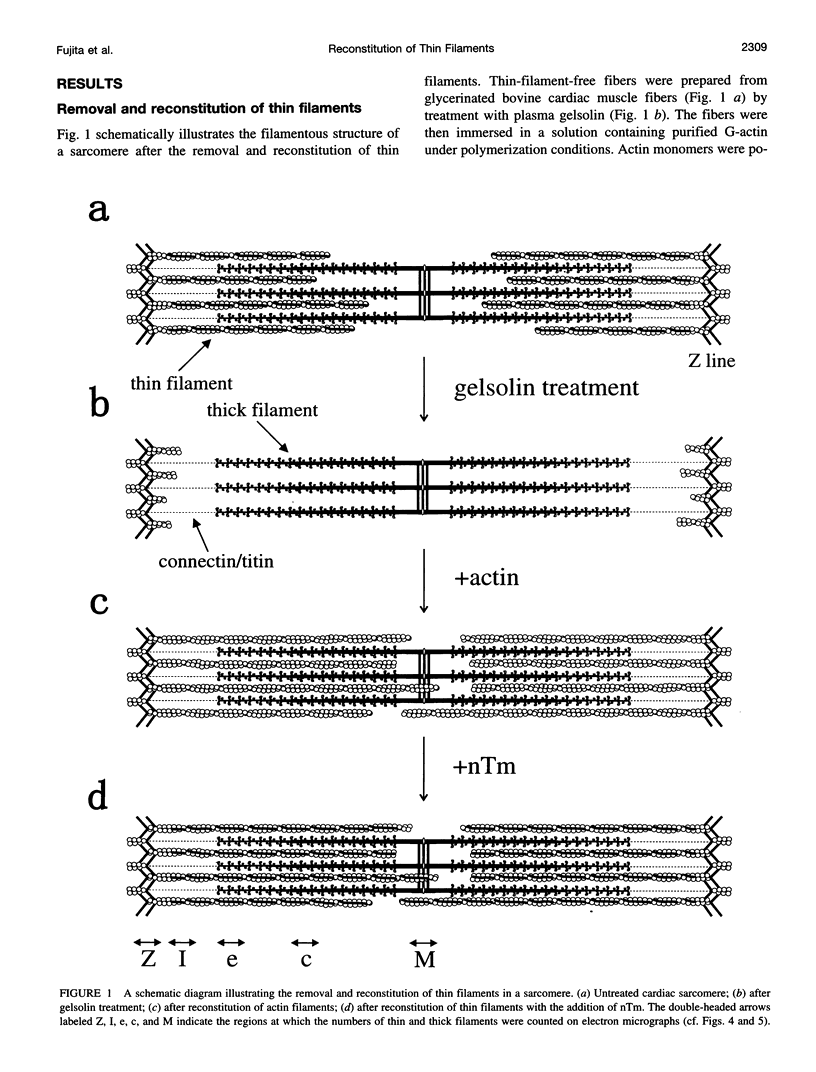
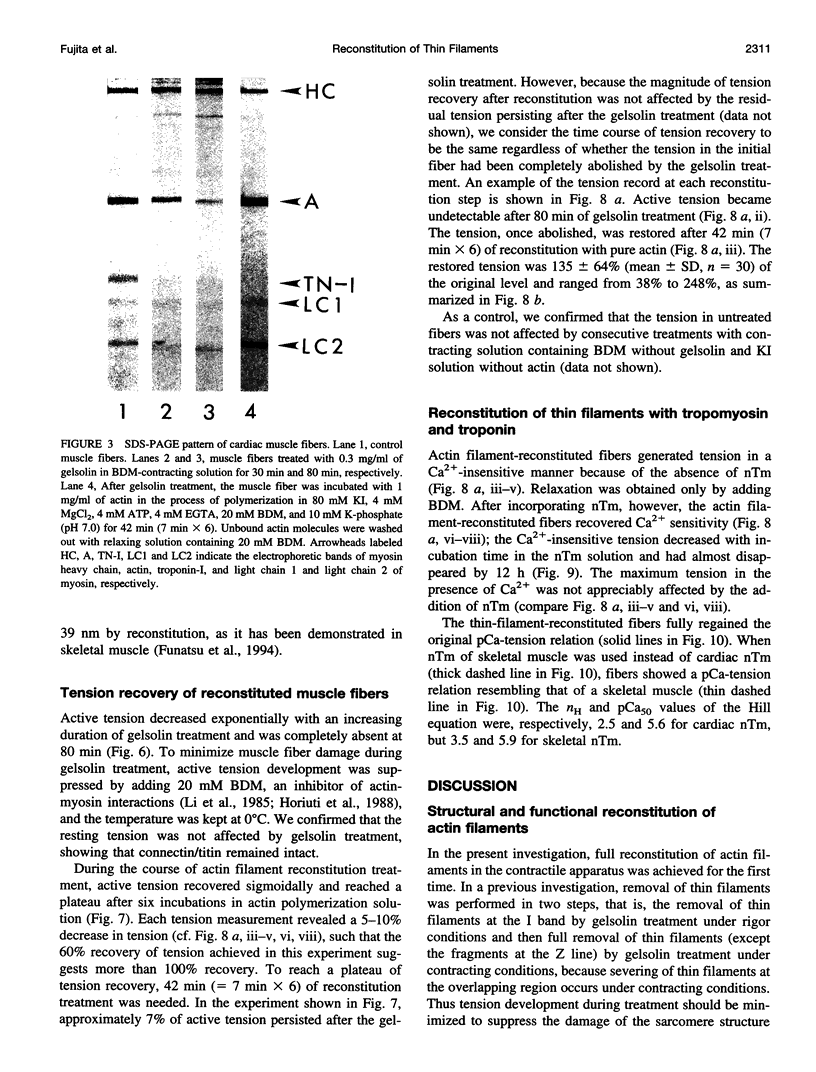
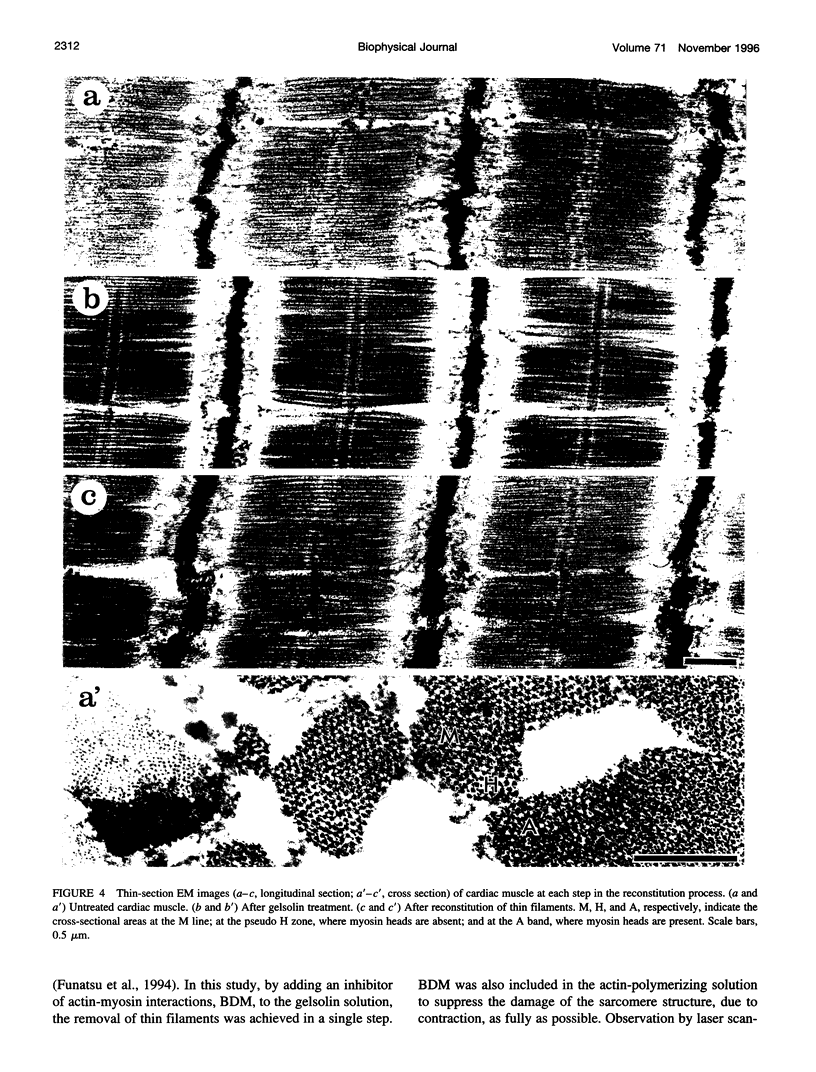
Abstract
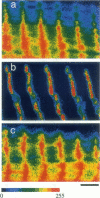
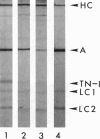
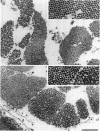
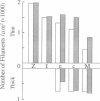
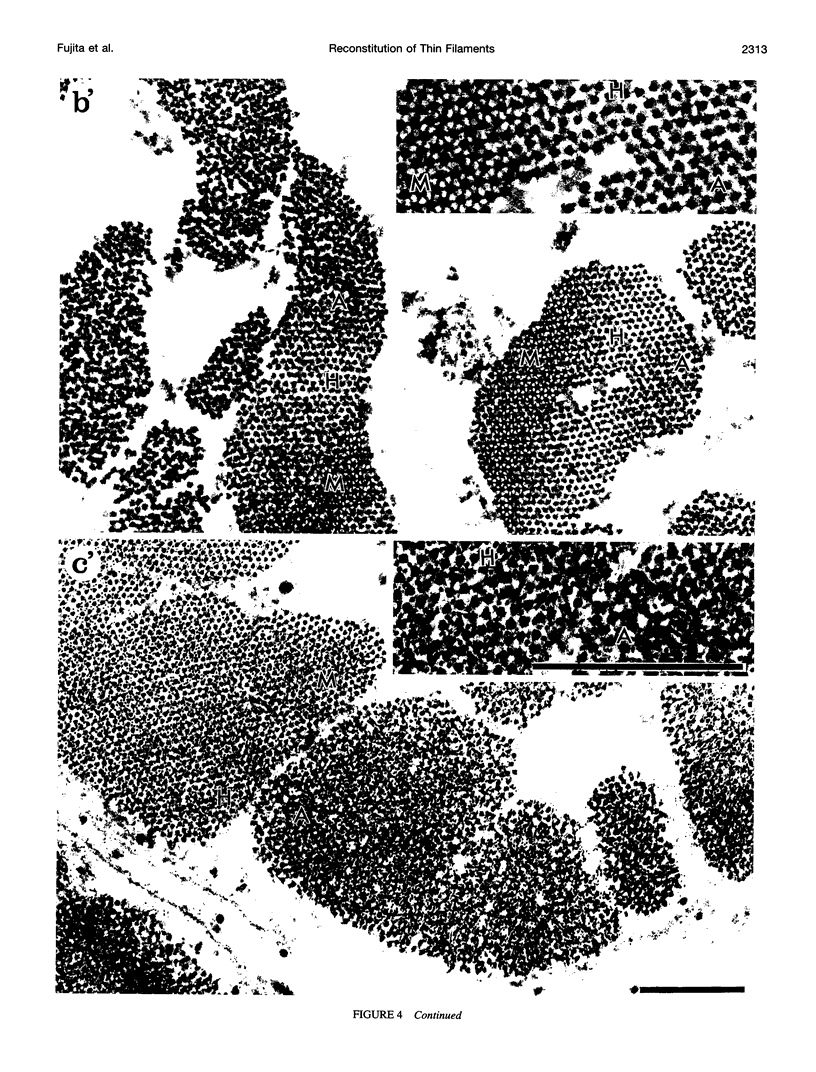
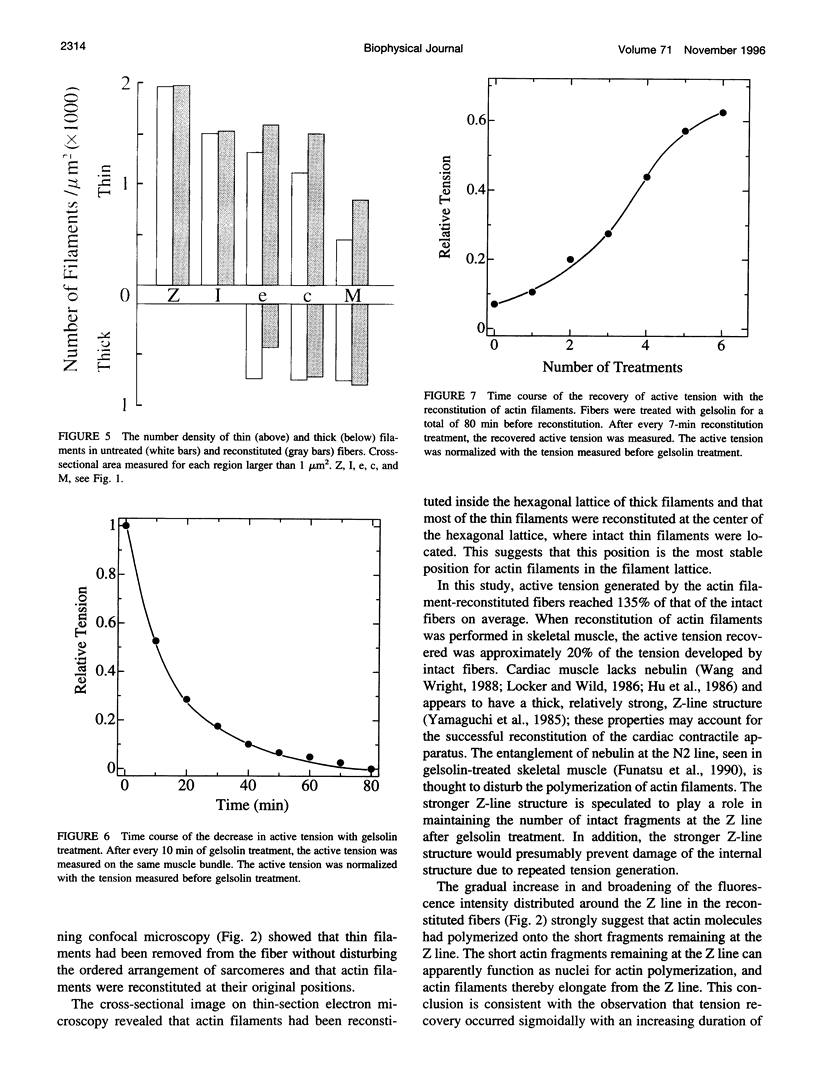
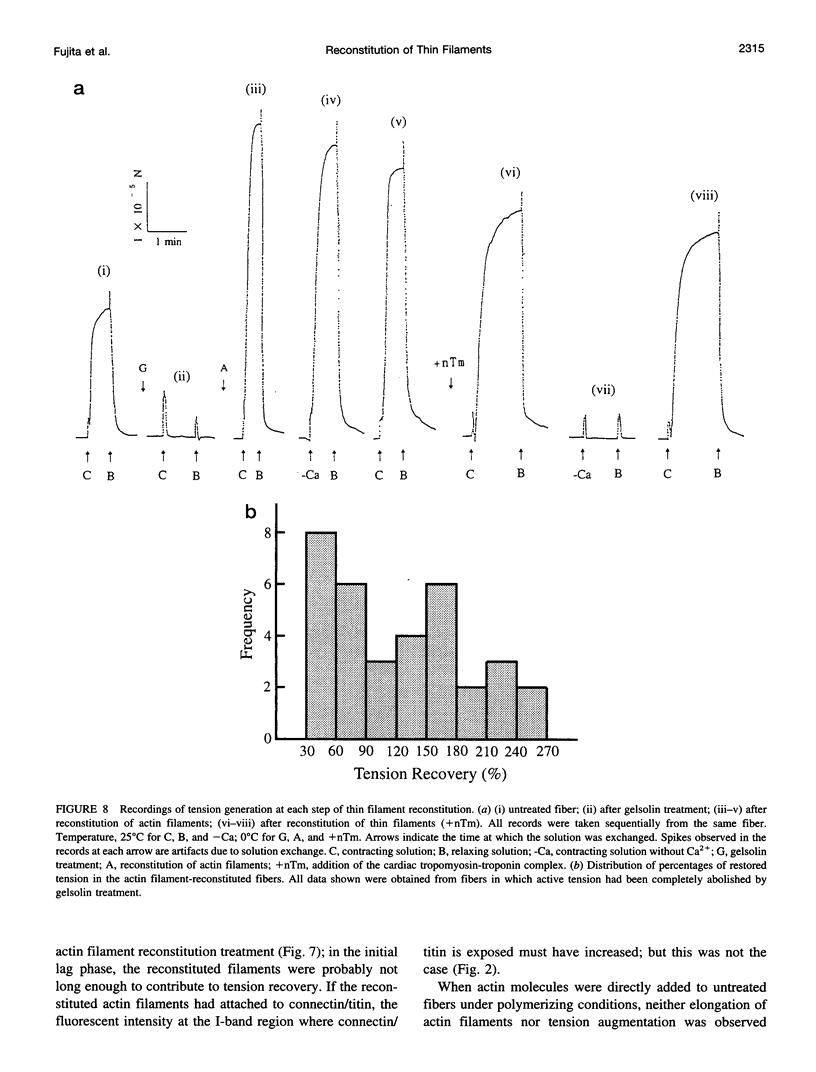
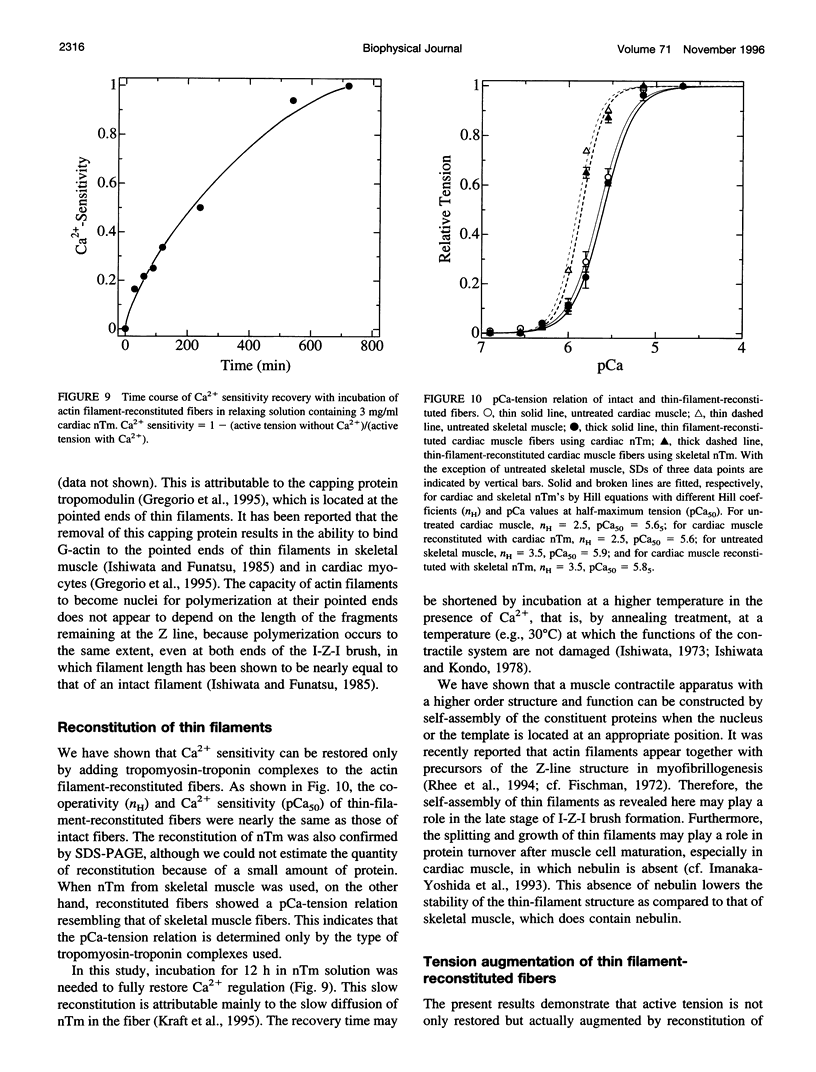
The muscle contractile apparatus has a highly ordered liquid crystalline structure. The molecular mechanism underlying the formation of this apparatus remains, however, to be elucidated. Selective removal and reconstitution of the components are useful means of examining this mechanism. In addition, this approach is a powerful technique for examining the structure and function of a specific component of the contractile system. In this study we have achieved the structural and functional reconstitution of thin filaments in the cardiac contractile apparatus. First, all thin filaments other than short fragments at the Z line were removed by treatment with gelsolin. Under these conditions no active tension could be generated. By incorporating exogenous actin into these thin filament-free fibers, actin filaments were reconstituted, and active tension, which was insensitive to Ca2+, was restored. The active tension after the reconstitution of thin filaments reached 135 +/- 64% of the original level. The augmentation of tension was attributable to the elongation of reconstituted filaments. As another possibility for augmented tension generation, we suggest the presence of an inhibitory system that was not reconstituted. In any case, the thin filaments of the cardiac contractile apparatus are considered to be assembled so as not to develop the highest degree of tension. Incorporation of the tropomyosin-troponin complex fully restored Ca2+ sensitivity without affecting maximum tension. The present results indicate that a muscle contractile apparatus with a higher order structure and function can be constructed by the self-assembly of constituent proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Kentish J. C. The cellular basis of the length-tension relation in cardiac muscle. J Mol Cell Cardiol. 1985 Sep;17(9):821–840. doi: 10.1016/s0022-2828(85)80097-3. [DOI] [PubMed] [Google Scholar]
- Bukatina A. E., Fuchs F. Effect of phalloidin on the ATPase activity of striated muscle myofibrils. J Muscle Res Cell Motil. 1994 Feb;15(1):29–36. doi: 10.1007/BF00123830. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Kodama A., Ebashi F. Troponin. I. Preparation and physiological function. J Biochem. 1968 Oct;64(4):465–477. doi: 10.1093/oxfordjournals.jbchem.a128918. [DOI] [PubMed] [Google Scholar]
- Fraenkel-Conrat H., Williams R. C. RECONSTITUTION OF ACTIVE TOBACCO MOSAIC VIRUS FROM ITS INACTIVE PROTEIN AND NUCLEIC ACID COMPONENTS. Proc Natl Acad Sci U S A. 1955 Oct 15;41(10):690–698. doi: 10.1073/pnas.41.10.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funatsu T., Anazawa T., Ishiwata S. Structural and functional reconstitution of thin filaments in skeletal muscle. J Muscle Res Cell Motil. 1994 Apr;15(2):158–171. doi: 10.1007/BF00130426. [DOI] [PubMed] [Google Scholar]
- Funatsu T., Higuchi H., Ishiwata S. Elastic filaments in skeletal muscle revealed by selective removal of thin filaments with plasma gelsolin. J Cell Biol. 1990 Jan;110(1):53–62. doi: 10.1083/jcb.110.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funatsu T., Kono E., Higuchi H., Kimura S., Ishiwata S., Yoshioka T., Maruyama K., Tsukita S. Elastic filaments in situ in cardiac muscle: deep-etch replica analysis in combination with selective removal of actin and myosin filaments. J Cell Biol. 1993 Feb;120(3):711–724. doi: 10.1083/jcb.120.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorio C. C., Weber A., Bondad M., Pennise C. R., Fowler V. M. Requirement of pointed-end capping by tropomodulin to maintain actin filament length in embryonic chick cardiac myocytes. Nature. 1995 Sep 7;377(6544):83–86. doi: 10.1038/377083a0. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F., NIEDERGERKE R. Structural changes in muscle during contraction; interference microscopy of living muscle fibres. Nature. 1954 May 22;173(4412):971–973. doi: 10.1038/173971a0. [DOI] [PubMed] [Google Scholar]
- HUXLEY H., HANSON J. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature. 1954 May 22;173(4412):973–976. doi: 10.1038/173973a0. [DOI] [PubMed] [Google Scholar]
- Horiuti K., Higuchi H., Umazume Y., Konishi M., Okazaki O., Kurihara S. Mechanism of action of 2, 3-butanedione 2-monoxime on contraction of frog skeletal muscle fibres. J Muscle Res Cell Motil. 1988 Apr;9(2):156–164. doi: 10.1007/BF01773737. [DOI] [PubMed] [Google Scholar]
- Hu D. H., Kimura S., Maruyama K. Sodium dodecyl sulfate gel electrophoresis studies of connectin-like high molecular weight proteins of various types of vertebrate and invertebrate muscles. J Biochem. 1986 May;99(5):1485–1492. doi: 10.1093/oxfordjournals.jbchem.a135618. [DOI] [PubMed] [Google Scholar]
- Imanaka-Yoshida K., Sanger J. M., Sanger J. W. Contractile protein dynamics of myofibrils in paired adult rat cardiomyocytes. Cell Motil Cytoskeleton. 1993;26(4):301–312. doi: 10.1002/cm.970260405. [DOI] [PubMed] [Google Scholar]
- Ishiwata S. A study on the F-actin-tropomyosin-troponin complex. I. Gel-filament transformation. Biochim Biophys Acta. 1973 Mar 23;303(1):77–89. doi: 10.1016/0005-2795(73)90150-5. [DOI] [PubMed] [Google Scholar]
- Ishiwata S., Funatsu T. Does actin bind to the ends of thin filaments in skeletal muscle? J Cell Biol. 1985 Jan;100(1):282–291. doi: 10.1083/jcb.100.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwata S., Kondo H. Studies on the F-actin.tropomyosin.troponin complex. II. Partial reconstitution of thin filament by F-actin, tropomyosin and the tropomyosin binding component of troponin (TN-T). Biochim Biophys Acta. 1978 Jun 21;534(2):341–349. doi: 10.1016/0005-2795(78)90017-x. [DOI] [PubMed] [Google Scholar]
- Katsura I., Noda H. Assembly of myosin molecules into the structure of thick filaments of muscle. Adv Biophys. 1973;5(0):177–202. [PubMed] [Google Scholar]
- Kondo H., Ishiwata S. Uni-directional growth of F-actin. J Biochem. 1976 Jan;79(1):159–171. doi: 10.1093/oxfordjournals.jbchem.a131043. [DOI] [PubMed] [Google Scholar]
- Kraft T., Messerli M., Rothen-Rutishauser B., Perriard J. C., Wallimann T., Brenner B. Equilibration and exchange of fluorescently labeled molecules in skinned skeletal muscle fibers visualized by confocal microscopy. Biophys J. 1995 Oct;69(4):1246–1258. doi: 10.1016/S0006-3495(95)80018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa H., Fujii W., Ohmi K., Sakurai T., Nonomura Y. Simple and rapid purification of brevin. Biochem Biophys Res Commun. 1990 Apr 30;168(2):451–457. doi: 10.1016/0006-291x(90)92342-w. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li T., Sperelakis N., Teneick R. E., Solaro R. J. Effects of diacetyl monoxime on cardiac excitation-contraction coupling. J Pharmacol Exp Ther. 1985 Mar;232(3):688–695. [PubMed] [Google Scholar]
- Locker R. H., Wild D. J. A comparative study of high molecular weight proteins in various types of muscle across the animal kingdom. J Biochem. 1986 May;99(5):1473–1484. doi: 10.1093/oxfordjournals.jbchem.a135617. [DOI] [PubMed] [Google Scholar]
- Moncman C. L., Wang K. Nebulette: a 107 kD nebulin-like protein in cardiac muscle. Cell Motil Cytoskeleton. 1995;32(3):205–225. doi: 10.1002/cm.970320305. [DOI] [PubMed] [Google Scholar]
- Nomura M. Assembly of bacterial ribosomes. J Supramol Struct. 1974;2(2-4):163–165. doi: 10.1002/jss.400020210. [DOI] [PubMed] [Google Scholar]
- Rhee D., Sanger J. M., Sanger J. W. The premyofibril: evidence for its role in myofibrillogenesis. Cell Motil Cytoskeleton. 1994;28(1):1–24. doi: 10.1002/cm.970280102. [DOI] [PubMed] [Google Scholar]
- Robinson T. F., Winegrad S. Variation of thin filament length in heart muscles. Nature. 1977 May 5;267(5606):74–75. doi: 10.1038/267074a0. [DOI] [PubMed] [Google Scholar]
- Saeki Y., Kawai M., Zhao Y. Comparison of crossbridge dynamics between intact and skinned myocardium from ferret right ventricles. Circ Res. 1991 Mar;68(3):772–781. doi: 10.1161/01.res.68.3.772. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Taniguchi M., Ishikawa H. In situ reconstitution of myosin filaments within the myosin-extracted myofibril in cultured skeletal muscle cells. J Cell Biol. 1982 Feb;92(2):324–332. doi: 10.1083/jcb.92.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawada K., Yoshida A., Morita K. Myosin-free ghosts of single fibers and an attempt to re-form myosin filaments in the ghost fibers. J Biochem. 1976 Jul;80(1):121–127. doi: 10.1093/oxfordjournals.jbchem.a131243. [DOI] [PubMed] [Google Scholar]
- Wang K., Wright J. Architecture of the sarcomere matrix of skeletal muscle: immunoelectron microscopic evidence that suggests a set of parallel inextensible nebulin filaments anchored at the Z line. J Cell Biol. 1988 Dec;107(6 Pt 1):2199–2212. doi: 10.1083/jcb.107.6.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M., Izumimoto M., Robson R. M., Stromer M. H. Fine structure of wide and narrow vertebrate muscle Z-lines. A proposed model and computer simulation of Z-line architecture. J Mol Biol. 1985 Aug 20;184(4):621–643. doi: 10.1016/0022-2836(85)90308-0. [DOI] [PubMed] [Google Scholar]
- Yasuda K., Anazawa T., Ishiwata S. Microscopic analysis of the elastic properties of nebulin in skeletal myofibrils. Biophys J. 1995 Feb;68(2):598–608. doi: 10.1016/S0006-3495(95)80221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]