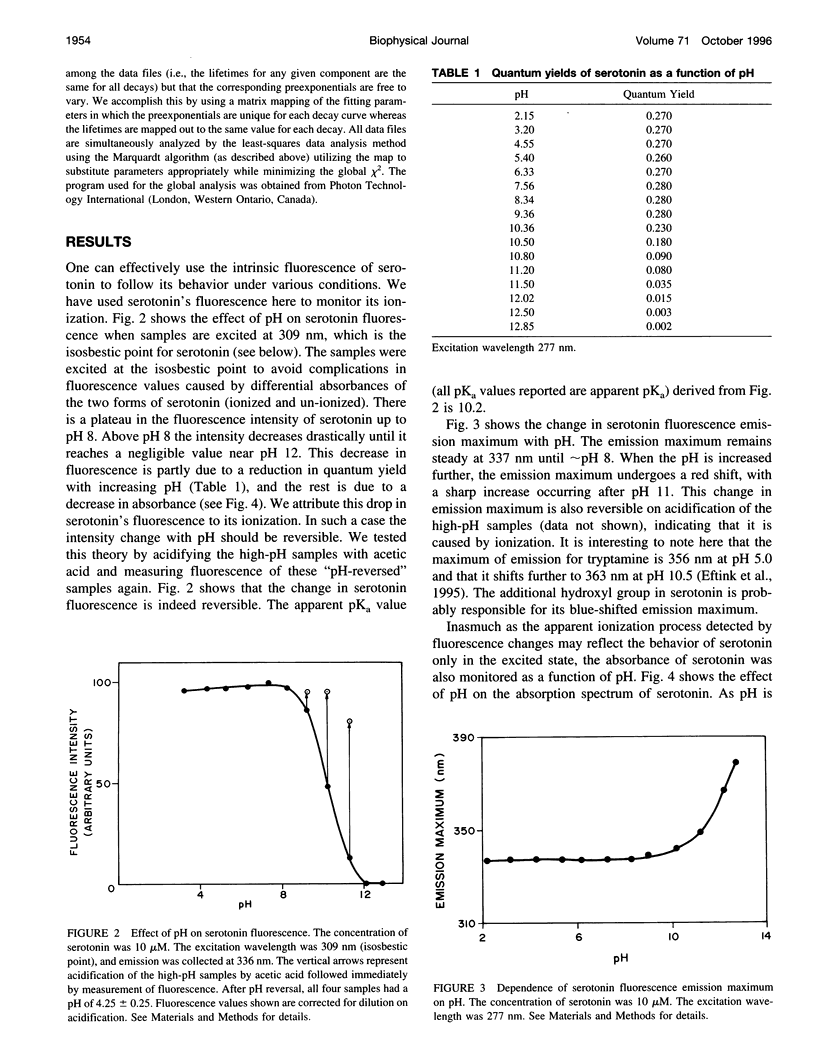
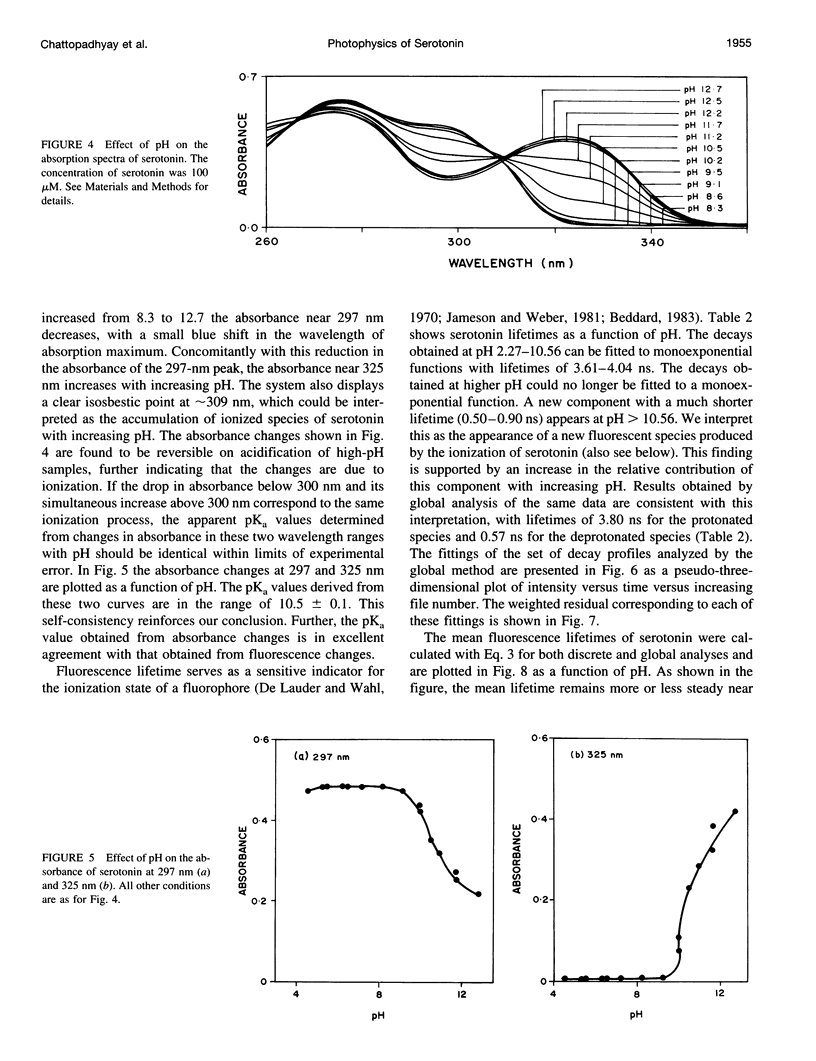
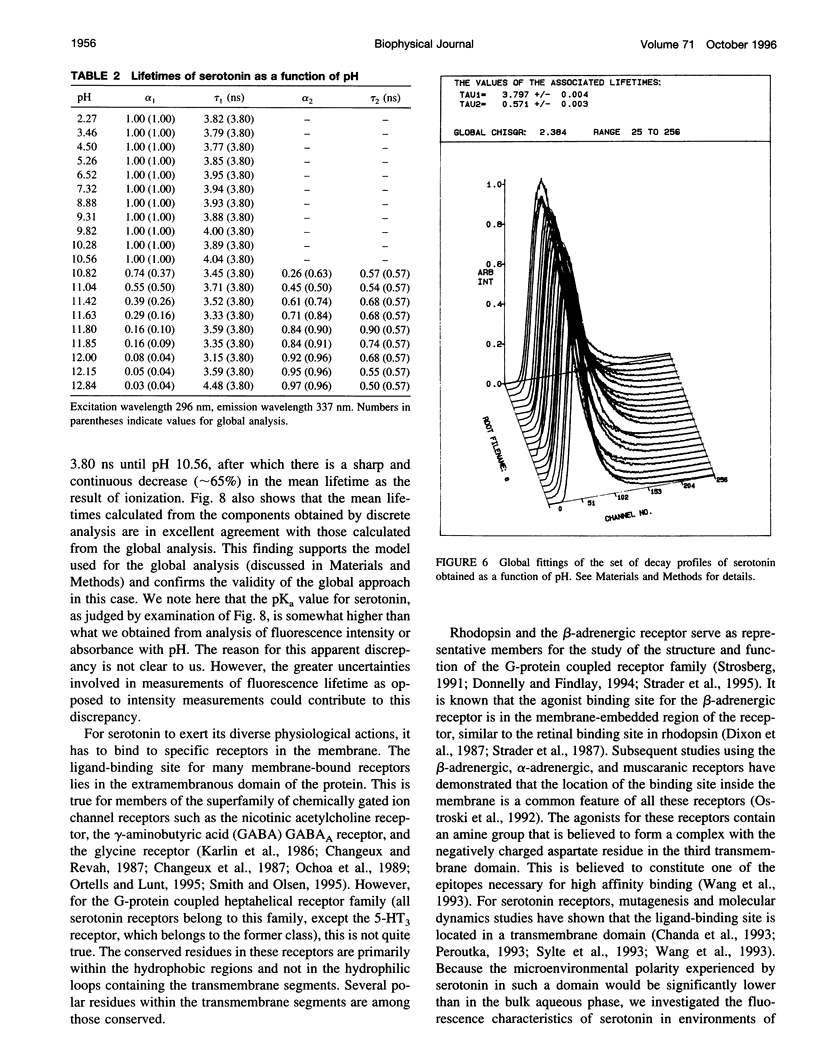
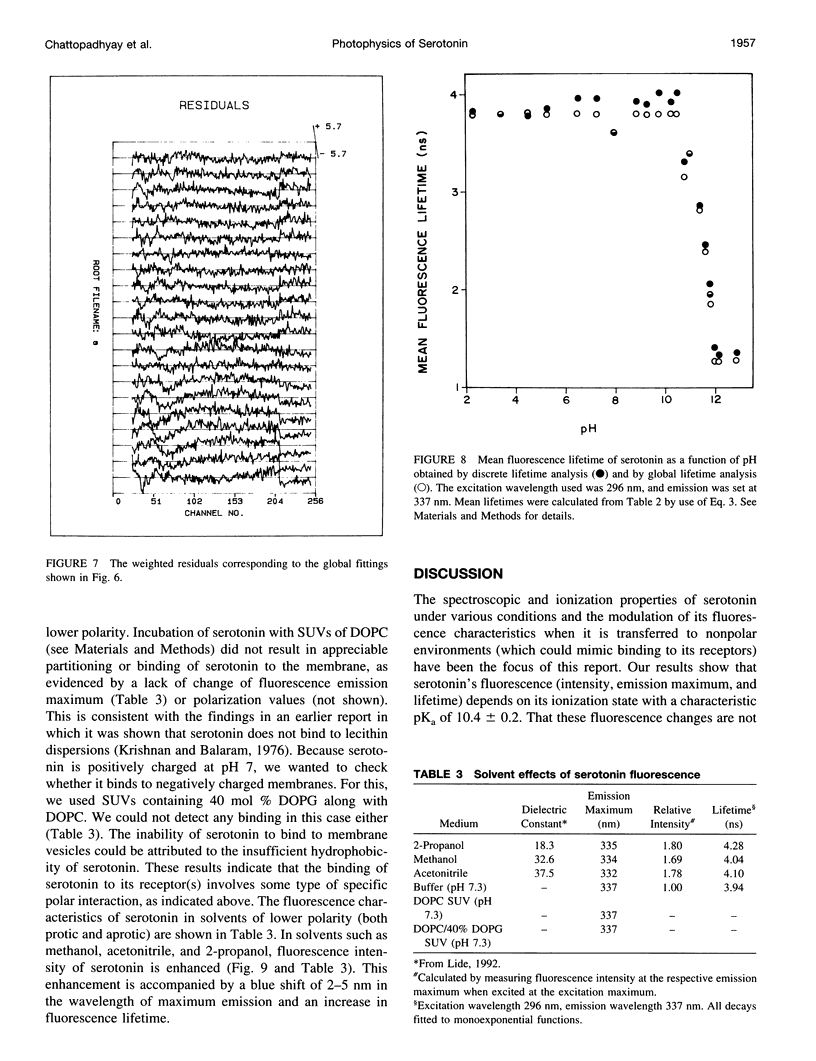
Abstract
The neurotransmitter serotonin plays a modulatory role in the regulation of various cognitive and behavioral functions such as sleep, mood, pain, depression, anxiety, and learning by binding to a number of serotonin receptors present upon the cell surface. The spectroscopic properties of serotonin and their modulation with ionization state have been studied. Results show that serotonin fluorescence, as measured by its intensity, emission maximum, and lifetime, is pH dependent. These results are further supported by absorbance changes that show very similar pH dependence. Changes in fluorescence intensity and absorbance as a function of pH are consistent with a pK(a) of 10.4 +/- 0.2. The ligand-binding site for serotonin receptors is believed to be located in one of the transmembrane domains of the receptors. To develop a basis for monitoring the binding of serotonin to its receptors, its fluorescence in nonpolar media has been studied. No significant binding or partitioning of serotonin to membranes under physiological conditions was observed. Serotonin fluorescence in solvents of lower polarity is characterized by an enhancement in intensity and a blue shift in emission maximum, although the solvatochromism is much less pronounced than in tryptophan. In view of the multiple roles played by the serotonergic systems in the central and peripheral nervous systems, these results are relevant to future studies of serotonin and its binding to its receptors.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beechem J. M. A second generation global analysis program for the recovery of complex inhomogeneous fluorescence decay kinetics. Chem Phys Lipids. 1989 Jun;50(3-4):237–251. doi: 10.1016/0009-3084(89)90052-2. [DOI] [PubMed] [Google Scholar]
- Beechem J. M., Brand L. Time-resolved fluorescence of proteins. Annu Rev Biochem. 1985;54:43–71. doi: 10.1146/annurev.bi.54.070185.000355. [DOI] [PubMed] [Google Scholar]
- Beechem J. M. Global analysis of biochemical and biophysical data. Methods Enzymol. 1992;210:37–54. doi: 10.1016/0076-6879(92)10004-w. [DOI] [PubMed] [Google Scholar]
- Cases O., Seif I., Grimsby J., Gaspar P., Chen K., Pournin S., Müller U., Aguet M., Babinet C., Shih J. C. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995 Jun 23;268(5218):1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda P. K., Minchin M. C., Davis A. R., Greenberg L., Reilly Y., McGregor W. H., Bhat R., Lubeck M. D., Mizutani S., Hung P. P. Identification of residues important for ligand binding to the human 5-hydroxytryptamine1A serotonin receptor. Mol Pharmacol. 1993 Apr;43(4):516–520. [PubMed] [Google Scholar]
- Chen R. F. Fluorescence quantum yield measurements: vitamin B6 compounds. Science. 1965 Dec 17;150(3703):1593–1595. doi: 10.1126/science.150.3703.1593. [DOI] [PubMed] [Google Scholar]
- De Lauder W. B., Wahl P. pH dependence of the fluorescence decay of tryptophan. Biochemistry. 1970 Jun 23;9(13):2750–2754. doi: 10.1021/bi00815a025. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Sigal I. S., Candelore M. R., Register R. B., Scattergood W., Rands E., Strader C. D. Structural features required for ligand binding to the beta-adrenergic receptor. EMBO J. 1987 Nov;6(11):3269–3275. doi: 10.1002/j.1460-2075.1987.tb02645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftink M. R. Fluorescence techniques for studying protein structure. Methods Biochem Anal. 1991;35:127–205. doi: 10.1002/9780470110560.ch3. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Steinberg I. Z. On the analysis of fluorescence decay kinetics by the method of least-squares. Anal Biochem. 1974 Jun;59(2):583–598. doi: 10.1016/0003-2697(74)90312-1. [DOI] [PubMed] [Google Scholar]
- Hen R. Of mice and flies: commonalities among 5-HT receptors. Trends Pharmacol Sci. 1992 Apr;13(4):160–165. doi: 10.1016/0165-6147(92)90054-a. [DOI] [PubMed] [Google Scholar]
- Jacobs B. L., Azmitia E. C. Structure and function of the brain serotonin system. Physiol Rev. 1992 Jan;72(1):165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Krishnan K. S., Balaram P. A nuclear magnetic resonance study of the interaction of serotonin with gangliosides. FEBS Lett. 1976 Apr 1;63(2):313–315. doi: 10.1016/0014-5793(76)80119-6. [DOI] [PubMed] [Google Scholar]
- López-Ibor J. J., Jr The involvement of serotonin in psychiatric disorders and behaviour. Br J Psychiatry Suppl. 1988 Sep;(3):26–39. [PubMed] [Google Scholar]
- Ochoa E. L., Chattopadhyay A., McNamee M. G. Desensitization of the nicotinic acetylcholine receptor: molecular mechanisms and effect of modulators. Cell Mol Neurobiol. 1989 Jun;9(2):141–178. doi: 10.1007/BF00713026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortells M. O., Lunt G. G. Evolutionary history of the ligand-gated ion-channel superfamily of receptors. Trends Neurosci. 1995 Mar;18(3):121–127. doi: 10.1016/0166-2236(95)93887-4. [DOI] [PubMed] [Google Scholar]
- Ostrowski J., Kjelsberg M. A., Caron M. G., Lefkowitz R. J. Mutagenesis of the beta 2-adrenergic receptor: how structure elucidates function. Annu Rev Pharmacol Toxicol. 1992;32:167–183. doi: 10.1146/annurev.pa.32.040192.001123. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J. 5-Hydroxytryptamine receptors. J Neurochem. 1993 Feb;60(2):408–416. doi: 10.1111/j.1471-4159.1993.tb03166.x. [DOI] [PubMed] [Google Scholar]
- Smith G. B., Olsen R. W. Functional domains of GABAA receptors. Trends Pharmacol Sci. 1995 May;16(5):162–168. doi: 10.1016/s0165-6147(00)89009-4. [DOI] [PubMed] [Google Scholar]
- Strader C. D., Fong T. M., Graziano M. P., Tota M. R. The family of G-protein-coupled receptors. FASEB J. 1995 Jun;9(9):745–754. [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Register R. B., Candelore M. R., Rands E., Dixon R. A. Identification of residues required for ligand binding to the beta-adrenergic receptor. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4384–4388. doi: 10.1073/pnas.84.13.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland E. H., Billups C. Oscillator strengths of the 1La and 1Lb absorption bands of tryptophan and several other indoles. Biopolymers. 1973;12(9):1989–1995. doi: 10.1002/bip.1973.360120906. [DOI] [PubMed] [Google Scholar]
- Strosberg A. D. Structure/function relationship of proteins belonging to the family of receptors coupled to GTP-binding proteins. Eur J Biochem. 1991 Feb 26;196(1):1–10. doi: 10.1111/j.1432-1033.1991.tb15778.x. [DOI] [PubMed] [Google Scholar]
- Sylte I., Edvardsen O., Dahl S. G. Molecular dynamics of the 5-HT1a receptor and ligands. Protein Eng. 1993 Sep;6(7):691–700. doi: 10.1093/protein/6.7.691. [DOI] [PubMed] [Google Scholar]
- Tecott L. H., Sun L. M., Akana S. F., Strack A. M., Lowenstein D. H., Dallman M. F., Julius D. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995 Apr 6;374(6522):542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- Wang C. D., Gallaher T. K., Shih J. C. Site-directed mutagenesis of the serotonin 5-hydroxytrypamine2 receptor: identification of amino acids necessary for ligand binding and receptor activation. Mol Pharmacol. 1993 Jun;43(6):931–940. [PubMed] [Google Scholar]
- Yeh S. R., Fricke R. A., Edwards D. H. The effect of social experience on serotonergic modulation of the escape circuit of crayfish. Science. 1996 Jan 19;271(5247):366–369. doi: 10.1126/science.271.5247.366. [DOI] [PubMed] [Google Scholar]


