi: 10.1073/pnas.181181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laroche A., Chaire V., Algeo M.P., Karanian M., Fourneaux B., Italiano A. MDM2 antagonists synergize with PI3K/mTOR inhibition in well-differentiated/dedifferentiated liposarcomas. Oncotarget. 2017;8(33):53968–53977. doi: 10.18632/oncotarget.16345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chae H., Kim D., Yoo C., et al. Therapeutic relevance of targeted sequencing in management of patients with advanced biliary tract cancer: DNA damage repair gene mutations as a predictive biomarker. Eur J Cancer. 2019;120:31–39. doi: 10.1016/j.ejca.2019.07.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kaplan-Meier estimates of progression-free survival (PFS). (A) PFS for patients treated with second-line 5-FU-based chemotherapy. (B) PFS for patients treated with second-line immune-checkpoint-inhibitor monotherapy. MDM2-amp/TP53-WT versus others. amp, amplification; CI, confidence interval; HR, hazard ratio; WT, wild-type.