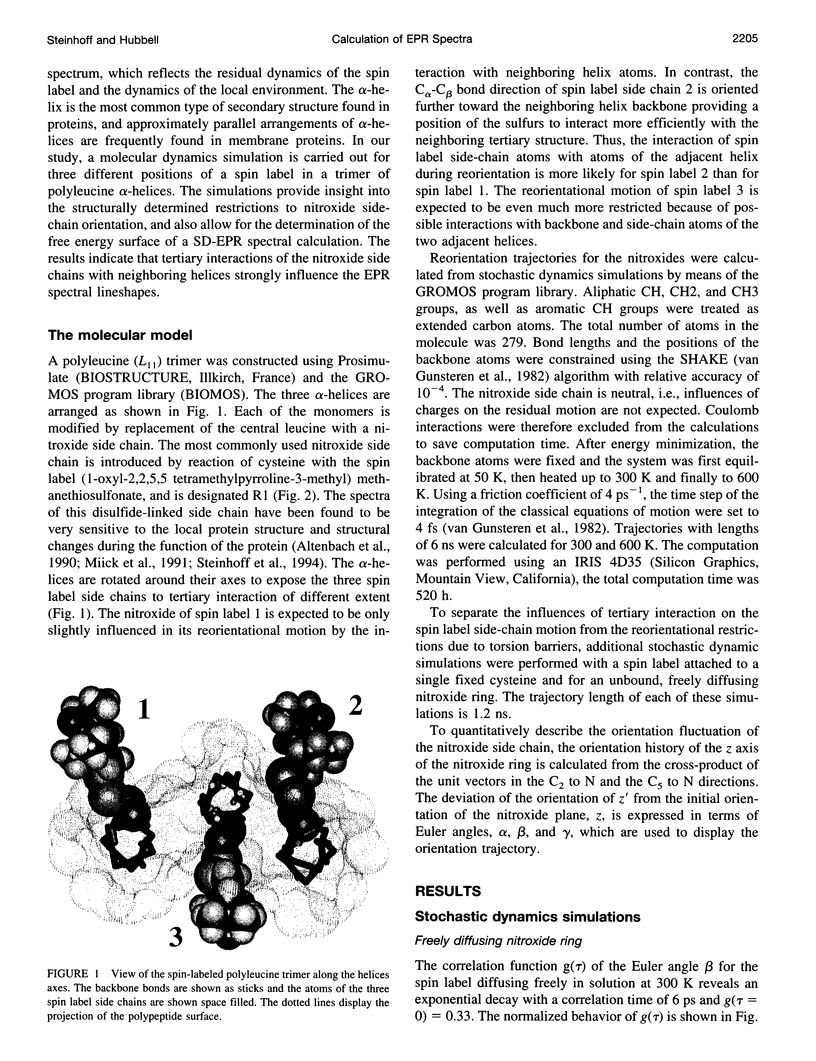
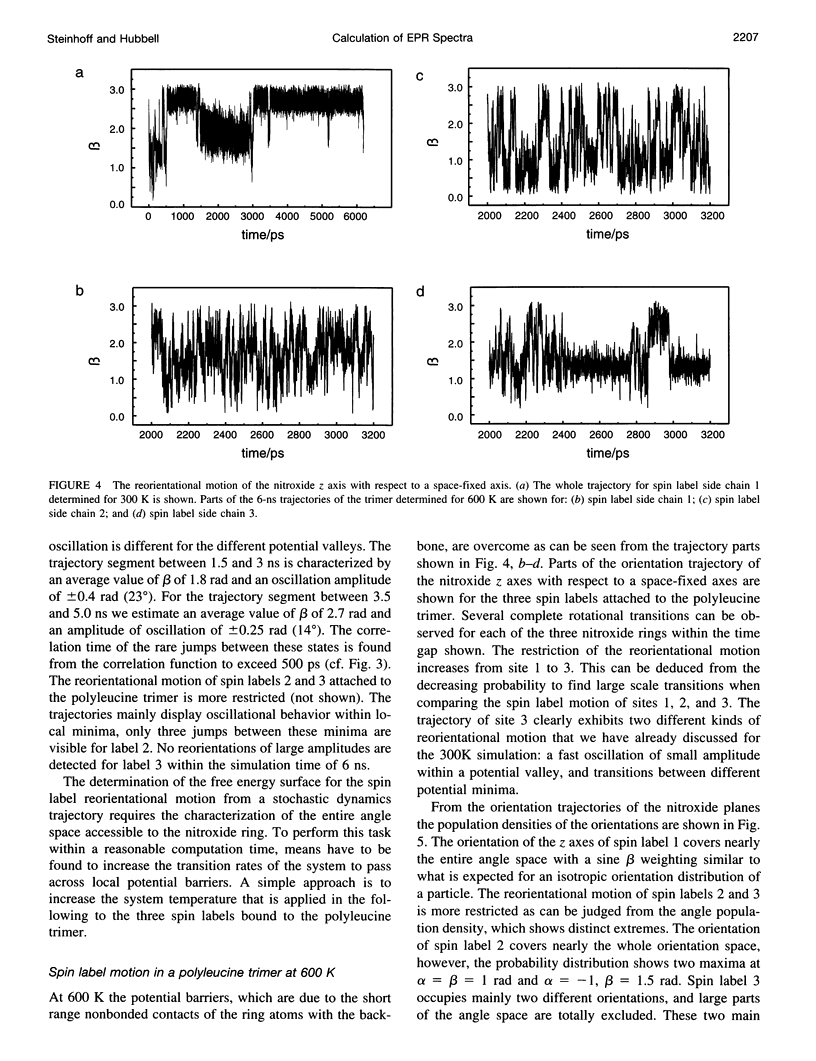
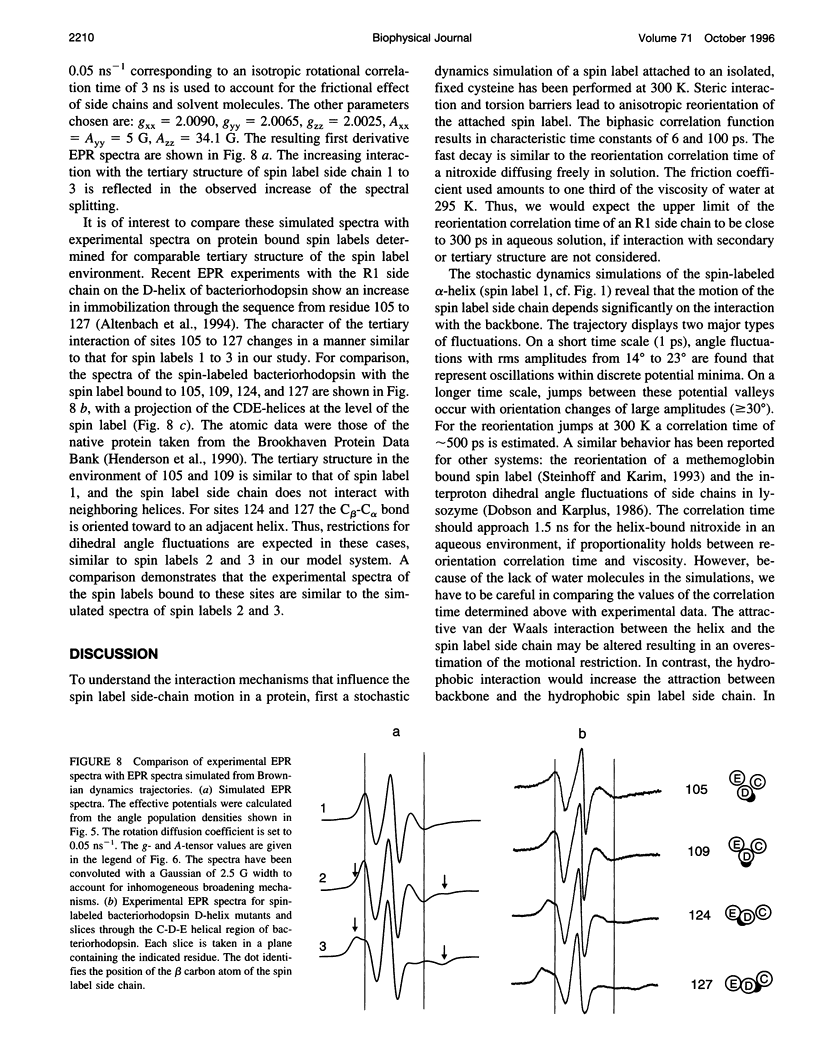
Abstract
We present a method to simulate electron paramagnetic resonance spectra of spin-labeled proteins that explicitly includes the protein structure in the vicinity of the attached spin label. The method is applied to a spin-labeled polyleucine alpha-helix trimer. From short (6 ns) stochastic dynamics simulations of this trimer, an effective potential energy function is calculated. Interaction with secondary and tertiary structures determine the reorientational motion of the spin label side chains. After reduction to a single particle problem, long stochastic dynamic trajectories (700 ns) of the spin label side-chain reorientation are calculated from which the Lamor frequency trajectory and subsequently the electron paramagnetic resonance spectrum is determined. The simulated spectra agree well with experimental electron paramagnetic resonance spectra of bacteriorhodopsin mutants with spin labels in similar secondary and tertiary environments as in the polyleucine.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenbach C., Greenhalgh D. A., Khorana H. G., Hubbell W. L. A collision gradient method to determine the immersion depth of nitroxides in lipid bilayers: application to spin-labeled mutants of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1667–1671. doi: 10.1073/pnas.91.5.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenbach C., Marti T., Khorana H. G., Hubbell W. L. Transmembrane protein structure: spin labeling of bacteriorhodopsin mutants. Science. 1990 Jun 1;248(4959):1088–1092. doi: 10.1126/science.2160734. [DOI] [PubMed] [Google Scholar]
- Brooks C. L., 3rd Methodological advances in molecular dynamics simulations of biological systems. Curr Opin Struct Biol. 1995 Apr;5(2):211–215. doi: 10.1016/0959-440x(95)80078-6. [DOI] [PubMed] [Google Scholar]
- Ge M., Freed J. H. An electron spin resonance study of interactions between gramicidin A' and phosphatidylcholine bilayers. Biophys J. 1993 Nov;65(5):2106–2123. doi: 10.1016/S0006-3495(93)81255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelin B. R., Karplus M. Side-chain torsional potentials: effect of dipeptide, protein, and solvent environment. Biochemistry. 1979 Apr 3;18(7):1256–1268. doi: 10.1021/bi00574a022. [DOI] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Miick S. M., Casteel K. M., Millhauser G. L. Experimental molecular dynamics of an alanine-based helical peptide determined by spin label electron spin resonance. Biochemistry. 1993 Aug 10;32(31):8014–8021. doi: 10.1021/bi00082a024. [DOI] [PubMed] [Google Scholar]
- Miick S. M., Todd A. P., Millhauser G. L. Position-dependent local motions in spin-labeled analogues of a short alpha-helical peptide determined by electron spin resonance. Biochemistry. 1991 Oct 1;30(39):9498–9503. doi: 10.1021/bi00103a016. [DOI] [PubMed] [Google Scholar]
- Steinhoff H. J. A simple method for determination of rotational correlation times and separation of rotational and polarity effects from EPR spectra of spin-labeled biomolecules in a wide correlation time range. J Biochem Biophys Methods. 1988 Dec;17(4):237–247. doi: 10.1016/0165-022x(88)90047-4. [DOI] [PubMed] [Google Scholar]
- Steinhoff H. J., Kramm B., Hess G., Owerdieck C., Redhardt A. Rotational and translational water diffusion in the hemoglobin hydration shell: dielectric and proton nuclear relaxation measurements. Biophys J. 1993 Oct;65(4):1486–1495. doi: 10.1016/S0006-3495(93)81217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff H. J., Mollaaghababa R., Altenbach C., Hideg K., Krebs M., Khorana H. G., Hubbell W. L. Time-resolved detection of structural changes during the photocycle of spin-labeled bacteriorhodopsin. Science. 1994 Oct 7;266(5182):105–107. doi: 10.1126/science.7939627. [DOI] [PubMed] [Google Scholar]
- Steinhoff H. J., Mollaaghababa R., Altenbach C., Khorana H. G., Hubbell W. L. Site directed spin labeling studies of structure and dynamics in bacteriorhodopsin. Biophys Chem. 1995 Sep-Oct;56(1-2):89–94. doi: 10.1016/0301-4622(95)00019-t. [DOI] [PubMed] [Google Scholar]
- Steinhoff H. J. Residual motion of hemoglobin-bound spin labels and protein dynamics: viscosity dependence of the rotational correlation times. Eur Biophys J. 1990;18(1):57–62. doi: 10.1007/BF00185420. [DOI] [PubMed] [Google Scholar]
- Steinhoff H., Lieutenant K., Schlitter J. Residual motion of hemoglobin-bound spin labels as a probe for protein dynamics. Z Naturforsch C. 1989 Mar-Apr;44(3-4):280–288. doi: 10.1515/znc-1989-3-417. [DOI] [PubMed] [Google Scholar]
- Todd A. P., Cong J., Levinthal F., Levinthal C., Hubbell W. L. Site-directed mutagenesis of colicin E1 provides specific attachment sites for spin labels whose spectra are sensitive to local conformation. Proteins. 1989;6(3):294–305. doi: 10.1002/prot.340060312. [DOI] [PubMed] [Google Scholar]
- Todd A. P., Millhauser G. L. ESR spectra reflect local and global mobility in a short spin-labeled peptide throughout the alpha-helix----coil transition. Biochemistry. 1991 Jun 4;30(22):5515–5523. doi: 10.1021/bi00236a026. [DOI] [PubMed] [Google Scholar]



