Abstract
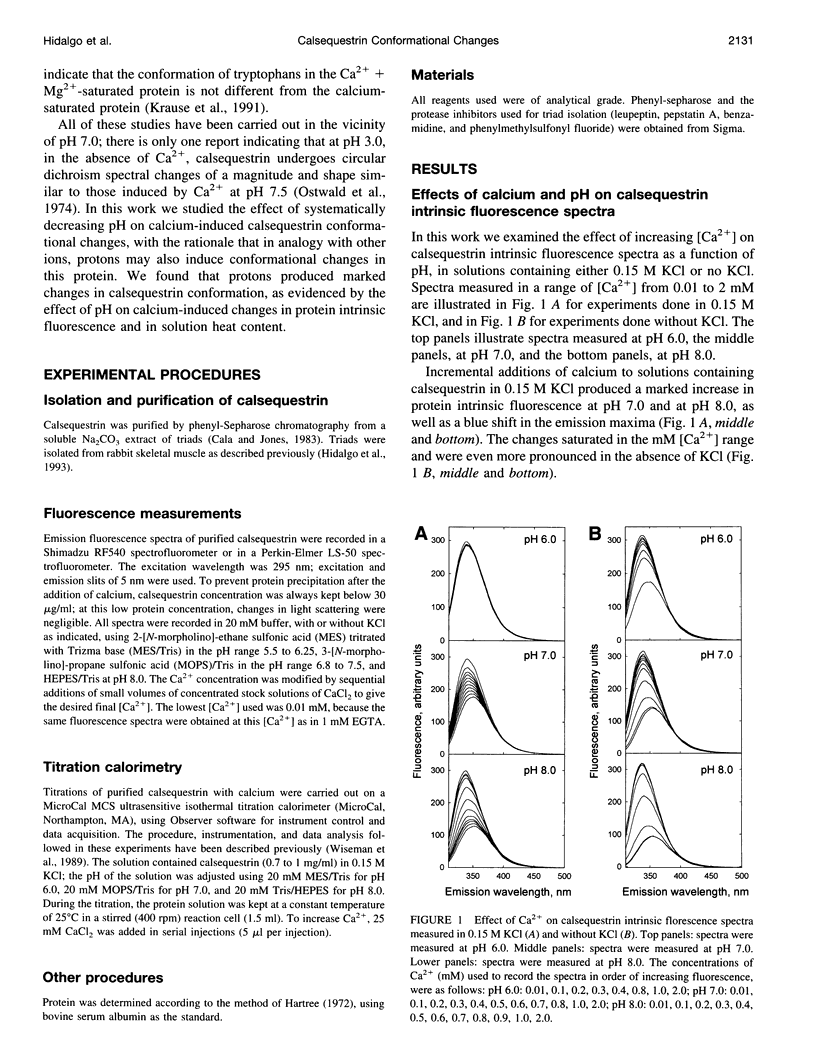
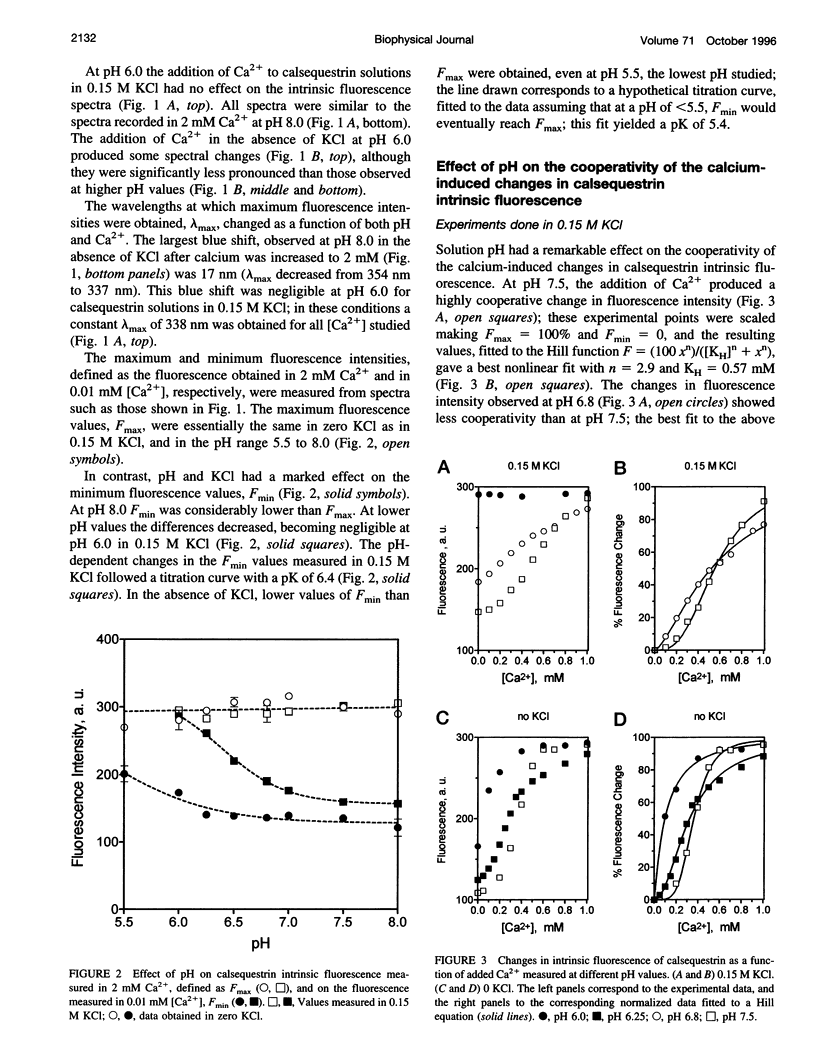
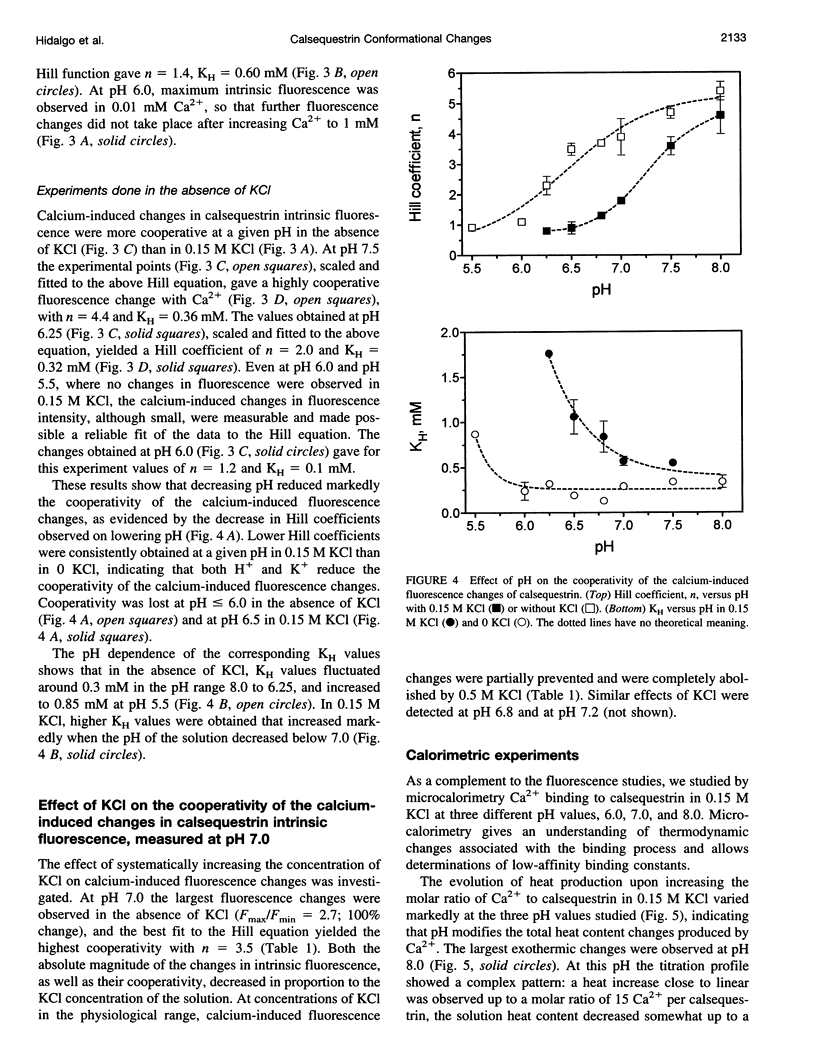
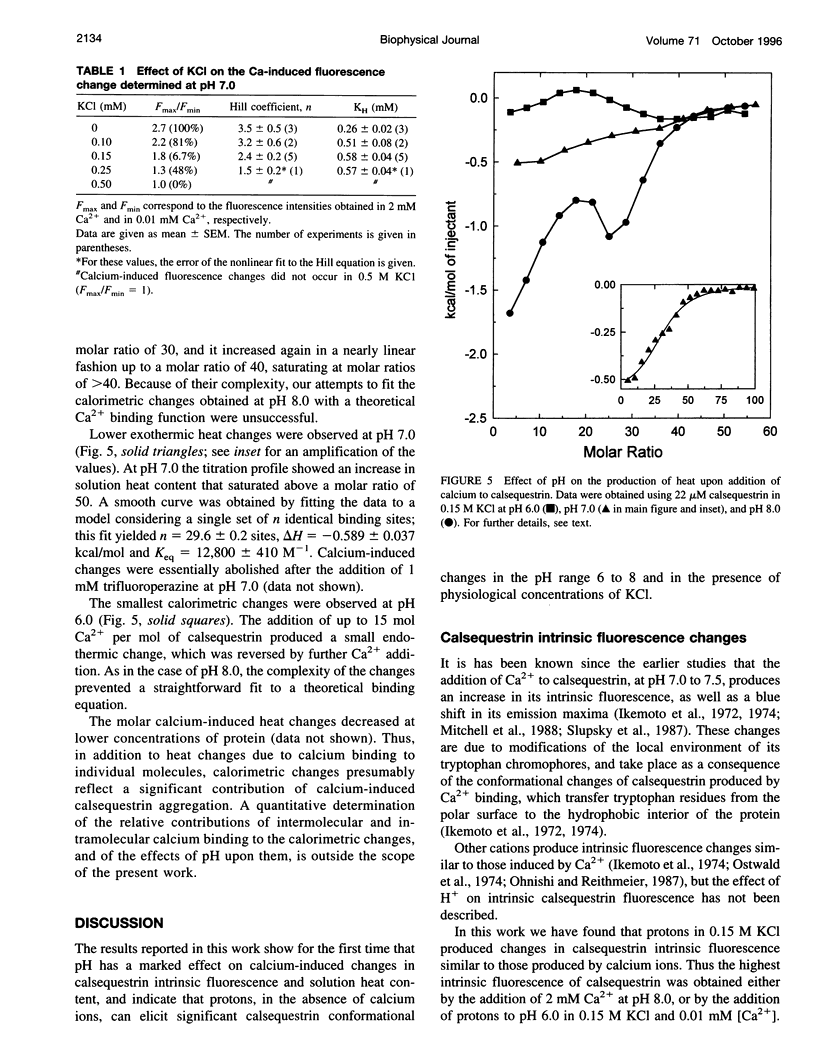
Calsequestrin, a high-capacity, intermediate-affinity, calcium-binding protein present in the lumen of sarcoplasmic reticulum, undergoes extensive calcium-induced conformational changes at neutral pH that cause distinct intrinsic fluorescence changes. The results reported in this work indicate that pH has a marked effect on these calcium-induced intrinsic fluorescence changes, as well as on calorimetric changes produced by the addition of Ca(2+) to calsequestrin. The addition of Ca(2+) at neutral pH produced a marked and cooperative increase in calsequestrin intrinsic fluorescence. In contrast, at pH 6.0 calsequestrin's intrinsic fluorescence was not affected by the addition of Ca(2+), and the same intrinsic fluorescence as that measured in millimolar calcium at neutral pH was obtained. The magnitude and the cooperativity of the calcium-induced intrinsic fluorescence changes decreased as either [H+] or [K+] increased. The evolution of heat production, determined by microcalorimetry, observed upon increasing the molar ratio of Ca(2+) to calsequestrin in 0.15 M KCl, decreased markedly as the pH decreased from pH 8.0 to pH 6.0, indicating that pH modifies the total heat content changes produced by Ca(2+). We propose that protons bind to calsequestrin and induce protein conformational changes that are responsible for the observed proton-induced intrinsic fluorescence and calorimetric changes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaron B. M., Oikawa K., Reithmeier R. A., Sykes B. D. Characterization of skeletal muscle calsequestrin by 1H NMR spectroscopy. J Biol Chem. 1984 Oct 10;259(19):11876–11881. [PubMed] [Google Scholar]
- Cala S. E., Jones L. R. Rapid purification of calsequestrin from cardiac and skeletal muscle sarcoplasmic reticulum vesicles by Ca2+-dependent elution from phenyl-sepharose. J Biol Chem. 1983 Oct 10;258(19):11932–11936. [PubMed] [Google Scholar]
- Cozens B., Reithmeier R. A. Size and shape of rabbit skeletal muscle calsequestrin. J Biol Chem. 1984 May 25;259(10):6248–6252. [PubMed] [Google Scholar]
- Damiani E., Margreth A. Specific protein-protein interactions of calsequestrin with junctional sarcoplasmic reticulum of skeletal muscle. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1253–1259. doi: 10.1016/0006-291x(90)91584-f. [DOI] [PubMed] [Google Scholar]
- Damiani E., Salvatori S., Zorzato F., Margreth A. Characteristics of skeletal muscle calsequestrin: comparison of mammalian, amphibian and avian muscles. J Muscle Res Cell Motil. 1986 Oct;7(5):435–445. doi: 10.1007/BF01753586. [DOI] [PubMed] [Google Scholar]
- Donoso P., Prieto H., Hidalgo C. Luminal calcium regulates calcium release in triads isolated from frog and rabbit skeletal muscle. Biophys J. 1995 Feb;68(2):507–515. doi: 10.1016/S0006-3495(95)80212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegel L., Ohnishi M., Carpenter M. R., Khanna V. K., Reithmeier R. A., MacLennan D. H. Amino acid sequence of rabbit fast-twitch skeletal muscle calsequestrin deduced from cDNA and peptide sequencing. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1167–1171. doi: 10.1073/pnas.84.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C., Kenney L. J., Varriano-Marston E. The structure of calsequestrin in triads of vertebrate skeletal muscle: a deep-etch study. J Cell Biol. 1987 Jul;105(1):49–56. doi: 10.1083/jcb.105.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist J. S., Belcastro A. N., Katz S. Intraluminal Ca2+ dependence of Ca2+ and ryanodine-mediated regulation of skeletal muscle sarcoplasmic reticulum Ca2+ release. J Biol Chem. 1992 Oct 15;267(29):20850–20856. [PubMed] [Google Scholar]
- Guo W., Campbell K. P. Association of triadin with the ryanodine receptor and calsequestrin in the lumen of the sarcoplasmic reticulum. J Biol Chem. 1995 Apr 21;270(16):9027–9030. doi: 10.1074/jbc.270.16.9027. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- He Z., Dunker A. K., Wesson C. R., Trumble W. R. Ca(2+)-induced folding and aggregation of skeletal muscle sarcoplasmic reticulum calsequestrin. The involvement of the trifluoperazine-binding site. J Biol Chem. 1993 Nov 25;268(33):24635–24641. [PubMed] [Google Scholar]
- Hidalgo C., Donoso P. Luminal calcium regulation of calcium release from sarcoplasmic reticulum. Biosci Rep. 1995 Oct;15(5):387–397. doi: 10.1007/BF01788370. [DOI] [PubMed] [Google Scholar]
- Hidalgo C., Jorquera J., Tapia V., Donoso P. Triads and transverse tubules isolated from skeletal muscle contain high levels of inositol 1,4,5-trisphosphate. J Biol Chem. 1993 Jul 15;268(20):15111–15117. [PubMed] [Google Scholar]
- Ikemoto N., Antoniu B., Kang J. J., Mészáros L. G., Ronjat M. Intravesicular calcium transient during calcium release from sarcoplasmic reticulum. Biochemistry. 1991 May 28;30(21):5230–5237. doi: 10.1021/bi00235a017. [DOI] [PubMed] [Google Scholar]
- Ikemoto N., Bhatnagar G. M., Nagy B., Gergely J. Interaction of divalent cations with the 55,000-dalton protein component of the sarcoplasmic reticulum. Studies of fluorescence and circular dichroism. J Biol Chem. 1972 Dec 10;247(23):7835–7837. [PubMed] [Google Scholar]
- Ikemoto N., Nagy B., Bhatnagar G. M., Gergely J. Studies on a metal-binding protein of the sarcoplasmic reticulum. J Biol Chem. 1974 Apr 25;249(8):2357–2365. [PubMed] [Google Scholar]
- Ikemoto N., Ronjat M., Mészáros L. G., Koshita M. Postulated role of calsequestrin in the regulation of calcium release from sarcoplasmic reticulum. Biochemistry. 1989 Aug 8;28(16):6764–6771. doi: 10.1021/bi00442a033. [DOI] [PubMed] [Google Scholar]
- Jones L. R., Zhang L., Sanborn K., Jorgensen A. O., Kelley J. Purification, primary structure, and immunological characterization of the 26-kDa calsequestrin binding protein (junctin) from cardiac junctional sarcoplasmic reticulum. J Biol Chem. 1995 Dec 22;270(51):30787–30796. doi: 10.1074/jbc.270.51.30787. [DOI] [PubMed] [Google Scholar]
- Jorgensen A. O., Shen A. C., Campbell K. P. Ultrastructural localization of calsequestrin in adult rat atrial and ventricular muscle cells. J Cell Biol. 1985 Jul;101(1):257–268. doi: 10.1083/jcb.101.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T., Kasai M. Regulation of calcium channel in sarcoplasmic reticulum by calsequestrin. Biochem Biophys Res Commun. 1994 Mar 30;199(3):1120–1127. doi: 10.1006/bbrc.1994.1347. [DOI] [PubMed] [Google Scholar]
- Krause K. H., Milos M., Luan-Rilliet Y., Lew D. P., Cox J. A. Thermodynamics of cation binding to rabbit skeletal muscle calsequestrin. Evidence for distinct Ca(2+)- and Mg(2+)-binding sites. J Biol Chem. 1991 May 25;266(15):9453–9459. [PubMed] [Google Scholar]
- MacLennan D. H., Wong P. T. Isolation of a calcium-sequestering protein from sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1231–1235. doi: 10.1073/pnas.68.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maylie J., Irving M., Sizto N. L., Boyarsky G., Chandler W. K. Calcium signals recorded from cut frog twitch fibers containing tetramethylmurexide. J Gen Physiol. 1987 Jan;89(1):145–176. doi: 10.1085/jgp.89.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G. Isolation and characterization of two types of sarcoplasmic reticulum vesicles. Biochim Biophys Acta. 1975 Apr 21;389(1):51–68. doi: 10.1016/0005-2736(75)90385-5. [DOI] [PubMed] [Google Scholar]
- Meissner G., Young R. C. Proton permeability of sarcoplasmic reticulum vesicles. J Biol Chem. 1980 Jul 25;255(14):6814–6819. [PubMed] [Google Scholar]
- Mitchell R. D., Simmerman H. K., Jones L. R. Ca2+ binding effects on protein conformation and protein interactions of canine cardiac calsequestrin. J Biol Chem. 1988 Jan 25;263(3):1376–1381. [PubMed] [Google Scholar]
- Ohnishi M., Reithmeier R. A. Fragmentation of rabbit skeletal muscle calsequestrin: spectral and ion binding properties of the carboxyl-terminal region. Biochemistry. 1987 Nov 17;26(23):7458–7465. doi: 10.1021/bi00397a039. [DOI] [PubMed] [Google Scholar]
- Ostwald T. J., MacLennan D. H., Dorrington K. J. Effects of cation binding on the conformation of calsequestrin and the high affinity calcium-binding protein of sarcoplasmic reticulum. J Biol Chem. 1974 Sep 25;249(18):5867–5871. [PubMed] [Google Scholar]
- Pape P. C., Konishi M., Hollingworth S., Baylor S. M. Perturbation of sarcoplasmic reticulum calcium release and phenol red absorbance transients by large concentrations of fura-2 injected into frog skeletal muscle fibers. J Gen Physiol. 1990 Sep;96(3):493–516. doi: 10.1085/jgp.96.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A., Seiler S., Chu A., Fleischer S. Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle. J Cell Biol. 1984 Sep;99(3):875–885. doi: 10.1083/jcb.99.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slupsky J. R., Ohnishi M., Carpenter M. R., Reithmeier R. A. Characterization of cardiac calsequestrin. Biochemistry. 1987 Oct 6;26(20):6539–6544. doi: 10.1021/bi00394a038. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Gonzalez-Serratos H. G., Shuman H., McClellan G., Somlyo A. P. Calcium release and ionic changes in the sarcoplasmic reticulum of tetanized muscle: an electron-probe study. J Cell Biol. 1981 Sep;90(3):577–594. doi: 10.1083/jcb.90.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. W., Beeler T. J. Secondary structure of calsequestrin in solutions and in crystals as determined by Raman spectroscopy. J Biol Chem. 1986 Sep 15;261(26):12408–12413. [PubMed] [Google Scholar]
- Wiseman T., Williston S., Brandts J. F., Lin L. N. Rapid measurement of binding constants and heats of binding using a new titration calorimeter. Anal Biochem. 1989 May 15;179(1):131–137. doi: 10.1016/0003-2697(89)90213-3. [DOI] [PubMed] [Google Scholar]
- Yano K., Zarain-Herzberg A. Sarcoplasmic reticulum calsequestrins: structural and functional properties. Mol Cell Biochem. 1994 Jun 15;135(1):61–70. doi: 10.1007/BF00925961. [DOI] [PubMed] [Google Scholar]


