Abstract
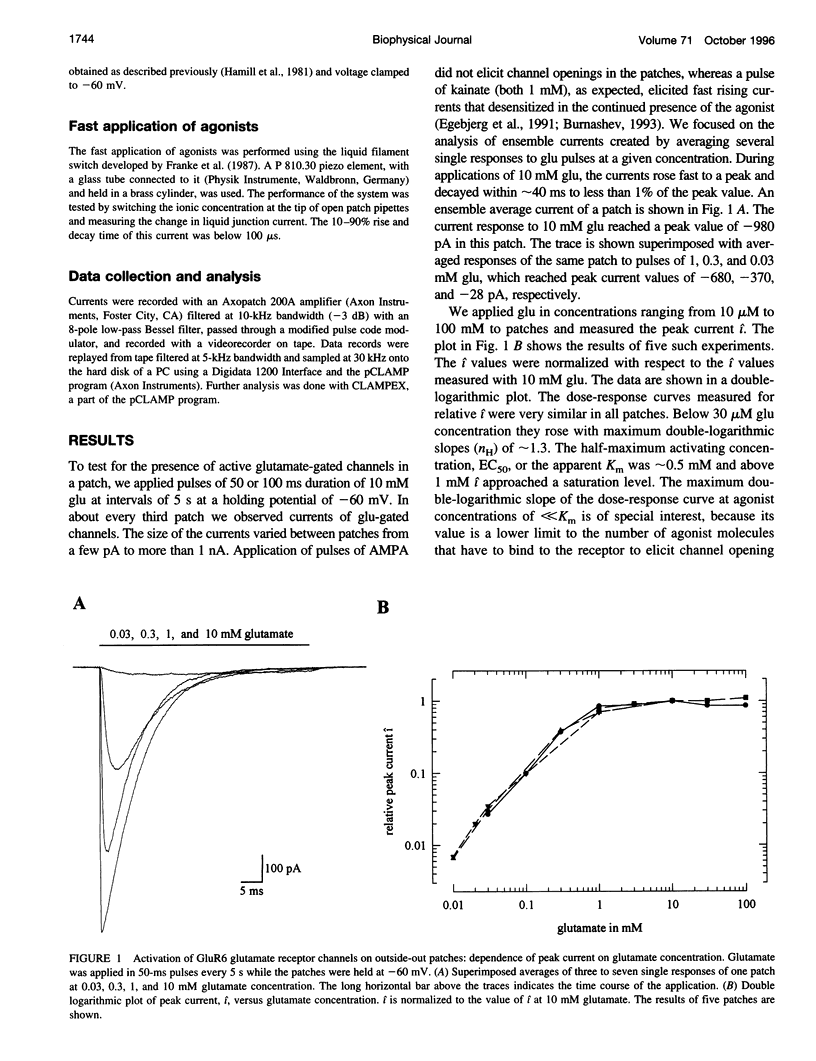
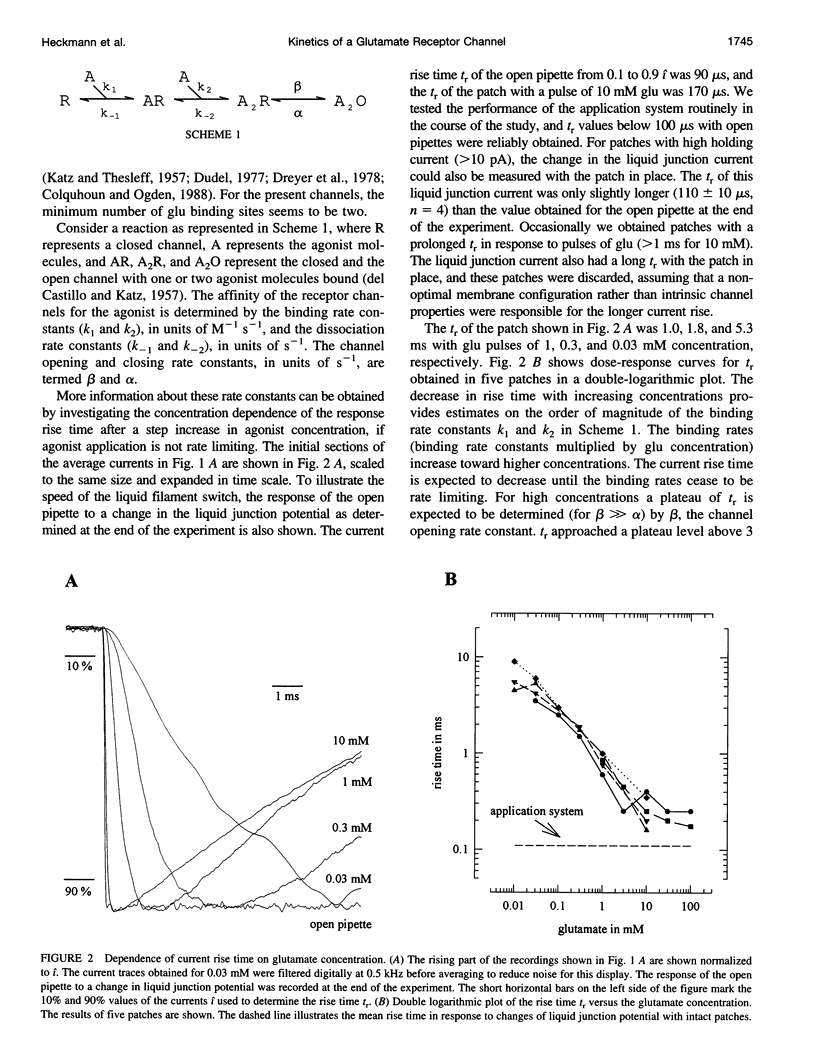
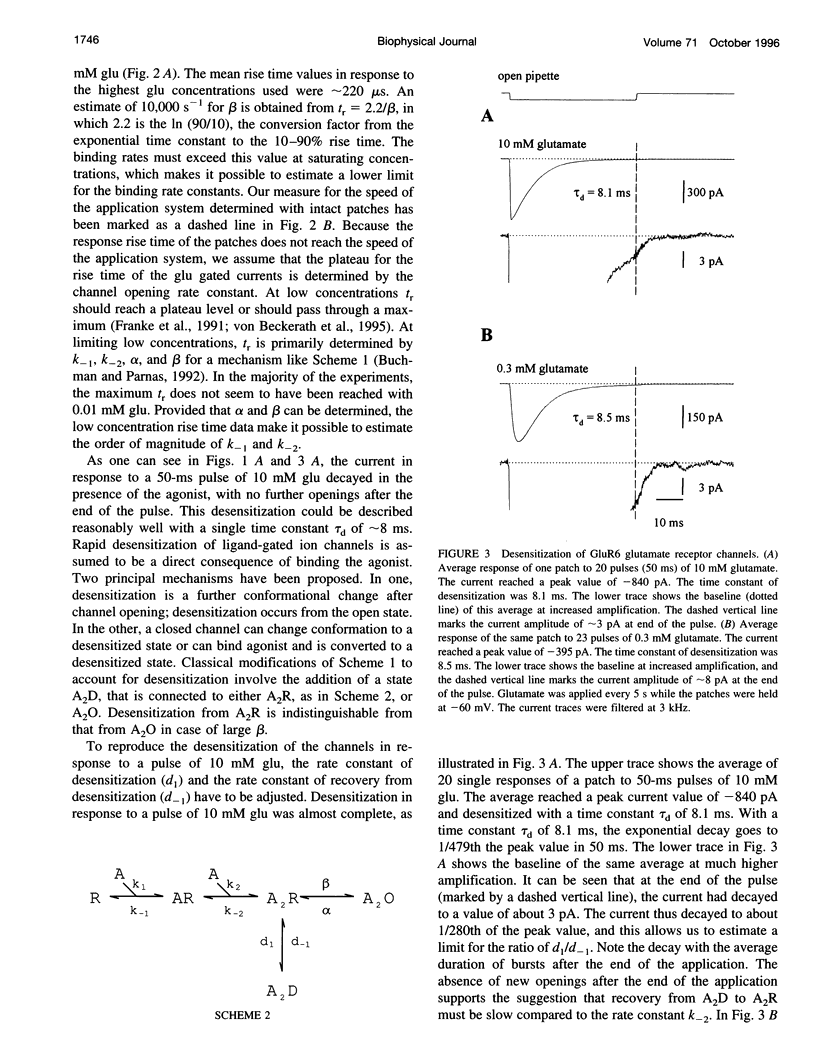
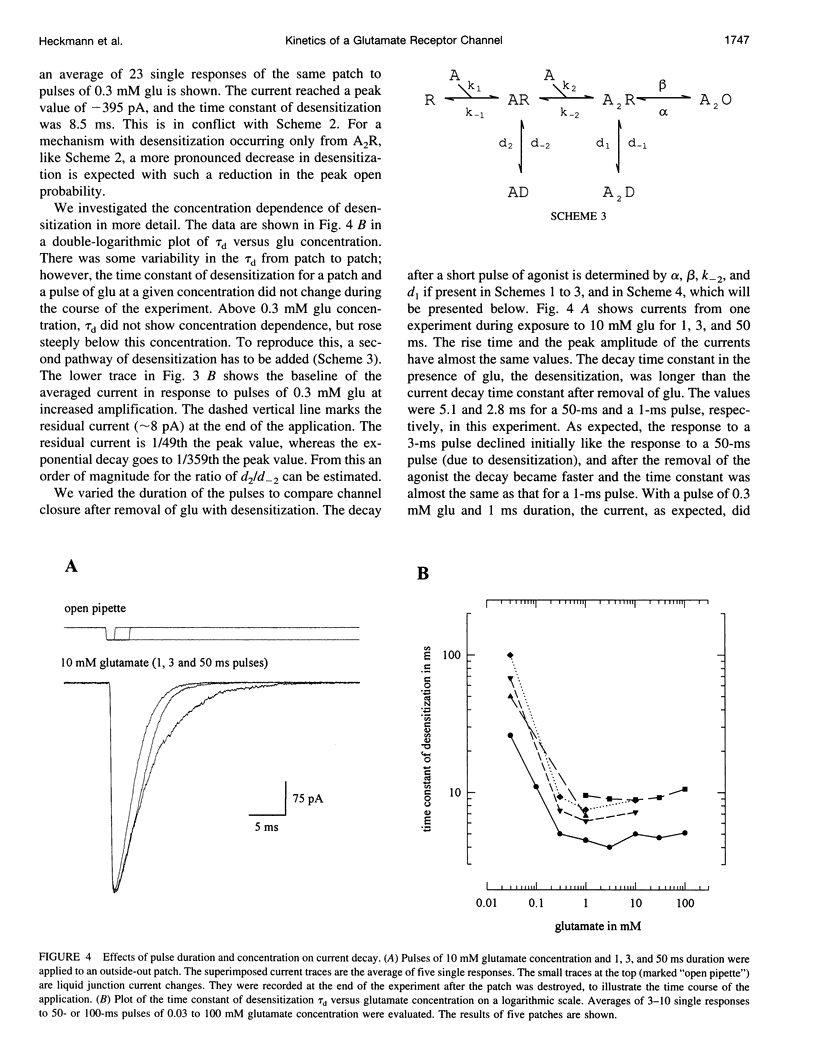
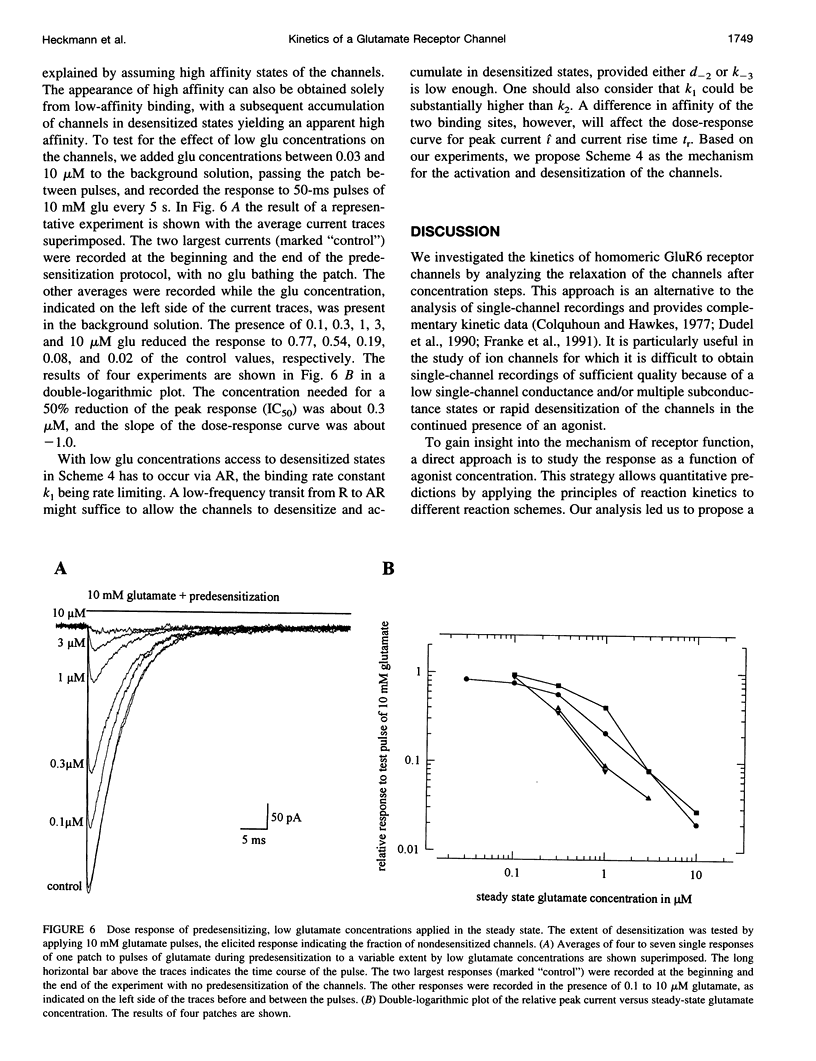
We studied the kinetics of the unedited version of rat GluR6 glutamate (glu) receptor channels, GluR6Q, in outside-out patches using a system for submillisecond solution exchange. Half-maximum activation of the channels was reached with approximately 0.5 microM glu. The maximum slope of the double-logarithmic plot of the peak current versus glu was approximately 1.3, indicating that at least two binding steps are necessary to open the channels. Currents in response to a pulse of 10 microM glu had a short rise time (10-90% of peak current) of approximately 220 microseconds at approximately 20 degrees C. The rise time increased with falling glu concentration, reaching approximately 6.0 ms with 10 microM glu. In the continued presence of glu, the channels desensitized, and this desensitization can be described with a single time constant of approximately 7.0 ms for a pulse of 10 microM glu. The steady-state current in response to a long pulse of 10 microM glu was below 1/280th of the peak current. The time constant of desensitization was found to be independent of concentration between 30.0 and 0.3 microM glu, but to be increased for lower concentrations. After a short pulse of 1 ms duration and 10 or 0.3 microM glu, currents decayed with a time constant of approximately 2.5 ms. Recovery from desensitization after a pulse took approximately 5 s, and the half-time of recovery was approximately 2.2 s. Continuous application of low concentrations of glutamate reduced the peak currents in response to a pulse of 10 microM glu markedly. Fifty percent response reduction was observed in the continuous presence of approximately 0.3 microM glu. Our results for homomeric GluR6 agree with a cyclical reaction scheme developed for completely desensitizing, glu-activated channels on crayfish muscles.
Full text
PDF
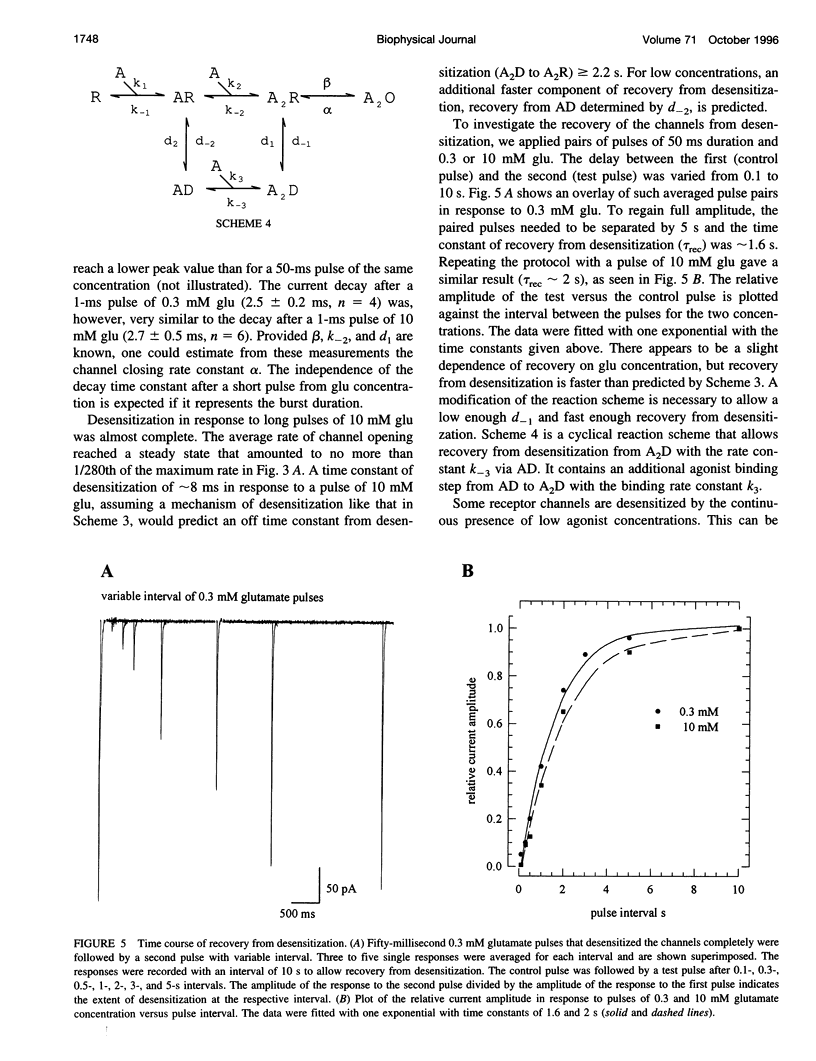

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Colquhoun D. Mechanisms of drug action at the voluntary muscle endplate. Annu Rev Pharmacol. 1975;15:307–325. doi: 10.1146/annurev.pa.15.040175.001515. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Ogden D. C. Activation of ion channels in the frog end-plate by high concentrations of acetylcholine. J Physiol. 1988 Jan;395:131–159. doi: 10.1113/jphysiol.1988.sp016912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Interaction at end-plate receptors between different choline derivatives. Proc R Soc Lond B Biol Sci. 1957 May 7;146(924):369–381. doi: 10.1098/rspb.1957.0018. [DOI] [PubMed] [Google Scholar]
- Dreyer F., Peper K., Sterz R. Determination of dose-response curves by quantitative ionophoresis at the frog neuromuscular junction. J Physiol. 1978 Aug;281:395–419. doi: 10.1113/jphysiol.1978.sp012430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J. Dose-response curve of glutamate applied by superfusion to crayfish muscle synapses. Pflugers Arch. 1977 Mar 11;368(1-2):49–54. doi: 10.1007/BF01063454. [DOI] [PubMed] [Google Scholar]
- Dudel J., Franke C., Hatt H. Rapid activation, desensitization, and resensitization of synaptic channels of crayfish muscle after glutamate pulses. Biophys J. 1990 Mar;57(3):533–545. doi: 10.1016/S0006-3495(90)82569-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J., Franke C., Luboldt W. Reaction scheme for the glutamate-ergic, quisqualate type, completely desensitizing channels on crayfish muscle. Neurosci Lett. 1993 Aug 20;158(2):177–180. doi: 10.1016/0304-3940(93)90258-m. [DOI] [PubMed] [Google Scholar]
- Egebjerg J., Bettler B., Hermans-Borgmeyer I., Heinemann S. Cloning of a cDNA for a glutamate receptor subunit activated by kainate but not AMPA. Nature. 1991 Jun 27;351(6329):745–748. doi: 10.1038/351745a0. [DOI] [PubMed] [Google Scholar]
- Franke C., Hatt H., Dudel J. Liquid filament switch for ultra-fast exchanges of solutions at excised patches of synaptic membrane of crayfish muscle. Neurosci Lett. 1987 Jun 15;77(2):199–204. doi: 10.1016/0304-3940(87)90586-6. [DOI] [PubMed] [Google Scholar]
- Franke C., Hatt H., Parnas H., Dudel J. Kinetic constants of the acetylcholine (ACh) receptor reaction deduced from the rise in open probability after steps in ACh concentration. Biophys J. 1991 Nov;60(5):1008–1016. doi: 10.1016/S0006-3495(91)82138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke C., Parnas H., Hovav G., Dudel J. A molecular scheme for the reaction between acetylcholine and nicotinic channels. Biophys J. 1993 Feb;64(2):339–356. doi: 10.1016/S0006-3495(93)81374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hollmann M., Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Jackson M. B. Perfection of a synaptic receptor: kinetics and energetics of the acetylcholine receptor. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2199–2203. doi: 10.1073/pnas.86.7.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg P. H. The TiPS/TINS lecture: the molecular biology of mammalian glutamate receptor channels. Trends Pharmacol Sci. 1993 Aug;14(8):297–303. doi: 10.1016/0165-6147(93)90047-N. [DOI] [PubMed] [Google Scholar]
- Werman R. An electrophysiological approach to drug-receptor mechanisms. Comp Biochem Physiol. 1969 Sep 15;30(6):997–1017. doi: 10.1016/0010-406x(69)91038-x. [DOI] [PubMed] [Google Scholar]
- von Beckerath N., Adelsberger H., Parzefall F., Franke C., Dudel J. GABAergic inhibition of crayfish deep extensor abdominal muscle exhibits a steep dose-response relationship and a high degree of cooperativity. Pflugers Arch. 1995 Apr;429(6):781–788. doi: 10.1007/BF00374801. [DOI] [PubMed] [Google Scholar]



