Abstract
PURPOSE:
Hypomagnesemia is a common side effect of platinum-based chemotherapy and predicts poor overall survival in some cancers. Standard magnesium replacement strategies are often inadequate for maintaining magnesium levels. We hypothesized that a daily dietary magnesium replacement approach through magnesium-rich foods would help maintain adequate magnesium levels during platinum-based treatment.
METHODS:
We conducted a prospective feasibility study of magnesium-rich diets in patients aged ≥18 years with previously untreated ovarian cancer scheduled to receive carboplatin-containing chemotherapy of at least 6 consecutive cycles. Education about magnesium-rich diets was provided at enrollment and then weekly during chemotherapy. Feasibility was defined as ≥60% completion of dietary recalls and ≥280 mg average daily dietary magnesium intake across all patients.
RESULTS:
Twenty-one of 26 patients enrolled completed at least 5 chemotherapy cycles and were included in the analysis. Adherence to the study diet was 76%. Daily dietary magnesium intake was 100.5 mg at baseline and increased throughout each cycle: 6% of patients at baseline, 24% after the first cycle, and 67% after the fifth cycle reached ≥280-mg/day magnesium intake. Seven (33%) of 21 had at least one incident of hypomagnesemia. Patients who were adherent had significantly lower incidence of hypomagnesemia (19% vs 80%, P=0.03) and less need for intravenous magnesium (6% vs 60%, P=0.03) than those who were nonadherent.
CONCLUSIONS:
The study achieved primary feasibility objectives of retention and adherence to the study intervention. Weekly education about magnesium-rich diets was effective in increasing dietary magnesium intake. Adequate dietary magnesium appeared to be protective against hypomagnesemia.
Keywords: Oncologic nutrition, nutritional support, hypomagnesemia, ovarian cancer, chemotherapy side effects
1. Introduction
Hypomagnesemia (serum magnesium level <1.8 mg/dL) is a common side effect of platinum-based chemotherapy. The incidence of hypomagnesemia is nearly 100% after the sixth cycle of cisplatin-based chemotherapy. Carboplatin causes less severe hypomagnesemia than cisplatin.1,2 In a retrospective study, 84% of patients with ovarian cancer who received carboplatin-based chemotherapy had at least one incident of hypomagnesemia during the treatment.3 Direct nephrotoxicity resulting in renal magnesium wasting is the main underlying mechanism of platinum-associated hypomagnesemia.4,5 Hypomagnesemia and compromised renal magnesium uptake, in turn, have an additive effect on cisplatin-induced renal toxicity in animal models.6,7 A vicious cycle of hypomagnesemia, renal toxicity, and further hypomagnesemia can develop if hypomagnesemia is not effectively remedied. In the retrospective study noted above, frequent occurrence of hypomagnesemia during carboplatin-based treatments was a strong predictor of poor overall survival, independent of International Federation of Gynecology and Obstetrics (FIGO) stage and completeness of tumor reduction.3 This association of platinum-induced hypomagnesemia with poor survival was replicated in patients with squamous cell carcinoma of the head and neck who had received concurrent chemoradiation with cisplatin or carboplatin independent of age, cancer type (human papillomavirus-related or unrelated), Eastern Cooperative Oncology Group performance status, and smoking history.8
When hypomagnesemia is detected at each treatment cycle, the current standard magnesium replacement regimen is intravenous magnesium sulfate. This intervention may be followed by oral magnesium salts, such as magnesium oxide or magnesium amino acids chelate, to prevent recurrent hypomagnesemia.9 However, this regimen has not been proven to maintain normal magnesium levels during the treatment intervals.3,9 High-dose intravenous magnesium often results in an abrupt elevation of the plasma magnesium concentration, which partially inhibits magnesium reabsorption in the loop of Henle and exacerbates renal magnesium leak.9 Up to 50% of the infused magnesium is excreted in the urine.10 Thus, the correction of magnesium level by intravenous magnesium is temporary and usually does not last beyond 72 hours.9 More frequent blood tests and infusions are a substantial inconvenience for patients and may negatively impact their quality of life. In addition, oral supplementation with magnesium salts carries a low bioavailability rate (33%) because intestinal magnesium absorption principally occurs in the small bowel through a saturable transport system.11 The remaining unabsorbed magnesium results in osmotic diarrhea, often making oral supplementation intolerable.9,12 Therefore, novel strategies are called for to provide adequate magnesium repletion and sustained correction of hypomagnesemia.
Magnesium is widely available from food sources such as legumes, nuts, whole grains, and vegetables, which makes the recommended daily intake of 400 mg readily attainable.13,14 In a study of healthy subjects, magnesium absorption from almonds, a naturally magnesium-rich food, was equal to that from soluble magnesium acetate and significantly better than that from enteric-coated magnesium chloride (Slow-Mag).15 Magnesium intake through food is likely better tolerated by patients than oral magnesium medications because the wide variety of magnesium-rich foods allows the dietary approach to be adjusted to reduce the occurrence of diarrhea without compromising magnesium repletion.
Despite our knowledge of food sources rich in magnesium and the frequent presence of hypomagnesemia related to many antineoplastic therapies, dietary recommendations for the prevention and management of hypomagnesemia are not common and should be further explored. The current study assessed the feasibility of dietary replacement of magnesium for prevention of hypomagnesemia in patients with ovarian cancer receiving carboplatin-based chemotherapy and explored the association of dietary magnesium intake with development of hypomagnesemia. We hypothesized that dietary magnesium replacement through daily consumption of magnesium-rich foods is feasible and would provide constant magnesium replacement and maintain a more stable magnesium level.
2. Materials and Methods
2.1. Patient selection and study design
This prospective study was approved by The University of Texas MD Anderson Cancer Center Institutional Review Board in accordance with an assurance filed with, and approved by, the Department of Health and Human Services. Patients with a carboplatin chemotherapy schedule were identified through an institutional electronic schedule and screened for a new diagnosis of ovarian cancer. Eligible patients were primarily recruited from the Gynecologic Oncology Center or Ambulatory Treatment Center at MD Anderson. Inclusion criteria were as follows: 1) diagnosed with ovarian cancer, 2) aged 18 years or older, 3) planning to undergo carboplatin-based chemotherapy and tumor-reductive surgery, and 4) able to tolerate an oral diet. Exclusion criteria were as follows: 1) prior platinum-based chemotherapy, 2) serum creatinine level >1.4 mg/dL prior to the first cycle of treatment, or 3) artificial nutrition accounting for >50% of total calorie intake.
Informed consent was obtained by research assistants before or at the time the patients arrived for their first cycle of carboplatin-based chemotherapy. Patients were provided with a food reference list based on United States Department of Agriculture Food Composition Databases13 and instructed to consume at least 400 mg daily of elemental magnesium from food.14 Detailed explanations and handouts with information about food names, processing method (cooked or raw), serving size, and quantity of elemental magnesium per serving were provided (Table 1).13 The patients were encouraged to eat whole grains and legumes daily for at least part of the total dietary magnesium intake to avoid overconsumption of one particular food group, such as nuts. The overall dietary structure, such as total calorie and protein intake, followed American Institute for Cancer Research dietary guidelines for cancer patients. Adherence to the dietary regimen was monitored and reinforced via weekly phone calls, online communications, or interviews during the patients’ chemotherapy visits, until the end of sixth cycle of treatments (15 total dietary entries).
Table 1. Magnesium daily intake source and serving size information provided to patients, based on the United States Department of Agriculture National Nutrient Database for Standard Reference Release 28.
The recommended magnesium daily intake is ≥400 mg, and the foods shown in boldface are recommended daily to ensure adequate magnesium intake. Patients are also instructed to consume at least 2 cereal or grain servings per day, at least 1 legume serving per day, and at least 2 vegetable servings per day.
| Food source | Serving size | Magnesium, mg |
|---|---|---|
| Dairy, drinks, additives | ||
| Soymilk, unfortified | 1 cup (8 oz) | 61 |
| Coconut water | 1 cup (8 oz) | 60 |
| Molasses, unsulphured, blackstrap | 1 tablespoon | 51 |
| Yogurt, low fat, plain | 1 container (6 oz) | 29 |
| Nuts and seeds | ||
| Hemp seed, hulled | 3 tablespoons | 210 |
| Pumpkin seeds, roasted | ¼ cup | 162 |
| Flax seed, whole | ¼ cup | 160 |
| Chia seed | 3 tablespoons | 133 |
| Almonds, roasted | ¼ cup | 96 |
| Cashews, roasted | ¼ cup | 89 |
| Vegetables | ||
| Spinach, cooked, drained | 1 cup | 157 |
| Chard, Swiss, boiled | 1 cup | 150 |
| Okra, cooked | 1 cup | 88 |
| Turnip greens, cooked, drained | 1 cup | 43 |
| Broccoli, boiled, drained | 1 cup | 32 |
| Grains/cereal | ||
| Wheat germ, toasted, plain | 2 tablespoons (1 oz) | 91 |
| Bran flakes, plain | ¾ cup | 69 |
| Oatmeal, unenriched, cooked | 1 cup | 63 |
| Amaranth grain, cooked | 1 cup | 160 |
| Quinoa, cooked | 1 cup | 118 |
| Pasta, 51% whole wheat, cooked | 1 cup | 116 |
| Buckwheat groats, cooked | 1 cup | 86 |
| Brown rice, cooked | 1 cup | 86 |
| Bread, whole-wheat | 1 slice (1 oz) | 28 |
| Rolls, dinner, whole-wheat | 1 roll (1 oz) | 24 |
| Legumes | ||
| Soybean, boiled | ½ cup | 74 |
| Black beans, boiled | ½ cup | 60 |
| Beans, white, boiled | ½ cup | 57 |
| Mung beans, boiled | ½ cup | 49 |
| Beans, navy, boiled | ½ cup | 48 |
| Beans, pinto, boiled | ½ cup | 43 |
| Beans, lima, boiled | ½ cup | 41 |
| Beans, kidney, boiled | ½ cup | 40 |
| Chickpeas, boiled | ½ cup | 40 |
| Beans, fava, boiled | ½ cup | 37 |
| Split peas, boiled | ½ cup | 36 |
| Lentils, boiled | ½ cup | 36 |
Patient demographic data, including age and race; International Federation of Gynecology and Obstetrics (FIGO) stage; and pharmacy data, including chemotherapy regimen (dose and cycles) and magnesium supplementation (dose, route, and time), were extracted from medical records. Laboratory tests were routinely conducted before each treatment cycle and included complete blood counts (sodium, potassium, chloride, bicarbonate, blood urea nitrogen, creatinine, magnesium, calcium, phosphorus, and glucose levels) and liver function tests (total protein, albumin, alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, and total bilirubin). All tests were carried out by MD Anderson laboratories at the main campus and regional satellites.
Hypomagnesemia was defined as serum magnesium level <1.8 mg/dL (or <0.74 mmol/L). The primary oncology team managed all electrolyte abnormalities according to standard practice.
2.2. Statistical analysis
Our primary objective was to determine the feasibility of the intervention. Patient retention rate is defined as the percentage of dietary recalls completed by patients, and dietary adherence rate is defined as the ratio of actual dietary magnesium intake to the desired 400 mg. Feasibility in terms of retention will be achieved if the average dietary recall completion rate is ≥60% (number of dietary entries divided by 15). Feasibility in terms of adherence to the dietary intervention will be achieved if the average dietary magnesium intake across all patients is ≥70% of the instructed 400 mg dietary magnesium (280 mg). Patient adherence is defined as completing ≥60% of the dietary recalls and achieving an average daily dietary magnesium intake of at least 280 mg. This pre-specified intake of dietary magnesium is an estimation based on the Institute of Medicine Estimated Average Requirement of magnesium of 265 mg/day for women ages 51 to 70 years.16
Descriptive statistics (frequencies, proportions, means, standard deviations, and ranges), along with 95% confidence intervals for the means, were computed for the measures of interest for all primary objectives, which included retention and dietary adherence rates, and secondary objectives, which included occurrence of hypomagnesemia and the need for a pharmacy intervention with oral and intravenous magnesium dosage. Mean (and standard deviation) dietary magnesium intake by chemotherapy cycle was calculated. Baseline is before the first cycle of treatment; cycle 1 denotes the weeks between the first and the second cycle of treatments, and so on. Patient characteristics were compared between those who were adherent and those who were not adherent using the Wilcoxon rank-sum test for continuous variables and Fisher’s exact test for categorical variables. The Spearman correlation coefficient was obtained to evaluate the correlation between mean serum magnesium and mean magnesium intake, and a scatter plot was generated along with a fitted linear regression line (and an estimated Pearson correlation coefficient). P < 0.05 was considered statistically significant. SAS 9.4 (SAS Institute INC, Cary, NC) was used for statistical analysis.
3. Results
3.1. Patient demographic and clinic characteristics
The study recruited 26 of 28 patients who were approached. Twenty-one of the 26 patients (81%) completed at least 5 cycles of the planned carboplatin-based treatments and were included in our analysis (considering hypomagnesemia a dose-dependent side effect of carboplatin). Table 2 summarizes patient clinical and demographic characteristics. The mean age was 60 years (standard deviation, 13). Most patients (95%) had advanced ovarian cancer (FIGO stage II and higher). The mean retention rate for completing dietary recalls was 87% (IQR, 80%−100%). Sixteen patients (76%) were adherent to the instructed dietary magnesium intake, i.e., mean daily dietary magnesium intake of ≥ 280 mg.
Table 2.
Patient clinical and demographic characteristics for those who completed at least 5 chemotherapy cycles (n = 21).
| Continuous variable | Mean ± SD |
|---|---|
| Age at first chemotherapy cycle (years) | 60 ± 13 |
| Categorical variable | No. (%) |
| Race | |
| Asian | 2 (9) |
| Black | 4 (19) |
| White | 14 (67) |
| Other | 1 (5) |
| Stage | |
| Early stage | 1 (5) |
| Advanced stage (stage II and higher) | 20 (95) |
3.2. Dietary magnesium intake
Dietary magnesium intake increased over time (Table 3). The baseline mean magnesium intake was 100.5mg/day, with only 1 patient reporting ≥ 280 mg/day magnesium intake. However, after 12 weekly dietary counseling and reinforcement interventions, 67% of patients achieved at least 280 mg/day magnesium intake after the fifth cycle of chemotherapy.
Table 3.
Mean dietary magnesium intake by chemotherapy cycle*.
| Chemotherapy cycle | No. | Magnesium intake, mg | Patients with daily dietary magnesium intake ≥280 mg, %† | |||
|---|---|---|---|---|---|---|
| Mean | SD | Minimum | Maximum | |||
| Baseline | 16 | 100.53 | 101.88 | 0.00 | 347.67 | 6% |
| 1 | 21 | 299.64 | 153.87 | 0.00 | 664.69 | 24% |
| 2 | 19 | 437.37 | 185.08 | 64.50 | 805.67 | 42% |
| 3 | 18 | 375.25 | 202.60 | 0.00 | 785.34 | 44% |
| 4 | 20 | 396.52 | 220.46 | 0.00 | 936.80 | 60% |
| 5 | 18 | 428.30 | 230.33 | 132.00 | 956.00 | 67% |
| 6 | 3 | 244.72 | 224.62 | 0.00 | 441.50 | 33% |
Baseline is before the first cycle of treatment; cycle 1 denotes the weeks between the first and the second cycle of treatments, and so on.
For patients who turned in their recall diaries for that chemotherapy cycle.
3.3. Correlation between dietary magnesium intake and serum magnesium levels
Figure 1 illustrates the linear correlation between mean dietary magnesium intake and mean serum magnesium levels (Pearson correlation = 0.40, P = 0.07). A moderate positive correlation (Spearman correlation = 0.48, P = 0.03) was observed between the mean serum magnesium level and the mean dietary magnesium intake.
Figure 1.
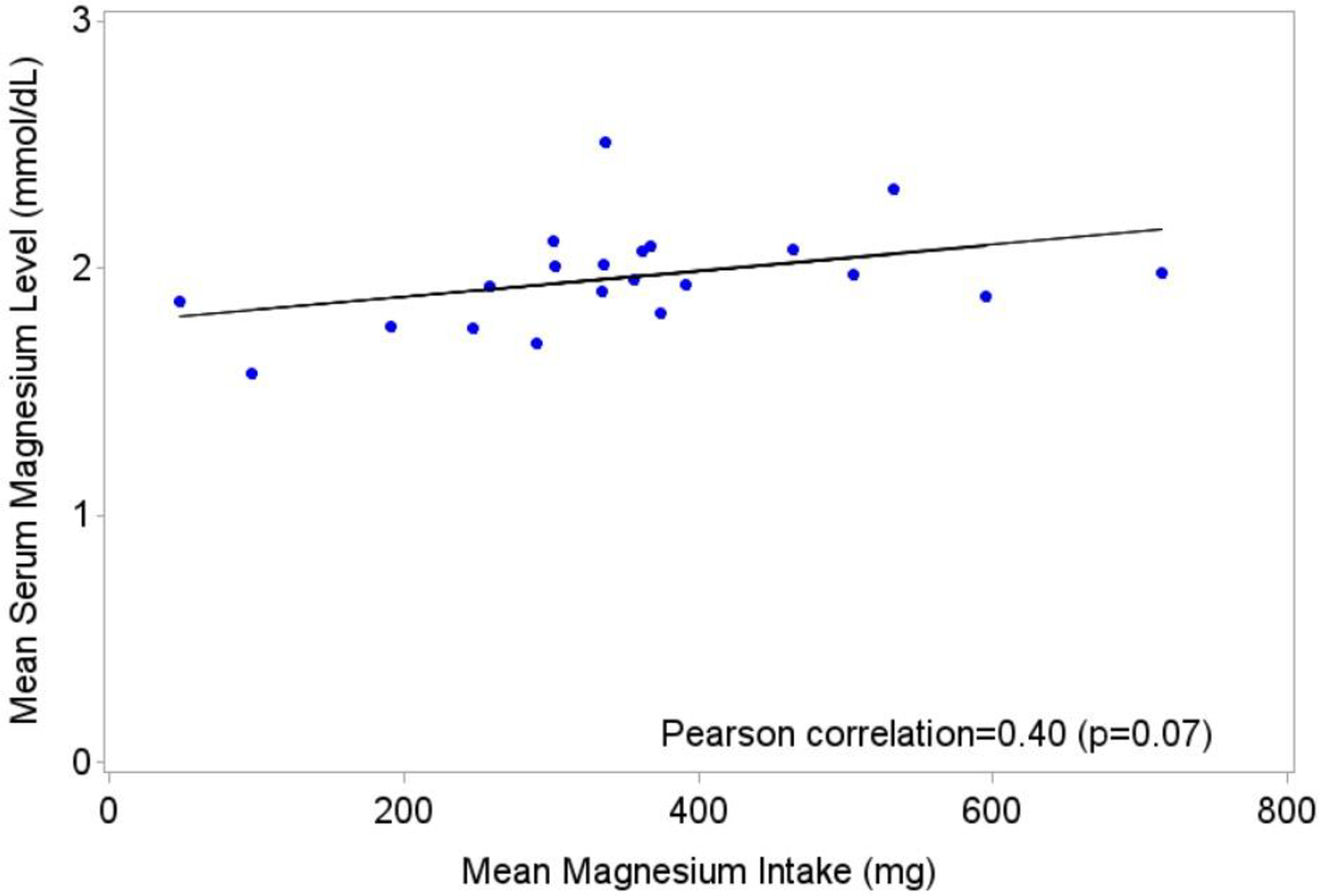
Linear regression of mean serum magnesium level by mean magnesium intake, calculated from dietary diaries.
3.4. Occurrence of hypomagnesemia and need of magnesium supplementation
Table 4 shows associations between adherence to the study intervention and occurrence of hypomagnesemia. Patients who completed ≥60% dietary recalls and achieved ≥280 mg average daily dietary magnesium intake (i.e., were considered adherent to the intervention) had a significantly lower incidence of hypomagnesemia (P = 0.03) and significantly lower use of intravenous magnesium infusions (P = 0.03) than those who were not adherent. No significant difference in the use of oral magnesium supplementation was observed. Mean serum magnesium level was significantly higher for adherent patients than for nonadherent patients (P = 0.009). A higher percentage of adherent patients completed six cycles of carboplatin chemotherapy compared to those not adherent (P = 0.429).
Table 4.
Patient characteristics by adherence to the study intervention.
| Variable | Nonadherent, n=5 | Adherent,* n=16 | P |
|---|---|---|---|
| Mean ± SD age at first chemotherapy cycle (in years) | 61 ± 11 | 60 ± 13 | 0.967 |
| Hypomagnesemia, no. (%) | 4 (80) | 3 (19) | 0.025 |
| Receipt of oral magnesium, no. (%) | 1 (20) | 1 (6) | 0.429 |
| Receipt of intravenous magnesium, no. (%) | 3 (60) | 1 (6) | 0.028 |
| Completed chemotherapy cycles, no. (%) | |||
| 5 | 1 (20) | 1 (6) | 0.429 |
| 6 | 4 (80) | 15 (94) | |
| Mean ± SD serum magnesium level (in mg) | 1.78 ± 0.14 | 2.02 ±0.19 | 0.009 |
Patients considered adherent to the study intervention had a mean dietary magnesium level of 280 mg or higher and diary adherence rate (number of diaries completed divided by 15) of 60% or higher.
4. Discussion
Hypomagnesemia is a frequent side effect of platinum-based treatments. The current practice of magnesium replacement has limited efficacy and sustainability in mitigating hypomagnesemia. Diet, as a daily life behavior, is an excellent opportunity for stable supplementation of a specific nutrient. However, dietary interventions that aim to prevent specific chemotherapy toxicities are not commonly practiced, and this may be partly due to a lack of clinical trials. The current dietary guidelines for cancer patients provide general guidance on caloric and protein requirements without individualization to specific cancer populations and related cancer treatments. Following nutritional guidelines alone is inadequate for patients with ovarian cancer who suffer from platinum-induced hypomagnesemia. To the best of our knowledge, the current study provides the first example of a dietary approach that targets a specific chemotherapy toxicity. Our study intervention was innovative and comprehensive in that it integrated information from the Recommended Daily Allowance of nutrients and United States Department of Agriculture Food Composition Databases into patients’ daily food choices. We believe this novel approach will add a new dimension to the current standard regimen for hypomagnesemia.
The study had high recruitment, retention, and dietary adherence rates and therefore achieved its primary objective of feasibility of the magnesium-rich dietary intervention. The racial profile of the study participants was consistent with that of our institution’s patient population with ovarian cancer. Our findings suggest that our dietary protocol was broadly accepted by patients and their oncologists. Dietary and lifestyle approaches to manage health conditions have become more appealing to cancer patients and the general population.17,18 A dietary guide to maintain and replete magnesium had not been researched or established for the population in need. Considering the essential role of magnesium in many disease processes, this clinical innovation may have broad applications that go beyond the cancer population.
The study intervention was intensive, with dietary education provided weekly. A national survey of dietary habits revealed generally inadequate dietary magnesium intake in US women (117–237 mg/day).19 Low dietary magnesium intake and/or hypomagnesemia is linked to the risk of many medical conditions, including diabetes, coronary artery disease, ischemic stroke, and cancer.20–22 Consistent with the national dietary survey, our patients’ baseline dietary magnesium intake was 100.5 mg/day. A knowledge deficit in nutrition is likely common among our cancer patients. Our weekly education on the magnesium-rich diet appeared effective in increasing dietary magnesium intake over time, with most patients achieving magnesium intake of more than 280 mg/day. Intensive education about dietary behavioral modifications is necessary for cancer patients. While a dietary magnesium intake exceeding 280 mg/day appears effective in averting hypomagnesemia among our patients undergoing carboplatin chemotherapy, it might not be sufficient to rectify the underlying issue of deficient magnesium reserves. Determining the optimal dietary magnesium level and intervention duration for individuals with ovarian cancer necessitates further research, which surpasses the scope of our current study.
Only 33% of participants in our study experienced any incident of hypomagnesemia. This rate is substantially lower than our historical report of 84% in the same patient population.3 The positive correlation of dietary magnesium intake and serum magnesium levels, as well as lower incidence of hypomagnesemia in patients who were adherent to the study intervention, indicates that dietary magnesium replacement is very likely effective in preventing platinum-induced hypomagnesemia. The adherent patients also, as hypothesized, had less need for magnesium infusion therapy. Our study also showed a higher percentage of adherent patients, though not statistically significant, completed all six cycles of planned carboplatin treatments compared with those who were nonadherent. Effective mitigation of chemotherapy side effects may prevent interruptions or premature termination of treatments which critically affect cancer outcomes.23 Platinum-induced hypomagnesemia is not only a prominent predictor of overall survival in several cancers3,8 but also mechanistically associated with long-term side effects of cancer treatments, such as osteonecrosis24,25 and osteoradionecrosis.26 Hypomagnesemia causes a wide range of clinical manifestations, from subtle and nonspecific signs and symptoms, such as loss of appetite, nausea, vomiting, fatigue, neuropathy, and weakness,27 to life-threatening arrhythmias. In addition, magnesium is crucial to a wide range of biochemical reactions required for energy metabolism, as well as cell proliferation, differentiation, angiogenesis, and apoptosis. The benefit of proactively preventing hypomagnesemia goes beyond reducing hypomagnesemia-related symptoms in patients undergoing platinum-based therapy because adequate and effective magnesium supplementation will in all probability improve patient outcomes. Our current practice of managing platinum-induced hypomagnesemia lacks effective preventive measures. Evidence on the efficacy of oral magnesium supplements in preventing hypomagnesemia is scarce. However, despite oral and/or intravenous magnesium replacement, typically over 80% of patients still experience hypomagnesemia.3 Magnesium replacement through daily food choices is a much-needed addition to the current practice.
The main limitation of this pilot feasibility trial was that it used a single-arm design, and thus the true incidence of hypomagnesemia in a control group could not be determined. We also do not know the effects of just providing the dietary information only at the start of treatment without further support. The trial was both small and limited in duration. Several critical clinical factors, including gastrointestinal loss, renal disease, medications, and other medical or treatment factors not assessed could potentially affect magnesium retention and treatment tolerance in patients. Consequently, these preliminary findings on the efficacy of the magnesium-rich dietary intervention need to be interpreted with caution. Future studies should incorporate these influential clinical factors to validate the advantages of maintaining magnesium levels through dietary means for the completion of chemotherapy. Future large randomized controlled trials are needed to confirm the efficacy of a magnesium-rich diet in preventing platinum-induced hypomagnesemia and to investigate the impact of effective mitigation of hypomagnesemia on quality of life, disease free survival and overall survival in cancer patients receiving platinum-based treatments.
5. Conclusions
The current study achieved the primary feasibility objectives of retention and adherence to a magnesium-rich dietary intervention. Weekly education about magnesium-rich foods was effective and likely necessary in increasing dietary magnesium intake. Adequate dietary magnesium intake appeared to be protective against occurrence of hypomagnesemia and the need for medical intervention. Future large randomized controlled trials are needed to investigate the effects of dietary magnesium replacement on clinical outcomes in cancer patients.
CONTEXT.
Key objective:
To assess the feasibility of a magnesium-rich dietary approach to prevent carboplatin-induced hypomagnesemia while investigating the link between dietary magnesium intake and the development of hypomagnesemia.
Knowledge generated:
Adherence to the magnesium-rich dietary intervention was feasible and effective at increasing dietary magnesium intake, revealing a positive correlation between magnesium dietary intake and mean serum magnesium levels. Patients who were adherent to the magnesium-rich diet had significantly lower incidence of hypomagnesemia and less need for intravenous magnesium than those who were nonadherent. Regular weekly education on a magnesium-rich diet is likely necessary to enhance and maintain adequate dietary magnesium intake.
Relevance:
Current practices of magnesium replacement have limitations in addressing hypomagnesemia, a common side effect of cancer treatments. Leveraging diet, a daily behavior, to support magnesium-rich foods presents an opportunity for consistent supplementation of specific nutrients. This study exemplifies a feasible dietary strategy to prevent or mitigate platinum-induced hypomagnesemia.
Acknowledgement:
The study abstract was presented at the 20th International Conference of the Society of Integrative Medicine in Banff, Canada, September 16, 2023.
Funding:
Supported in part by the Cancer Center Support Grant (NCI Grant P30 CA016672).
Footnotes
Conflicts of interest: The authors declare no potential conflicts of interest.
Ethics approval: This prospective study was approved by The University of Texas MD Anderson Cancer Center Institutional Review Board in accordance with an assurance filed with, and approved by, the Department of Health and Human Services.
Patient consent: Patients provided written informed consent to participate in this study.
REFERENCES
- 1.Hodgkinson E, Neville-Webbe HL, Coleman RE. Magnesium depletion in patients receiving cisplatin-based chemotherapy. Clinical Oncology. 2006;18(9):710–718. [DOI] [PubMed] [Google Scholar]
- 2.English MW, Skinner R, Pearson AD, Price L, Wyllie R, Craft AW. Dose-related nephrotoxicity of carboplatin in children. British Journal of Cancer. 1999;81(2):336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu W, Qdaisat A, Soliman PT, et al. Hypomagnesemia and survival in patients with ovarian cancer who received chemotherapy with carboplatin. The Oncologist. 2019;24(6):e312–e317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arany I, Safirstein RL. Cisplatin nephrotoxicity. Seminars in Nephrology. 2003;23(5):460–464. [DOI] [PubMed] [Google Scholar]
- 5.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney International. 2008;73(9):994–1007. [DOI] [PubMed] [Google Scholar]
- 6.Lajer H, Kristensen M, Hansen HH, et al. Magnesium depletion enhances cisplatin-induced nephrotoxicity. Cancer Chemotherapy and Pharmacology. 2005;56(5):535–542. [DOI] [PubMed] [Google Scholar]
- 7.Saito Y, Okamoto K, Kobayashi M, Narumi K, Yamada T, Iseki K. Magnesium attenuates cisplatin-induced nephrotoxicity by regulating the expression of renal transporters. European Journal of Pharmacology. 2017;811:191–198. [DOI] [PubMed] [Google Scholar]
- 8.Liu W, Qdaisat A, Ferrarotto R, et al. Hypomagnesemia and survival in patients with head and neck cancers who received primary concurrent chemoradiation. Cancer. 2021;127:528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang DM, Dennis K, Steinmetz A, et al. Management of epidermal growth factor receptor inhibitor-induced hypomagnesemia: a systematic review. Clinical Colorectal Cancer. 2016;15(3):e117–123. [DOI] [PubMed] [Google Scholar]
- 10.Hebert P, Mehta N, Wang J, Hindmarsh T, Jones G, Cardinal P. Functional magnesium deficiency in critically ill patients identified using a magnesium-loading test. Critical Care Medicine. 1997;25(5):749–755. [DOI] [PubMed] [Google Scholar]
- 11.Schweigel M, Martens H. Magnesium transport in the gastrointestinal tract. Frontiers in Bioscience. 2000;5:D666–677. [DOI] [PubMed] [Google Scholar]
- 12.Fine KD, Santa Ana CA, Fordtran JS. Diagnosis of magnesium-induced diarrhea. The New England Journal of Medicine. 1991;324(15):1012–1017. [DOI] [PubMed] [Google Scholar]
- 13.United States Department of Agriculture Agricultural Research service. USDA Food Composition Databases. https://ndb.nal.usda.gov/ndb/nutrients/index. Accessed March 2018.
- 14.Institute of Medicine Food and Nutrition Board. Dietary Reference Intakes: Calcium, Phosphorus, Magnesium, Vitamin D and Fluoride. Washington, DC: National Academy Press, 1997. [PubMed] [Google Scholar]
- 15.Fine KD, Santa Ana CA, Porter JL, Fordtran JS. Intestinal absorption of magnesium from food and supplements. The Journal of Clinical Investigation. 1991;88(2):396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington (DC): National Academies Press (US); 1997. [PubMed] [Google Scholar]
- 17.Mao JJ, Palmer CS, Healy KE, Desai K, Amsterdam J. Complementary and alternative medicine use among cancer survivors: a population-based study. Journal of Cancer Survivorship. 2011;5(1):8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke TC, Black LI, Stussman BJ, et al. : Trends in the use of complementary health approaches among adults: United States, 2002–2012. Natl Health Stat Report 79:1–16, 2015 [PMC free article] [PubMed] [Google Scholar]
- 19.Ford ES, Mokdad AH. Dietary magnesium intake in a national sample of US adults. The Journal of Nutrition. 2003;133(9):2879–2882. [DOI] [PubMed] [Google Scholar]
- 20.Bertinato J, Wang KC, Hayward S. Serum magnesium concentrations in the Canadian population and associations with diabetes, glycemic regulation, and insulin resistance. Nutrients. 2017;9(3)296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houston M. The role of magnesium in hypertension and cardiovascular disease. Journal of Clinical Hypertension. 2011;13(11):843–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahabir S, Wei Q, Barrera SL, et al. Dietary magnesium and DNA repair capacity as risk factors for lung cancer. Carcinogenesis. 2008;29(5):949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groome PA, O’Sullivan B, Mackillop WJ, et al. Compromised local control due to treatment interruptions and late treatment breaks in early glottic cancer: Population-based outcomes study supporting need for intensified treatment schedules. International Journal of Radiation Oncology. 2006. Mar 15;64(4):1002–12. [DOI] [PubMed] [Google Scholar]
- 24.Shim K, MacKenzie MJ, Winquist E. Chemotherapy-associated osteonecrosis in cancer patients with solid tumours: a systematic review. Drug Safety. 2008;31(5):359–371. [DOI] [PubMed] [Google Scholar]
- 25.Cook AM, Dzik-Jurasz AS, Padhani AR, Norman A, Huddart RA. The prevalence of avascular necrosis in patients treated with chemotherapy for testicular tumours. British Journal of Cancer. 2001;85(11):1624–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu W, Qdaisat A, Zhou S, et al. Hypomagnesemia and incidence of osteoradionecrosis in patients with head and neck cancer. Head & Neck. 2021;43(2):613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oronsky B, Caroen S, Oronsky A, et al. Electrolyte disorders with platinum-based chemotherapy: mechanisms, manifestations and management. Cancer Chemotherapy and Pharmacology. 2017;80(5):895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]


