Abstract
Maps of fluorescing red cell membrane components on a pipette-aspirated projection are quantitated in an effort to elucidate and unify the heterogeneous kinematics of deformation. Transient gradients of diffusing fluorescent lipid first demonstrate the fluidity of an otherwise uniform-density bilayer and corroborate a "universal" calibration scale for relative surface density. A steep but smooth and stable gradient in the densities of the skeleton components spectrin, actin, and protein 4.1 is used to estimate large elastic strains along the aspirated skeleton. The deformation fields are argued to be an unhindered response to loading in the surface normal direction. Density maps intermediate to those of the compressible skeleton and fluid bilayer are exhibited by particular transmembrane proteins (e.g., Band 3) and yield estimates for the skeleton-connected fractions. Such connected proteins appear to occupy a significant proportion of the undeformed membrane surface and can lead to steric exclusion of unconnected integral membrane proteins from regions of network condensation. Consistent with membrane repatterning kinematics in reversible deformation, final vesiculation of the projection tip produces a cell fragment concentrated in freely diffusing proteins but depleted of skeleton.
Full text
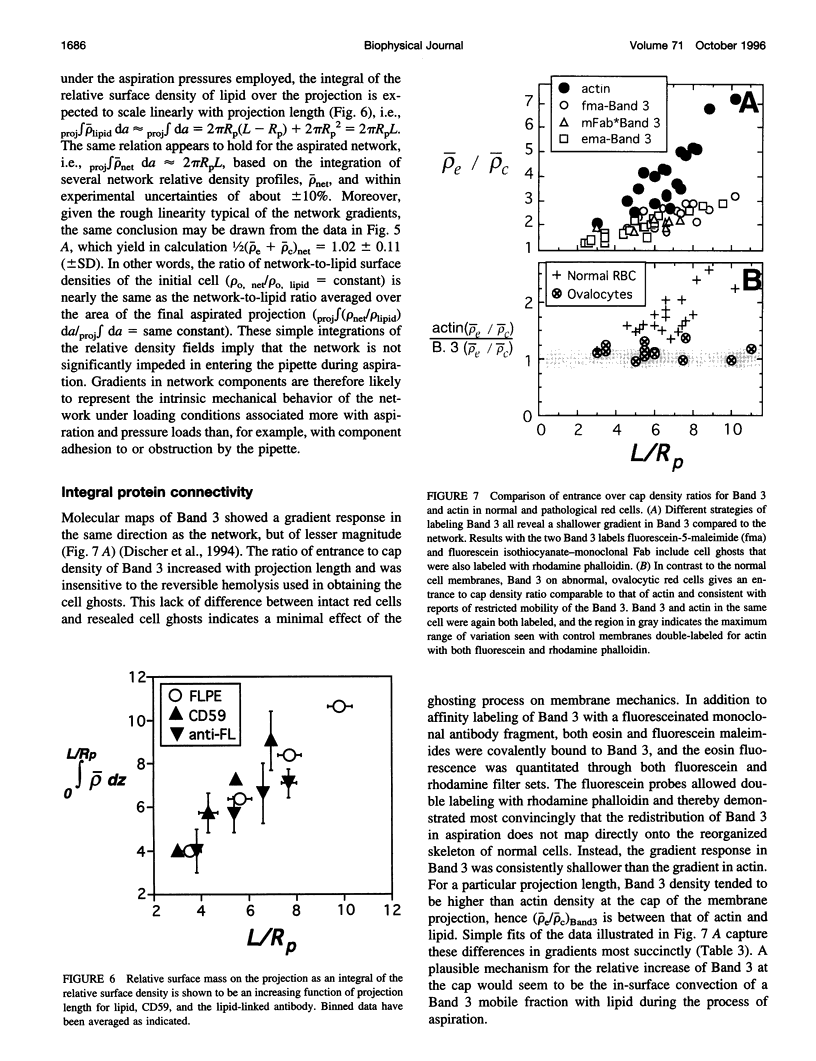
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk D. A., Clark A., Jr, Hochmuth R. M. Analysis of lateral diffusion from a spherical cell surface to a tubular projection. Biophys J. 1992 Jan;61(1):1–8. doi: 10.1016/S0006-3495(92)81810-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers T. J., Branton D. Visualization of the protein associations in the erythrocyte membrane skeleton. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6153–6157. doi: 10.1073/pnas.82.18.6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher D. E., Mohandas N., Evans E. A. Molecular maps of red cell deformation: hidden elasticity and in situ connectivity. Science. 1994 Nov 11;266(5187):1032–1035. doi: 10.1126/science.7973655. [DOI] [PubMed] [Google Scholar]
- Discher D. E., Winardi R., Schischmanoff P. O., Parra M., Conboy J. G., Mohandas N. Mechanochemistry of protein 4.1's spectrin-actin-binding domain: ternary complex interactions, membrane binding, network integration, structural strengthening. J Cell Biol. 1995 Aug;130(4):897–907. doi: 10.1083/jcb.130.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil R., Byers T. J., Branton D., Goldstein L. S., Kiehart D. P. Drosophilia spectrin. I. Characterization of the purified protein. J Cell Biol. 1987 Nov;105(5):2095–2102. doi: 10.1083/jcb.105.5.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A., Hochmuth R. M. A solid-liquid composite model of the red cell membrane. J Membr Biol. 1977 Jan 28;30(4):351–362. doi: 10.1007/BF01869676. [DOI] [PubMed] [Google Scholar]
- Evans E. A. Structure and deformation properties of red blood cells: concepts and quantitative methods. Methods Enzymol. 1989;173:3–35. doi: 10.1016/s0076-6879(89)73003-2. [DOI] [PubMed] [Google Scholar]
- Frye L. D., Edidin M. The rapid intermixing of cell surface antigens after formation of mouse-human heterokaryons. J Cell Sci. 1970 Sep;7(2):319–335. doi: 10.1242/jcs.7.2.319. [DOI] [PubMed] [Google Scholar]
- Fung Y. C., Tong P. Theory of the sphering of red blood cells. Biophys J. 1968 Feb;8(2):175–198. doi: 10.1016/S0006-3495(68)86484-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan D. E., Alecio M. R., Veatch W. R., Rando R. R. Lateral mobility of phospholipid and cholesterol in the human erythrocyte membrane: effects of protein-lipid interactions. Biochemistry. 1984 Jan 17;23(2):332–339. doi: 10.1021/bi00297a024. [DOI] [PubMed] [Google Scholar]
- Golan D. E., Veatch W. Lateral mobility of band 3 in the human erythrocyte membrane studied by fluorescence photobleaching recovery: evidence for control by cytoskeletal interactions. Proc Natl Acad Sci U S A. 1980 May;77(5):2537–2541. doi: 10.1073/pnas.77.5.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S. R., Krebs K. E., Whitfield C. F., Riederer B. M., Zagon I. S. Spectrin and related molecules. CRC Crit Rev Biochem. 1988;23(2):171–234. doi: 10.3109/10409238809088319. [DOI] [PubMed] [Google Scholar]
- Hagelberg C., Allan D. Restricted diffusion of integral membrane proteins and polyphosphoinositides leads to their depletion in microvesicles released from human erythrocytes. Biochem J. 1990 Nov 1;271(3):831–834. doi: 10.1042/bj2710831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka Y., Sedat J. W., Agard D. A. The use of a charge-coupled device for quantitative optical microscopy of biological structures. Science. 1987 Oct 2;238(4823):36–41. doi: 10.1126/science.3116667. [DOI] [PubMed] [Google Scholar]
- Hochmuth R. M., Mohandas N. Uniaxial loading of the red-cell membrane. J Biomech. 1972 Sep;5(5):501–509. doi: 10.1016/0021-9290(72)90007-3. [DOI] [PubMed] [Google Scholar]
- Katnik C., Waugh R. Alterations of the apparent area expansivity modulus of red blood cell membrane by electric fields. Biophys J. 1990 Apr;57(4):877–882. doi: 10.1016/S0006-3495(90)82607-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov M. M., Lerche D., Meier W. RBC membrane instability for large pipette deformation. A theoretical approach. Biorheology. 1988;25(6):843–856. doi: 10.3233/bir-1988-25605. [DOI] [PubMed] [Google Scholar]
- Laurent T. C., Granath K. A. Fractionation of dextran and Ficoll by chromatography on Sephadex G-200. Biochim Biophys Acta. 1967 Mar 22;136(2):191–198. doi: 10.1016/0304-4165(67)90063-3. [DOI] [PubMed] [Google Scholar]
- Markle D. R., Evans E. A., Hochmuth R. M. Force relaxation and permanent deformation of erythrocyte membrane. Biophys J. 1983 Apr;42(1):91–98. doi: 10.1016/S0006-3495(83)84372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas N., Evans E. Mechanical properties of the red cell membrane in relation to molecular structure and genetic defects. Annu Rev Biophys Biomol Struct. 1994;23:787–818. doi: 10.1146/annurev.bb.23.060194.004035. [DOI] [PubMed] [Google Scholar]
- Mohandas N., Winardi R., Knowles D., Leung A., Parra M., George E., Conboy J., Chasis J. Molecular basis for membrane rigidity of hereditary ovalocytosis. A novel mechanism involving the cytoplasmic domain of band 3. J Clin Invest. 1992 Feb;89(2):686–692. doi: 10.1172/JCI115636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama R., Ideguchi H., Lombardo C. R., Van Dort H. M., Low P. S. Structural and functional characterization of band 3 from Southeast Asian ovalocytes. J Biol Chem. 1992 Dec 25;267(36):25792–25797. [PubMed] [Google Scholar]
- Nakashima K., Beutler E. Effect of anti-spectrin antibody and ATP on deformability of resealed erythrocyte membranes. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3823–3825. doi: 10.1073/pnas.75.8.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A., Bron C., Girardet M., Cherry R. J. Band 3-glycophorin A association in erythrocyte membrane demonstrated by combining protein diffusion measurements with antibody-induced cross-linking. Biochemistry. 1980 Apr 29;19(9):1887–1893. doi: 10.1021/bi00550a024. [DOI] [PubMed] [Google Scholar]
- Noda M., Yoon K., Rodan G. A., Koppel D. E. High lateral mobility of endogenous and transfected alkaline phosphatase: a phosphatidylinositol-anchored membrane protein. J Cell Biol. 1987 Oct;105(4):1671–1677. doi: 10.1083/jcb.105.4.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan T. A., Myers J., Holowka D., Baird B., Webb W. W. Molecular crowding on the cell surface. Science. 1988 Jan 1;239(4835):61–64. doi: 10.1126/science.2962287. [DOI] [PubMed] [Google Scholar]
- Schmidt C. F., Svoboda K., Lei N., Petsche I. B., Berman L. E., Safinya C. R., Grest G. S. Existence of a flat phase in red cell membrane skeletons. Science. 1993 Feb 12;259(5097):952–955. doi: 10.1126/science.8438153. [DOI] [PubMed] [Google Scholar]
- Stokke B. T., Mikkelsen A., Elgsaeter A. The human erythrocyte membrane skeleton may be an ionic gel. I. Membrane mechanochemical properties. Eur Biophys J. 1986;13(4):203–218. doi: 10.1007/BF00260368. [DOI] [PubMed] [Google Scholar]
- Takakuwa Y., Tchernia G., Rossi M., Benabadji M., Mohandas N. Restoration of normal membrane stability to unstable protein 4.1-deficient erythrocyte membranes by incorporation of purified protein 4.1. J Clin Invest. 1986 Jul;78(1):80–85. doi: 10.1172/JCI112577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Test S. T., Woolworth V. S. Defective regulation of complement by the sickle erythrocyte: evidence for a defect in control of membrane attack complex formation. Blood. 1994 Feb 1;83(3):842–852. [PubMed] [Google Scholar]
- Tsuji A., Kawasaki K., Ohnishi S., Merkle H., Kusumi A. Regulation of band 3 mobilities in erythrocyte ghost membranes by protein association and cytoskeletal meshwork. Biochemistry. 1988 Sep 20;27(19):7447–7452. doi: 10.1021/bi00419a041. [DOI] [PubMed] [Google Scholar]
- Tsuji A., Ohnishi S. Restriction of the lateral motion of band 3 in the erythrocyte membrane by the cytoskeletal network: dependence on spectrin association state. Biochemistry. 1986 Oct 7;25(20):6133–6139. doi: 10.1021/bi00368a045. [DOI] [PubMed] [Google Scholar]
- Wang D. N., Sarabia V. E., Reithmeier R. A., Kühlbrandt W. Three-dimensional map of the dimeric membrane domain of the human erythrocyte anion exchanger, Band 3. EMBO J. 1994 Jul 15;13(14):3230–3235. doi: 10.1002/j.1460-2075.1994.tb06624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh R. E. Effects of abnormal cytoskeletal structure on erythrocyte membrane mechanical properties. Cell Motil. 1983;3(5-6):609–622. doi: 10.1002/cm.970030526. [DOI] [PubMed] [Google Scholar]
- Zhang F., Crise B., Su B., Hou Y., Rose J. K., Bothwell A., Jacobson K. Lateral diffusion of membrane-spanning and glycosylphosphatidylinositol-linked proteins: toward establishing rules governing the lateral mobility of membrane proteins. J Cell Biol. 1991 Oct;115(1):75–84. doi: 10.1083/jcb.115.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]











