Abstract
We report the results of microfluorometric measurements of physiological changes in optically trapped immotile Chinese hamster ovary cells (CHOs) and motile human sperm cells under continuous-wave (CW) and pulsed-mode trapping conditions at 1064 nm. The fluorescence spectra derived from the exogenous fluorescent probes laurdan, acridine orange, propidium iodide, and Snarf are used to assess the effects of optical confinement with respect to temperature, DNA structure, cell viability, and intracellular pH, respectively. In the latter three cases, fluorescence is excited via a two-photon process, using a CW laser trap as the fluorescence excitation source. An average temperature increase of < 0.1 +/- 0.30 degrees C/100 mW is measured for cells when held stationary with CW optical tweezers at powers of up to 400 mW. The same trapping conditions do not appear to alter DNA structure or cellular pH. In contrast, a pulsed 1064-nm laser trap (100-ns pulses at 40 microJ/pulse and average power of 40 mW) produced significant fluorescence spectral alterations in acridine orange, perhaps because of thermally induced DNA structural changes or laser-induced multiphoton processes. The techniques and results presented herein demonstrate the ability to perform in situ monitoring of cellular physiology during CW and pulsed laser trapping, and should prove useful in studying mechanisms by which optical tweezers and microbeams perturb metabolic function and cellular viability.
Full text
PDF

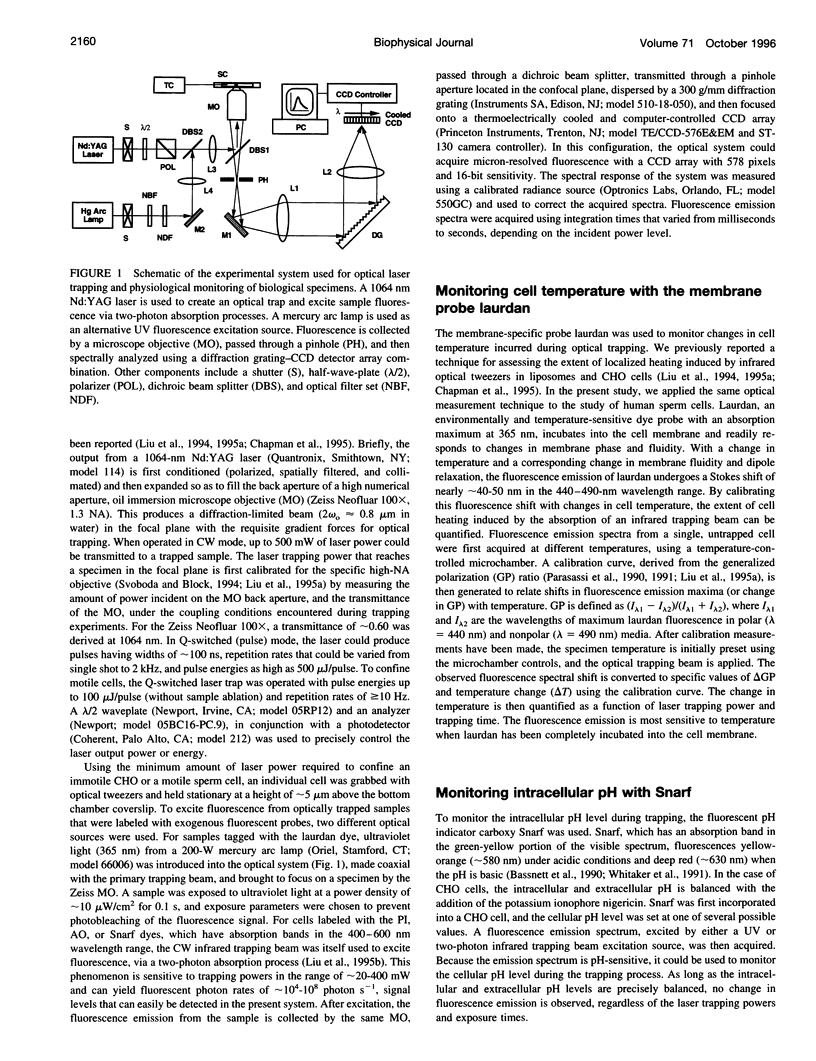

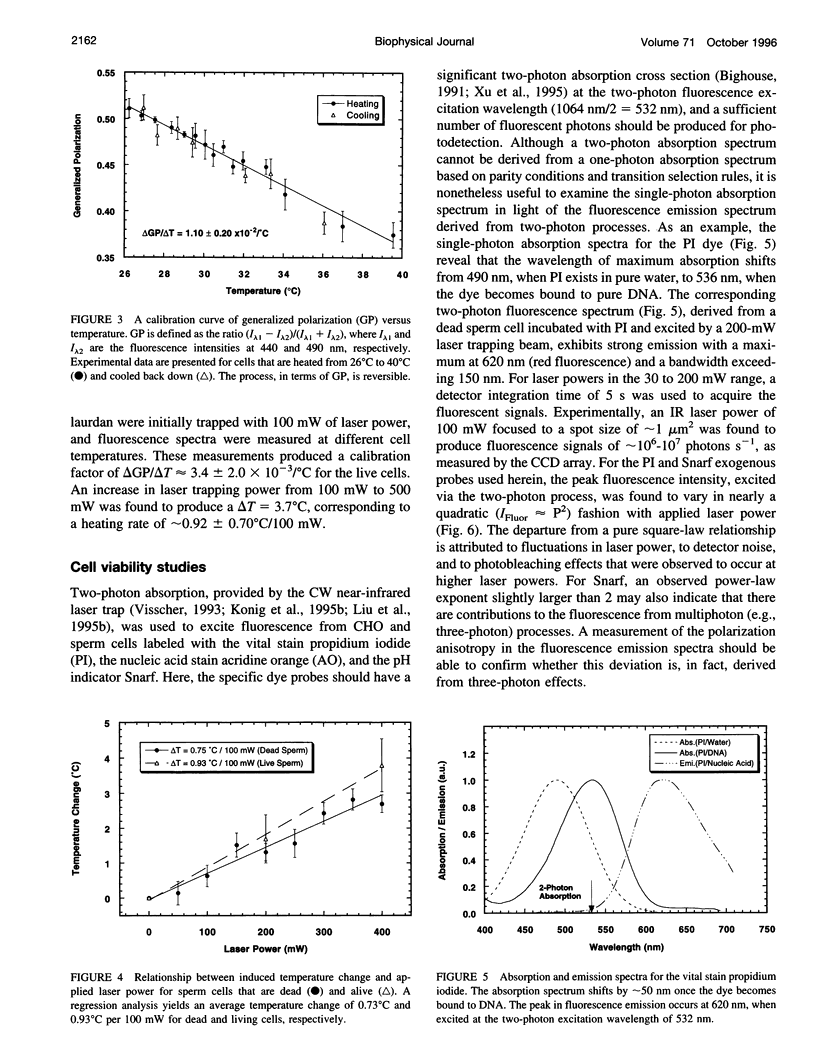
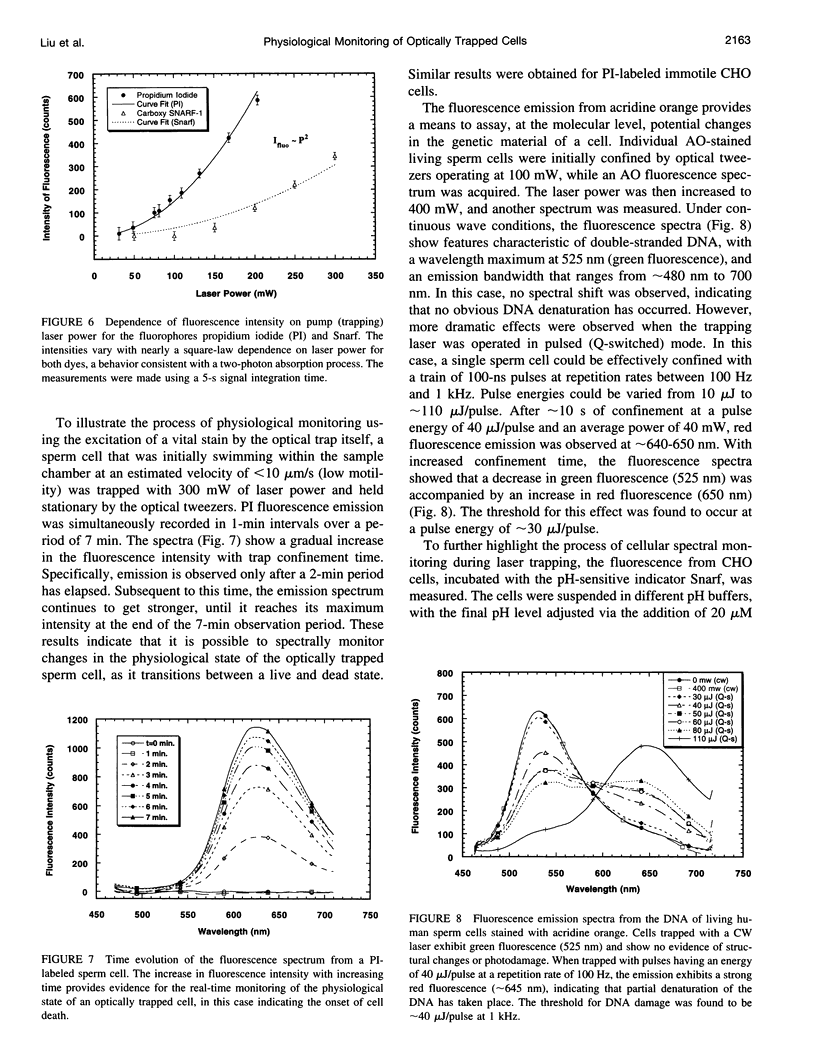
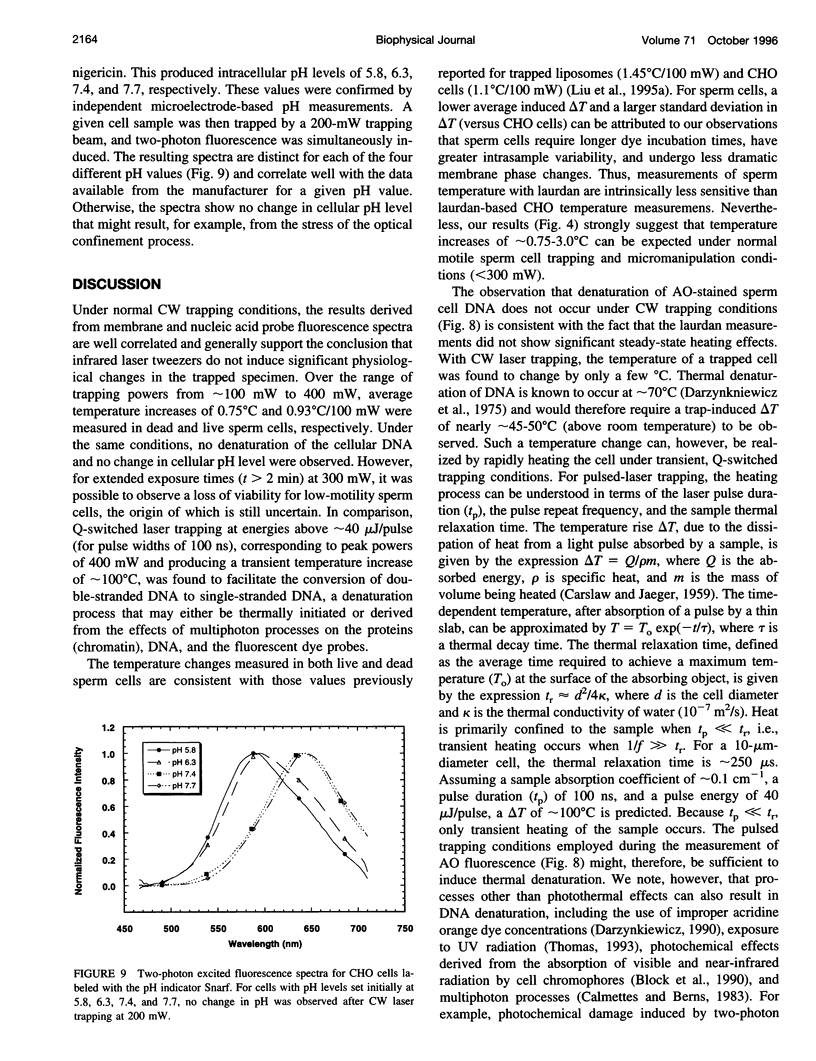



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashkin A., Dziedzic J. M. Internal cell manipulation using infrared laser traps. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7914–7918. doi: 10.1073/pnas.86.20.7914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkin A., Dziedzic J. M. Optical trapping and manipulation of viruses and bacteria. Science. 1987 Mar 20;235(4795):1517–1520. doi: 10.1126/science.3547653. [DOI] [PubMed] [Google Scholar]
- Ashkin A., Dziedzic J. M., Yamane T. Optical trapping and manipulation of single cells using infrared laser beams. Nature. 1987 Dec 24;330(6150):769–771. doi: 10.1038/330769a0. [DOI] [PubMed] [Google Scholar]
- Ashkin A., Schütze K., Dziedzic J. M., Euteneuer U., Schliwa M. Force generation of organelle transport measured in vivo by an infrared laser trap. Nature. 1990 Nov 22;348(6299):346–348. doi: 10.1038/348346a0. [DOI] [PubMed] [Google Scholar]
- Berland K. M., So P. T., Gratton E. Two-photon fluorescence correlation spectroscopy: method and application to the intracellular environment. Biophys J. 1995 Feb;68(2):694–701. doi: 10.1016/S0006-3495(95)80230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns M. W., Wright W. H., Tromberg B. J., Profeta G. A., Andrews J. J., Walter R. J. Use of a laser-induced optical force trap to study chromosome movement on the mitotic spindle. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4539–4543. doi: 10.1073/pnas.86.12.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block S. M., Goldstein L. S., Schnapp B. J. Bead movement by single kinesin molecules studied with optical tweezers. Nature. 1990 Nov 22;348(6299):348–352. doi: 10.1038/348348a0. [DOI] [PubMed] [Google Scholar]
- Calmettes P. P., Berns M. W. Laser-induced multiphoton processes in living cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7197–7199. doi: 10.1073/pnas.80.23.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman C. F., Liu Y., Sonek G. J., Tromberg B. J. The use of exogenous fluorescent probes for temperature measurements in single living cells. Photochem Photobiol. 1995 Sep;62(3):416–425. doi: 10.1111/j.1751-1097.1995.tb02362.x. [DOI] [PubMed] [Google Scholar]
- Colon J. M., Sarosi P., McGovern P. G., Askin A., Dziedzic J. M., Skurnick J., Weiss G., Bonder E. M. Controlled micromanipulation of human sperm in three dimensions with an infrared laser optical trap: effect on sperm velocity. Fertil Steril. 1992 Mar;57(3):695–698. doi: 10.1016/s0015-0282(16)54926-7. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z. Differential staining of DNA and RNA in intact cells and isolated cell nuclei with acridine orange. Methods Cell Biol. 1990;33:285–298. doi: 10.1016/s0091-679x(08)60532-4. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Sharpless T., Melamed M. R. Thermal denaturation of DNA in situ as studied by acridine orange staining and automated cytofluorometry. Exp Cell Res. 1975 Feb;90(2):411–428. doi: 10.1016/0014-4827(75)90331-6. [DOI] [PubMed] [Google Scholar]
- Delic J., Coppey J., Magdelenat H., Coppey-Moisan M. Impossibility of acridine orange intercalation in nuclear DNA of the living cell. Exp Cell Res. 1991 May;194(1):147–153. doi: 10.1016/0014-4827(91)90144-j. [DOI] [PubMed] [Google Scholar]
- Denk W., Strickler J. H., Webb W. W. Two-photon laser scanning fluorescence microscopy. Science. 1990 Apr 6;248(4951):73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- Finer J. T., Simmons R. M., Spudich J. A. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature. 1994 Mar 10;368(6467):113–119. doi: 10.1038/368113a0. [DOI] [PubMed] [Google Scholar]
- Kuo S. C., Sheetz M. P. Force of single kinesin molecules measured with optical tweezers. Science. 1993 Apr 9;260(5105):232–234. doi: 10.1126/science.8469975. [DOI] [PubMed] [Google Scholar]
- König K., Liang H., Berns M. W., Tromberg B. J. Cell damage by near-IR microbeams. Nature. 1995 Sep 7;377(6544):20–21. doi: 10.1038/377020a0. [DOI] [PubMed] [Google Scholar]
- König K., Liu Y., Sonek G. J., Berns M. W., Tromberg B. J. Autofluorescence spectroscopy of optically trapped cells. Photochem Photobiol. 1995 Nov;62(5):830–835. doi: 10.1111/j.1751-1097.1995.tb09143.x. [DOI] [PubMed] [Google Scholar]
- Liang H., Wright W. H., He W., Berns M. W. Micromanipulation of mitotic chromosomes in PTK2 cells using laser-induced optical forces ("optical tweezers"). Exp Cell Res. 1991 Nov;197(1):21–35. doi: 10.1016/0014-4827(91)90475-a. [DOI] [PubMed] [Google Scholar]
- Liang H., Wright W. H., Rieder C. L., Salmon E. D., Profeta G., Andrews J., Liu Y., Sonek G. J., Berns M. W. Directed movement of chromosome arms and fragments in mitotic newt lung cells using optical scissors and optical tweezers. Exp Cell Res. 1994 Jul;213(1):308–312. doi: 10.1006/excr.1994.1203. [DOI] [PubMed] [Google Scholar]
- Liu Y., Cheng D. K., Sonek G. J., Berns M. W., Chapman C. F., Tromberg B. J. Evidence for localized cell heating induced by infrared optical tweezers. Biophys J. 1995 May;68(5):2137–2144. doi: 10.1016/S0006-3495(95)80396-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasassi T., De Stasio G., Ravagnan G., Rusch R. M., Gratton E. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys J. 1991 Jul;60(1):179–189. doi: 10.1016/S0006-3495(91)82041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasassi T., De Stasio G., d'Ubaldo A., Gratton E. Phase fluctuation in phospholipid membranes revealed by Laurdan fluorescence. Biophys J. 1990 Jun;57(6):1179–1186. doi: 10.1016/S0006-3495(90)82637-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger S., Monajembashi S., Hutter K. J., Futterman G., Wolfrum J., Greulich K. O. Application of laser optical tweezers in immunology and molecular genetics. Cytometry. 1991;12(6):497–504. doi: 10.1002/cyto.990120606. [DOI] [PubMed] [Google Scholar]
- Svoboda K., Block S. M. Biological applications of optical forces. Annu Rev Biophys Biomol Struct. 1994;23:247–285. doi: 10.1146/annurev.bb.23.060194.001335. [DOI] [PubMed] [Google Scholar]
- Svoboda K., Schmidt C. F., Schnapp B. J., Block S. M. Direct observation of kinesin stepping by optical trapping interferometry. Nature. 1993 Oct 21;365(6448):721–727. doi: 10.1038/365721a0. [DOI] [PubMed] [Google Scholar]
- Tadir Y., Wright W. H., Vafa O., Ord T., Asch R. H., Berns M. W. Force generated by human sperm correlated to velocity and determined using a laser generated optical trap. Fertil Steril. 1990 May;53(5):944–947. doi: 10.1016/s0015-0282(16)53539-0. [DOI] [PubMed] [Google Scholar]
- Tadir Y., Wright W. H., Vafa O., Ord T., Asch R. H., Berns M. W. Micromanipulation of sperm by a laser generated optical trap. Fertil Steril. 1989 Nov;52(5):870–873. [PubMed] [Google Scholar]
- Thomas R. The denaturation of DNA. Gene. 1993 Dec 15;135(1-2):77–79. doi: 10.1016/0378-1119(93)90051-4. [DOI] [PubMed] [Google Scholar]
- Visscher K., Brakenhoff G. J. Single beam optical trapping integrated in a confocal microscope for biological applications. Cytometry. 1991;12(6):486–491. doi: 10.1002/cyto.990120604. [DOI] [PubMed] [Google Scholar]
- Vorobjev I. A., Liang H., Wright W. H., Berns M. W. Optical trapping for chromosome manipulation: a wavelength dependence of induced chromosome bridges. Biophys J. 1993 Feb;64(2):533–538. doi: 10.1016/S0006-3495(93)81398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker J. E., Haugland R. P., Prendergast F. G. Spectral and photophysical studies of benzo[c]xanthene dyes: dual emission pH sensors. Anal Biochem. 1991 May 1;194(2):330–344. doi: 10.1016/0003-2697(91)90237-n. [DOI] [PubMed] [Google Scholar]


