Abstract
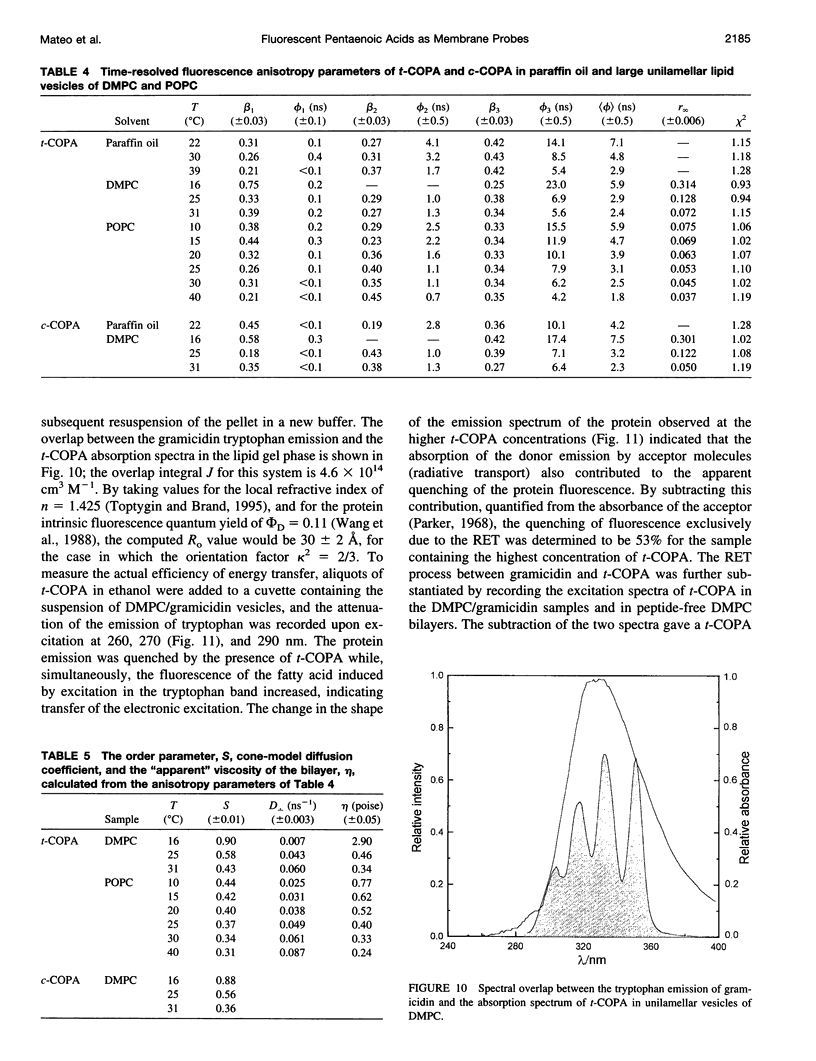
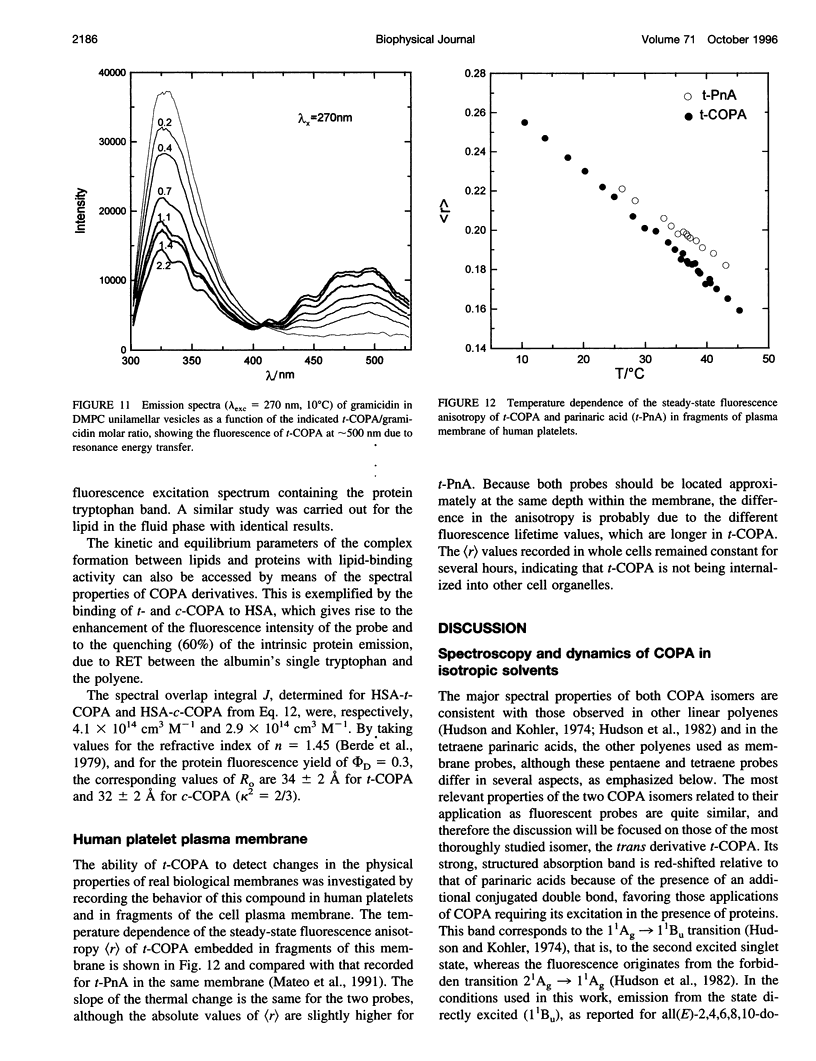
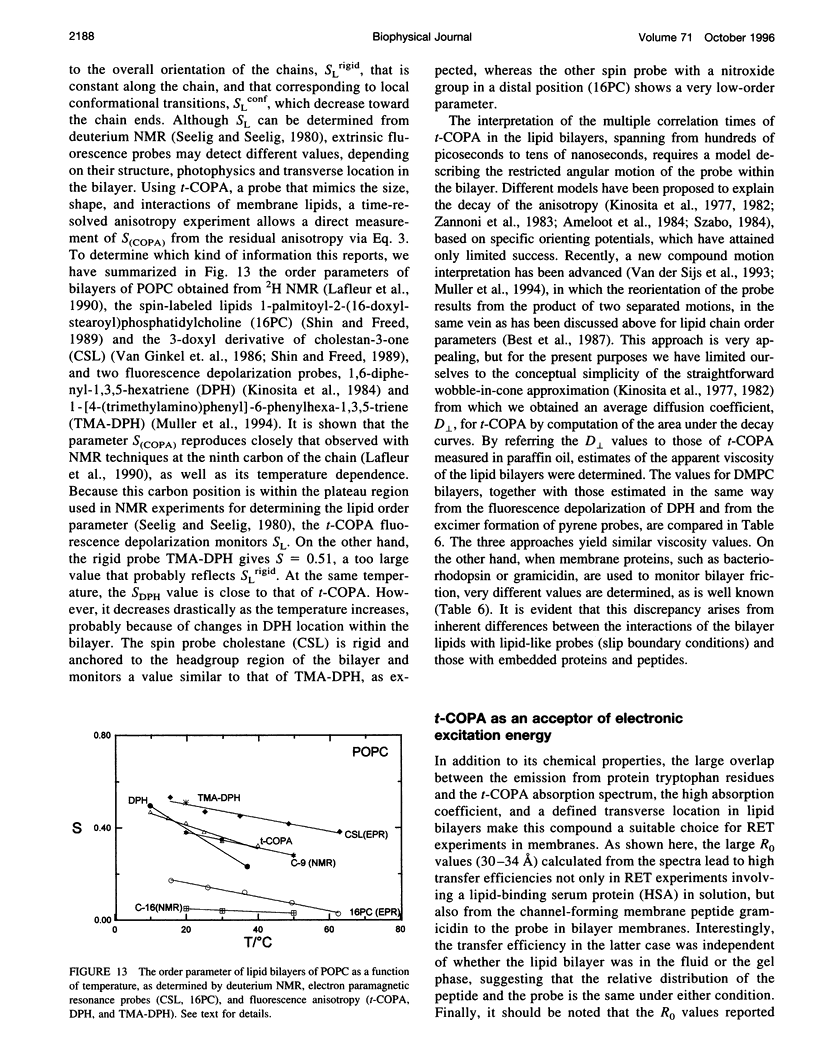
The chemical and spectroscopic properties of the new fluorescent acids all(E)-8, 10, 12, 14, 16-octadecapentaenoic acid (t-COPA) and its (8Z)-isomer (c-COPA) have been characterized in solvents of different polarity, synthetic lipid bilayers, and lipid/protein systems. These compounds are reasonably photostable in solution, present an intense UV absorption band (epsilon(350 nm) approximately 10(5) M(-1) cm(-1)) strongly overlapped by tryptophan fluorescence and their emission, centered at 470 nm, is strongly polarized (r(O) = 0.385 +/- 0.005) and decays with a major component (85%) of lifetime 23 ns and a faster minor one of lifetime 2 ns (D,L-alpha-dimyristoylphosphatidylcholine (DMPC), 15 degrees C). Both COPA isomers incorporate readily into vesicles and membranes (K(p) approximately 10(6)) and align parallel to the lipids. t-COPA distributes homogeneously between gel and fluid lipid domains and the changes in polarization accurately reflect the lipid T(m) values. From the decay of the fluorescence anisotropy in spherical bilayers of DMPC and POPC it is shown that t-COPA also correctly reflects the lipid order parameters, determined by 2H NMR techniques. Resonance energy transfer from tryptophan to the bound pentaenoic acid in serum albumin in solution, and from the tryptophan residues of gramicidin in lipid bilayers also containing the pentaenoic acid, show that this probe is a useful acceptor of protein tryptophan excitation, with R(O) values of 30-34 A.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ameloot M., Hendrickx H., Herreman W., Pottel H., Van Cauwelaert F., van der Meer W. Effect of orientational order on the decay of the fluorescence anisotropy in membrane suspensions. Experimental verification on unilamellar vesicles and lipid/alpha-lactalbumin complexes. Biophys J. 1984 Oct;46(4):525–539. doi: 10.1016/S0006-3495(84)84050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bañ M. C., Braco L., Abad C. Conformational transitions of gramicidin A in phospholipid model membranes. A high-performance liquid chromatography assessment. Biochemistry. 1991 Jan 29;30(4):886–894. doi: 10.1021/bi00218a002. [DOI] [PubMed] [Google Scholar]
- Berde C. B., Hudson B. S., Simoni R. D., Sklar L. A. Human serum albumin. Spectroscopic studies of binding and proximity relationships for fatty acids and bilirubin. J Biol Chem. 1979 Jan 25;254(2):391–400. [PubMed] [Google Scholar]
- Castanho M., Prieto M., Acuña A. U. The transverse location of the fluorescent probe trans-parinaric acid in lipid bilayers. Biochim Biophys Acta. 1996 Mar 13;1279(2):164–168. doi: 10.1016/0005-2736(95)00251-0. [DOI] [PubMed] [Google Scholar]
- Castanho M., Prieto M. Filipin fluorescence quenching by spin-labeled probes: studies in aqueous solution and in a membrane model system. Biophys J. 1995 Jul;69(1):155–168. doi: 10.1016/S0006-3495(95)79886-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay A., London E. Parallax method for direct measurement of membrane penetration depth utilizing fluorescence quenching by spin-labeled phospholipids. Biochemistry. 1987 Jan 13;26(1):39–45. doi: 10.1021/bi00375a006. [DOI] [PubMed] [Google Scholar]
- Cherry R. J., Godfrey R. E. Anisotropic rotation of bacteriorhodopsin in lipid membranes. Comparison of theory with experiment. Biophys J. 1981 Oct;36(1):257–276. doi: 10.1016/S0006-3495(81)84727-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. J., Fleming G. R. Analysis of time-resolved fluorescence anisotropy decays. Biophys J. 1984 Jul;46(1):45–56. doi: 10.1016/S0006-3495(84)83997-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. E., Chen L. A., Brand L. Rotational relaxation of the "microviscosity" probe diphenylhexatriene in paraffin oil and egg lecithin vesicles. J Biol Chem. 1977 Nov 10;252(21):7500–7510. [PubMed] [Google Scholar]
- Engel L. W., Prendergast F. G. Values for and significance of order parameters and "cone angles" of fluorophore rotation in lipid bilayers. Biochemistry. 1981 Dec 22;20(26):7338–7345. doi: 10.1021/bi00529a003. [DOI] [PubMed] [Google Scholar]
- Heyn M. P. Order and viscosity of membranes: analysis by time-resolved fluorescence depolarization. Methods Enzymol. 1989;172:462–471. doi: 10.1016/s0076-6879(89)72029-2. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Tecoma E. S., Wolber P. K., Hudson B. S., Wickner W. T., Simoni R. D. Protein-lipid interactions. Studies of the M13 coat protein in dimyristoylphosphatidylcholine vesicles using parinaric acid. Biochemistry. 1979 Dec 25;18(26):5874–5880. doi: 10.1021/bi00593a018. [DOI] [PubMed] [Google Scholar]
- Kinosita K., Jr, Ikegami A., Kawato S. On the wobbling-in-cone analysis of fluorescence anisotropy decay. Biophys J. 1982 Feb;37(2):461–464. doi: 10.1016/S0006-3495(82)84692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinosita K., Jr, Kawato S., Ikegami A. A theory of fluorescence polarization decay in membranes. Biophys J. 1977 Dec;20(3):289–305. doi: 10.1016/S0006-3495(77)85550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinosita K., Jr, Kawato S., Ikegami A. Dynamic structure of biological and model membranes: analysis by optical anisotropy decay measurement. Adv Biophys. 1984;17:147–203. doi: 10.1016/0065-227x(84)90027-3. [DOI] [PubMed] [Google Scholar]
- Lafleur M., Cullis P. R., Bloom M. Modulation of the orientational order profile of the lipid acyl chain in the L alpha phase. Eur Biophys J. 1990;19(2):55–62. doi: 10.1007/BF00185086. [DOI] [PubMed] [Google Scholar]
- Lentz B. R. Use of fluorescent probes to monitor molecular order and motions within liposome bilayers. Chem Phys Lipids. 1993 Sep;64(1-3):99–116. doi: 10.1016/0009-3084(93)90060-g. [DOI] [PubMed] [Google Scholar]
- Lipari G., Szabo A. Effect of librational motion on fluorescence depolarization and nuclear magnetic resonance relaxation in macromolecules and membranes. Biophys J. 1980 Jun;30(3):489–506. doi: 10.1016/S0006-3495(80)85109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoGrasso P. V., Moll F., 3rd, Cross T. A. Solvent history dependence of gramicidin A conformations in hydrated lipid bilayers. Biophys J. 1988 Aug;54(2):259–267. doi: 10.1016/S0006-3495(88)82955-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabrey S., Sturtevant J. M. Investigation of phase transitions of lipids and lipid mixtures by sensitivity differential scanning calorimetry. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3862–3866. doi: 10.1073/pnas.73.11.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald P. M., Seelig J. Dynamic properties of gramicidin A in phospholipid membranes. Biochemistry. 1988 Apr 5;27(7):2357–2364. doi: 10.1021/bi00407a017. [DOI] [PubMed] [Google Scholar]
- Martínez M. C., García de la Torre J. Brownian dynamics simulation of restricted rotational diffusion. Biophys J. 1987 Aug;52(2):303–310. doi: 10.1016/S0006-3495(87)83217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo C. R., Lillo M. P., González-Rodríguez J., Acuña A. U. Molecular order and fluidity of the plasma membrane of human platelets from time-resolved fluorescence depolarization. Eur Biophys J. 1991;20(1):41–52. doi: 10.1007/BF00183278. [DOI] [PubMed] [Google Scholar]
- Mukherjee S., Chattopadhyay A. Motionally restricted tryptophan environments at the peptide-lipid interface of gramicidin channels. Biochemistry. 1994 May 3;33(17):5089–5097. doi: 10.1021/bi00183a012. [DOI] [PubMed] [Google Scholar]
- Reyes Mateo C., Brochon J. C., Pilar Lillo M., Ulises Acuña A. Lipid clustering in bilayers detected by the fluorescence kinetics and anisotropy of trans-parinaric acid. Biophys J. 1993 Nov;65(5):2237–2247. doi: 10.1016/S0006-3495(93)81257-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes Mateo C., Ulises Acuña A., Brochon J. C. Liquid-crystalline phases of cholesterol/lipid bilayers as revealed by the fluorescence of trans-parinaric acid. Biophys J. 1995 Mar;68(3):978–987. doi: 10.1016/S0006-3495(95)80273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigler R., Ehrenberg M. Molecular interactions and structure as analysed by fluorescence relaxation spectroscopy. Q Rev Biophys. 1973 May;6(2):139–199. doi: 10.1017/s003358350000113x. [DOI] [PubMed] [Google Scholar]
- Ruggiero A., Hudson B. Analysis of the anisotropy decay of trans-parinaric acid in lipid bilayers. Biophys J. 1989 Jun;55(6):1125–1135. doi: 10.1016/S0006-3495(89)82909-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero A., Hudson B. Critical density fluctuations in lipid bilayers detected by fluorescence lifetime heterogeneity. Biophys J. 1989 Jun;55(6):1111–1124. doi: 10.1016/S0006-3495(89)82908-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig J., Seelig A. Lipid conformation in model membranes and biological membranes. Q Rev Biophys. 1980 Feb;13(1):19–61. doi: 10.1017/s0033583500000305. [DOI] [PubMed] [Google Scholar]
- Shin Y. K., Freed J. H. Dynamic imaging of lateral diffusion by electron spin resonance and study of rotational dynamics in model membranes. Effect of cholesterol. Biophys J. 1989 Mar;55(3):537–550. doi: 10.1016/S0006-3495(89)82847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar L. A., Hudson B. S., Petersen M., Diamond J. Conjugated polyene fatty acids on fluorescent probes: spectroscopic characterization. Biochemistry. 1977 Mar 8;16(5):813–819. doi: 10.1021/bi00624a001. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Hudson B. S., Simoni R. D. Conjugated polyene fatty acids as fluorescent probes: synthetic phospholipid membrane studies. Biochemistry. 1977 Mar 8;16(5):819–828. doi: 10.1021/bi00624a002. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Miljanich G. P., Bursten S. L., Dratz E. A. Thermal lateral phase separations in bovine retinal rod outer segment membranes and phospholipids as evidenced by parinaric acid fluorescence polarization and energy transfer. J Biol Chem. 1979 Oct 10;254(19):9583–9591. [PubMed] [Google Scholar]
- Sklar L. A. The partition of cis-parinaric acid and trans-parinaric acid among aqueous, fluid lipid, and solid lipid phases. Mol Cell Biochem. 1980 Nov 20;32(3):169–177. doi: 10.1007/BF00227444. [DOI] [PubMed] [Google Scholar]
- Vanderkooi J. M., Callis J. B. Pyrene. A probe of lateral diffusion in the hydrophobic region of membranes. Biochemistry. 1974 Sep 10;13(19):4000–4006. doi: 10.1021/bi00716a028. [DOI] [PubMed] [Google Scholar]
- Wallace B. A. Gramicidin channels and pores. Annu Rev Biophys Biophys Chem. 1990;19:127–157. doi: 10.1146/annurev.bb.19.060190.001015. [DOI] [PubMed] [Google Scholar]
- Wang S., Martin E., Cimino J., Omann G., Glaser M. Distribution of phospholipids around gramicidin and D-beta-hydroxybutyrate dehydrogenase as measured by resonance energy transfer. Biochemistry. 1988 Mar 22;27(6):2033–2039. doi: 10.1021/bi00406a033. [DOI] [PubMed] [Google Scholar]
- Wardlaw J. R., Sawyer W. H., Ghiggino K. P. Vertical fluctuations of phospholipid acyl chains in bilayers. FEBS Lett. 1987 Oct 19;223(1):20–24. doi: 10.1016/0014-5793(87)80502-1. [DOI] [PubMed] [Google Scholar]
- Zachariasse K. A., Vaz W. L., Sotomayor C., Kühnle W. Investigation of human erythrocyte ghost membranes with intramolecular excimer probes. Biochim Biophys Acta. 1982 Jun 14;688(2):323–332. doi: 10.1016/0005-2736(82)90343-1. [DOI] [PubMed] [Google Scholar]